Abstract
Purpose of Review
The goal of this paper is to review current literature on the gut microbiome within the context of host response to surgery and subsequent risk of developing complications, particularly anastomotic leak. We provide background on the relationship between host and gut microbiota with description of the role of the intestinal mucus layer as an important regulator of host health.
Recent Findings
Despite improvements in surgical technique and adherence to the tenets of creating a tension-free anastomosis with adequate blood flow, the surgical community has been unable to decrease rates of anastomotic leak using the current paradigm. Rather than adhere to empirical strategies of decontamination, it is imperative to focus on the interaction between the human host and the gut microbiota that live within us. The gut microbiome has been found to play a potential role in development of post-operative complications, including but not limited to anastomotic leak. Evidence suggests that peri-operative interventions may have a role in instigating or mitigating the impact of the gut microbiota via disruption of the protective mucus layer, use of multiple medications, and activation of virulence factors.
Summary
The microbiome plays a potential role in the development of surgical complications and can be modulated by peri-operative interventions. As such, further research into this relationship is urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite major advances in surgical technology and technique, anastomotic leak (AL) remains a feared complication after intestinal resection and is responsible for high morbidity and mortality. AL is caused by a disruption in the healing gut epithelium that allows for intestinal contents—succus or stool—to leak into the peritoneal cavity. The consequences range from subtle subclinical leaks to abscesses requiring percutaneous drainage to potentially fatal systemic sepsis. Leakage is associated with increased risk of severe consequences, including permanent stoma, pelvic sepsis, fistula, and increased all-cause mortality. The overall incidence of AL in colorectal surgery is approximately 11% with a range of 1 to 24% [1, 2], with increasing incidence for more distal colorectal and coloanal anastomoses [3]. AL contributes to greater length-of-stay, cost, poor quality of life, and need for further interventions; it is also associated with increased recurrence and decreased survival after oncologic resection [4, 5]. The overall mortality rate from AL is typically reported as high as 15% [6].
Despite extensive research into possible risk factors and optimal surgical technique, the pathogenesis of AL remains unknown [2]. The development of AL has been attributed to multiple patient variables including age, comorbidities, obesity, diet, smoking, radiation therapy, and immunosuppression among others. The success of an anastomosis is historically thought to be secondary to surgical technique and adherence to the basic tenets of ensuring good blood flow, a tension-free connection, and carefully executed apposition of mucosa either via a hand-sewn or stapled technique. While there is no doubt that these three factors are important for a healthy anastomosis, this paradigm does not account for the role of the endogenous microbiome and the protective mucus layer that lines the gut epithelium. Recent evidence suggests a potential relationship between microbial virulence and anastomotic breakdown.
Advancements in sequencing technology over the past three decades have exponentially expanded our ability to characterize the gut microbiota (i.e., microbial strains) and have identified correlations between gut microbiota composition and a host of conditions, including diet, environment, and disease state (e.g., inflammatory bowel disease, colorectal cancer, autism, obesity, and metabolic syndrome). As we move toward microbiota-based therapies, it is critical that we employ additional technologies (e.g., metabolomics, proteomics), high-dimensional analytics, culture-based techniques, and animal models to determine the functional role of the microbiota in effecting host physiology [7,8,9,10]. We have begun to understand that nuances and inter-patient variability of the microbiome may contribute to the development of post-operative complications. This review will highlight areas to focus future research for limiting iatrogenic perturbations to the host-microbiota relationship during the peri-operative period. To improve outcomes and make surgery safer, we need to increase our understanding of the molecular, genetic, and functional response of the host in reaction to alterations in microbiota [11]. Over the course of this review, we will begin to explore how surgical intervention initiates alterations in the host microenvironment that can cause a shift in microbiota composition and behavior that subsequently may confer increased risk of developing post-operative complications.
Background
The Microbiome and Its Metabolome
The human gut is home to a diverse microbial community (i.e., bacteria, viruses, fungi); the term microbiome refers to these microbes, their genes, and their metabolic products. More than 1500 bacterial species make up the gut microbiota; the majority are anaerobic and belong to the Firmicutes and Bacteroidetes phyla [12, 13]. However, there is substantial inter-individual variability largely based on environmental factors (diet, age, exercise, antibiotic use, smoking, inflammation, etc.) [14•, 15, 16, 17•] and genetic inheritance [18]. In addition to this vast taxonomic diversity, the gut microbiota contains an extraordinary genomic potential; the metagenome of the gut microbial community is approximately 150-fold larger than the human gene complement [19]. The bacterial community—both the structure of the community and the metabolic activities of individual taxa—is impacted by substrate delivered from the diet. The microbiota uses diet as the substrate to produce metabolites that in turn influence host physiology [20,21,22,23,24]. Consisting of tens of trillions of bacterial cells in the colon, the gut microbiota is the main source of thousands of small bioactive molecules that can trigger host metabolism and immunity [25]. Collectively, host-derived small-molecule compounds and these bacterial-derived metabolites within the host’s blood stream are referred to as the systemic metabolome.
The vast majority of human microbiome studies have used fecal samples as a proxy for the gut microbiome. However, it is important to note that the composition, diversity, and function of the gut microbiota vary both longitudinally (i.e., along the GI tract from the mouth to anus) and radially (i.e., from tissue-mucosal interface to the lumen) within the GI tract [26]. Longitudinally, although only a short physical distance separates the small intestine and the colon, they contain vastly different microbiota. Contrary to the highly diverse and relatively stable colonic microbiota, the small bowel microbiota has a lower diversity (up to 88.3% of bacteria from one genus [27, 28]) and is subject to sub-daily fluctuations [29]. These fluctuations are likely driven by a response to dietary variation, as metatranscriptomic analysis has shown that the metabolic focus of the small bowel microbiota is transport and metabolism of simple carbohydrate substrates [23]. Radially, microbiota composition varies between the epithelial cell layer, the mucus layer, and the lumen [30].
This anatomic and spatial diversity impacts microbial interaction between different species of bacteria and between bacteria and host. In addition to being influenced by lifestyle factors and host biology, bacteria can impact each other through intra- and inter-species communication via quorum sensing [31]. For example, the virulence of Pseudomonas aeruginosa is modulated by the presence of specific fermentation products [32]. Additionally, host defense and immunity play an important role in creating specific niches for bacterial colonization via the production of secretory immunoglobulins and antimicrobial peptides, as well as the activation of bactericidal mechanisms in response to mucosal injury.
In healthy subjects, the commensal bacteria in the human GI tract exist symbiotically with their host and contribute to the development of metabolism, immunity, epithelial cell growth and development, energy harvesting, gut motility, barrier function, and absorption of nutrients [33]. While daily variations in lifestyle—including diet, exercise, pets, and environment—can cause marked alterations in the microbiome, composition and function of the gut microbiome in healthy humans remain relatively stable [34]. However, upon injury—including elective surgery—the gut microbiota can undergo a swift phenotypic shift and dramatic change in microbial density and metabolite production. Over the course of a few hours after an insult, the microbiome demonstrates a 90% reduction in Bacteroidetes and Firmicutes species with a concomitant rise in potentially pathogenic gamma-Proteobacteria within the GI tract, which includes virulent phenotypes of Escherichia coli, Enterococcus faecalis, and P. aeruginosa that are often associated with post-operative infections [11]. These changes have been demonstrated to persist up to 90 days after surgery in a mouse model [35•]. Notwithstanding, the human response to surgical injury can be positively influenced through early administration of IV fluids, oral nutrition, and judicious medication use, which can support refaunation of the commensal microbiota [36].
The Intestinal Mucus Layer
The intestinal mucosa is lined by epithelial cells that are covered by a functional mucus system [37]. Colonic mucus is composed of two layers [38]: an inner layer of net-like sheets of MUC2—gel-forming mucin polymers—that is impenetrable to bacteria in healthy hosts [39], and a non-attached outer mucus layer that serves as a habitat for distinct colonic microbiota. The mucus layer is essential for protecting the colonic mucosa from injury and is regenerated by goblet cells through the production and secretion of mucin on an hourly basis [38]. Alterations to the host can affect this process; for example, in an ischemia-reperfusion model, colonic ischemia led to detachment of mucus that placed bacteria in direct contact with the intestinal epithelium. Following reperfusion, crypt goblet cells secreted stored mucus and effectively cleared the bacteria that had come in contact with the epithelium [40]. Additionally, mucus secretion is in part regulated by microbiota, including through bacterial metabolites generated from diet, prostaglandins, and certain bacterial strains [41]. The thickness of the mucus layer, which is rich in polysaccharides [38], has been positively correlated with microbial community diversity [42] and negatively correlated with dietary fiber intake [43]. A defective mucus layer that allows bacteria to contact the mucosa may be a pathophysiological mechanism for infectious disease, metabolic syndrome, and colitis [44, 45].
Host mucus production also impacts the composition of the microbiota, as specific microorganisms are capable of utilizing mucus glycoproteins as a nutrient for growth [46, 47]. While similar bacterial species exist in both the outer mucus layer and the lumen, differential resource utilization and genetic expression are observed between the separate compartments [30]. In healthy hosts, an increased presence of Actinobacteria and Proteobacteria is found in the mucosa-associated microbiome. This increase in aerotolerant organisms could, in part, be based on a radial oxygen gradient between aerobic tissue and the anaerobic lumen [48, 49]. Mucosally associated microbes differ significantly from luminal microbiota [50] and perform a distinct role in the host-microbiota relationship. Molecular exchange of bioactive molecules occurs bidirectionally between the host and the outer mucus layer [30].
The Impact of Peri-operative Interventions on the Microbiome-Host Relationship
Despite extensive studies linking the human gut microbiome with human health and disease (e.g., obesity, inflammatory diseases, malignancy), our understanding of its role in surgical diseases and outcomes remains limited. It will be essential to find a balance between our evolving understanding of the microbiome’s role in immune function and wound repair with the traditional dogma of intestinal antisepsis before gastrointestinal surgery. Surgeons make multiple peri-operative perturbations (e.g., fasting—nil per os [NPO], mechanical bowel preparation [MBP], and antibiotic use—both oral and intravenous administration) that impact the microbiota with minimal understanding of the impact on the structure or function(s) of the bacterial community [51, 52]. Evidence suggests that the microbiome may play an integral role in the development of post-operative complications, particularly AL and surgical site infections (SSI). While our understanding of the microbiome’s impact on the repair of anastomotic tissues is in its infancy, emerging data suggests that the insult of surgery itself may alter host-microbiota virulence and increase the risk of post-op complications, especially AL.
Peri-operative medications can also alter microbiome composition. Antacids neutralize gastric secretions, which disrupt the balance of acid-sensitive organisms in the foregut. Vasoactive medications, which are often used in critically ill patients, impact perfusion and oxygen delivery to the bowel lumen and induce luminal hypoxia and hypercarbia, which can induce a shift in bacterial virulence. Opioids disrupt peristalsis and impair GI motility, thereby decreasing the mechanical removal of luminal material (including bacteria). This can result in ileus, dysbiosis, and/or bacterial overgrowth. The host-pathogen balance is also altered in the setting of colonic enteral nutrient deprivation—either due to fasting, total parenteral nutrition, or highly processed foods whose absorption is complete in the small bowel. This imbalance is characterized by an absence of commensal bacteria, a plethora of highly virulent microorganisms, and a dampened immune response with alterations in intestinal barrier function that ultimately can lead to systemic inflammation (e.g., gut-derived sepsis) [53].
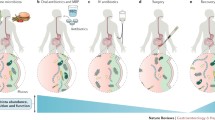
Peri-operative interventions have the potential to induce a bloom of virulent bacterial taxa (e.g., Enterococcus, Pseudomonas) that are able to shift toward a more aggressive and tissue-destroying phenotype in response to environmental cues [54••, 55]. For example, catecholamines secreted in response to stress can affect the growth of certain bacterial taxa, including E. coli, P. aeruginosa, Salmonella typhi, Yersinia enterocolitica, and Campylobacter jejuni, as well as their expression of virulence factors [56]. Likewise, intermittent intestinal hypoperfusion and subsequent reperfusion injury can alter the established oxygen gradient within the lumen of the GI tract and lead to increased nitrogen concentration, which favors the growth of opportunistic pathogens [48]. These alterations may contribute to the pathogenesis of AL (Fig. 1).
Pre-operative Manipulation of the Microbiome and the Mucus Layer
Pre-operative manipulations have the potential to have long-lasting consequences for the gut microbiota and the host. Both enteral and parenteral antibiotics shift the composition of the bacterial community, alter host-microbe symbiosis, and decrease protection against pathogenic bacteria conferred by commensal microbes [57, 58]. There are multiple mechanisms by which commensal organisms confer protection against pathogenic infections, including competitive niche exclusion (i.e., competition for space and nutrients), production of inhibitory compounds (e.g., antimicrobial peptides), and intra- and inter-species communication (i.e., quorum sensing). Antibiotic use can disrupt this defense mechanism via depletion and/or alteration of the commensal gut microbiota [30]. Although the microbiota recovers quickly following termination of antibiotic use, alterations can persist for up to 2 years after treatment, including decreased diversity, alteration of taxonomic composition, and upregulation of antibiotic resistance genes [59].
MBP can be employed with or without oral antibiotics. There are two mechanisms of action by which MBP alters the gut microbiota. Increased fluid in the colonic lumen washes out fecal content and stimulates gut motility in response to distension. This increase in gut motility also leads to a decrease in bacterial load and alteration in community structure [51, 60, 61]. The specific impact(s) of lavage itself on the colonic microbiota is poorly understood; however, there are differing effects on the mucosa-associated and luminal colonic (i.e., stool) bacteria [62]. In addition to clearing bulky matter and improving visualization, purgative cleansing preparations also induce mucosal inflammation and create a transient shift in microbial composition [51, 63]. A randomized controlled trial evaluating the effect of pre-op MBP on fecal flora found significant reduction in total number of bacteria, including Clostridium, Bifidobacteria, Lactobacillus, and Enterobacteriaceae. In contrast, there was no demonstrable decrease in the potentially pathogenic taxa Enterococcus and Staphylococcus [64, 65]. MBP has also been shown to impact the mucus layer, decrease the concentration of SCFAs in the lumen, and stimulate a bloom in Proteobacteria by altering luminal pH [66••, 67]. Alterations to the mucosa can ultimately impact intracellular signaling pathways.
While nearly all colorectal surgeons in the USA reported using MBP in 2003, there has been a slight decline over the past 15 years due to increasing scrutiny over the efficacy of MBP [68, 69•]. Several recent trials were unable to show a statistically significant difference in SSI between patients operated on with or without MBP (however, this analysis did not include the use of oral antibiotics) [68, 70, 71]. Further, a 2011 Cochrane review [72] showed no decrease in AL, post-operative complications, or mortality in the colorectal population following MBP alone. Since then, there is conflicting information from multiple studies regarding use of MBP with or without oral antibiotics in the era of prophylactic parenteral antibiotics.
In large retrospective analyses, pre-operative MBP combined with oral antibiotics significantly reduced (~ 50%) the rate of complications following colorectal surgery, including surgical site infection, ileus, and AL; however, this study did not address if both interventions are necessary for this outcome [62]. An analysis of the Veterans Affairs Surgical Quality Improvement Program (VASQIP) revealed no difference between the rate of SSI for patients receiving oral antibiotics, irrespective of whether they also had MBP (9% vs. 8%, with and without MBP, respectively); however, for the group that did not receive oral antibiotics, MBP alone was not effective in preventing infections (SSI rate doubled to 20%). After adjusted analysis, they found that MBP with oral antibiotics was associated with a 57% decrease in SSI (OR = 0.43, 95% CI 0.34–0.55). Even more striking, oral antibiotics alone resulted in a 67% decrease in SSI (OR = 0.33, 95% CI 0.21–0.5 0) [73]. This finding was re-demonstrated in Morris et al.’s evaluation of NSQIP data from 2011 to 2012, which demonstrated a 50% reduction in SSI with oral antibiotics versus no oral antibiotics (6.5% vs. 13%, respectively) [74].
These analyses suggest that benefits of oral antibiotics may outweigh their effects on the microbiota; it is less clear if the benefits of MBP, beyond making surgery technically easier, outweigh the impact on the microbiome. However, further work examining the specific mechanisms by which these interventions alter the microbiota in the setting of surgical interventions, as well as the functional consequences, is required before clinical protocols are significantly altered.
Surgical Injury and Host Response: Disruption of Microbiome Has Functional Consequences
Anastomotic Tissues—Bacterial and Host Response
Despite efforts to minimize trauma to tissues and adherence to the tenets of robust blood flow, decreased tension, and proper construction of anastomoses, the rates of AL have not changed in the twenty-first century [2]. Further, while grounded in pragmatism, these tenets are not based on level 1 evidence, which suggests the need to expand our understanding of the dynamic response to the creation of an anastomosis. Commensal microbes are influenced by surgical injury and peri-operative management; microbes continuously sample their microenvironment and respond to host cues to optimize their own survival. Tissue ischemia has been demonstrated to impact local microbiota composition. In a mouse model, mesenteric ischemia and subsequent reperfusion induced a relative increase in E. coli and decrease in Lactobacillus of the ileum and colon. This change persisted for approximately 6 h after reperfusion until colonic microbiota began to recover [75]. This shift in colonic microbiota is accompanied by breakdown in intestinal barrier function with loss of mucus layer integrity, which allows for clinically relevant localized tissue inflammation and translocation of potentially pathogenic bacterial species [11]. The transient ischemia endured during the process of creating an anastomosis induces reversible damage to the mucus layer as long as reperfusion occurs in under an hour [40]. Upon reperfusion, Goblet cells rapidly replenish the mucus layer via mass exocytosis of mucin. Because mucin biosynthesis takes time, the anastomosis should be protected from an additional period of ischemia.
Over 60 years ago, Cohn and Rives developed a dog model of colonic anastomosis, which included ligation of mesenteric vessels to induce ischemia of the bowel. They then used an indwelling catheter to infuse either topical antibiotics or saline directly at the site of anastomosis. Animals that received antibiotics demonstrated complete healing of anastomosis, as opposed to animals receiving saline alone who developed major leakage, peritonitis, and death [76]. The topical administration of antibiotics clearly interacted with the bacterial species in a beneficial way. The protective effect of non-absorbable enteric antibiotics was re-demonstrated by Cohen et al. in 1984 who also showed avoidance of AL despite mesenteric ischemia [77]. A decade later, Schardey and colleagues used a rat model to identify a potentially causative species of AL, P. aeruginosa [78]. In this model of total gastrectomy with esophagoduodenostomy, oral gavage of P. aeruginosa was compared to oral antibiotic treatment. The introduction of pathogenic Pseudomonas resulted in significantly greater transmural histological defect at the anastomosis and functionally resulted in lower bursting pressure—two features that reflect increased risk of leak rate. These experiments demonstrate the detrimental role that virulent microbiota can play in development of AL.
The process of resecting bowel and creating a new anastomosis induces a response in the host that causes release of soluble products. These chemical messengers are able to attract microbes and immune cells to the site of injury and send “cues” that induce a phenotypic shift among pathogenic bacteria, including Pseudomonas and Enterococcus [79•]. To understand how the normal dynamic response to surgery might lead to increased microbial virulence, Shogan et al. used 16S sequencing to characterize microbiota changes following partial colectomy and primary anastomosis [79•]. From post-operative day 0 to day 7, their group found a 200- and 500-fold increase in relative abundance of Escherichia/Shigella and Enterococcus, respectively. When examined on a functional level, there was a predominance in expression of bacterial virulence-associated pathways near the anastomotic tissue. Significantly, this bacterial gene expression was not present in the stool or luminal contents, which implies that there may induction of a phenotypic shift leading to increased local adherence of invasive bacteria to the anastomotic tissues.
Intestinal P. aeruginosa is capable of responding to host signals released during stress. By utilizing quorum sensing, opportunistic pathogens can sense host environmental changes (i.e., stress or injury) and respond by inducing a phenotypic shift in their own virulence [80]. In vitro, it has been demonstrated that products of hypoxic intestinal epithelial cells, like adenosine and dynorphin, can directly activate quorum sensing. For P. aeruginosa, this response is characterized by a shift to a more aggressive and barrier disrupting phenotype with high collagen-degradation activity [54••, 81] and increased intestinal tight junction permeability [82]. Theoretically, this may also occur during times of ischemia and reperfusion in vivo. This is further supported by both hemorrhagic shock and ischemia-reperfusion models using germ-free mice that demonstrate improved survival compared to conventional control animals [83, 84]. In mice, morphine exposure led to a shift to a more virulent phenotype of P. aeruginosa that expressed greater biofilm formation, increased antibiotic resistance, and the ability to cause lethal gut-derived sepsis. Further, in the presence of morphine, these bacteria shifted to a mucus-suppressing phenotype, which disrupted the mucus layer and subsequently degraded gut epithelial integrity [85]. The increased virulence in P. aeruginosa has been attributed to a SNP mutation in the mexT gene that displays increased tissue destruction, collagenase expression, and swarming motility [86].
E. faecalis has been found to be highly prevalent in anastomotic tissues, likely due to its high adherence affinity to extracellular matrix proteins, including collagen. E. faecalis is capable of producing gelatinase (GelE), which contributes to the development of AL by breaking down collagen and activating intestinal matrix metalloproteinases (MMP), which are capable of degrading collagen. Interestingly, researchers were able to suppress MMP9 activation via direct application of topical antibiotics to intestinal tissues, but this protective effect was not replicated with IV antibiotics [54••]. This again suggests that the local interaction of mucus-associated microbes at the site of tissue injury with soluble factors may be causing a phenotypic shift to more virulent strains that contribute to collagen breakdown and thus increasing likelihood of developing AL. Shogan et al. concluded that incidence of AL is associated with microbiota (i.e., E. faecalis) that has both increased production of collagenase (aka gelatinase) and increased capacity to activate host intestinal MMP [54••]. They went on to examine the impact of a topical antibiotic regimen on an anastomotic model in rats and found decreased activation of MMP and collagen breakdown among rats treated with antibiotics, which correlated with decreased incidence of AL. However, this relationship was not conserved in rats that received intravenous (IV) Cefoxitin. Rather, administration of systemic IV Cefoxitin was associated with a bloom in high collagenase producing E. faecalis, which may explain why this group did not have a reduction in AL [54••]. Together, this again suggests that bacteria play a role in the development of AL via bacterial collagenase expression and excessive activation of host intestinal MMP leading to robust collagen degradation.
Alverdy and colleagues have proposed that AL is a product of the “right bacteria (E. faecalis, P. aeruginosa), with the right virulence genes (collagenase), expressed, in vivo, by the right activating cues (long operation, blood loss, difficult dissection), existing within a critically deficient microbiome (history of smoking, alcohol use, pre-operative chemoradiation, antibiotic use),” that upregulate the inflammatory process and allow for the development of necrosis and dehiscence [87••, 88].
Recovery After Surgical Injury
Modulation of the microbiome offers an opportunity to improve patient recovery following surgical interventions. A number of interventions, including dietary therapy [89], antibiotics [89], prebiotics [90], probiotics [91], and synbiotics (i.e., prebiotics and probiotics given together), could potentially be used to influence patient recovery through the microbiota.
One example of the link between intervention, microbiome composition, and host health is inflammatory bowel disease (IBD). In the case of IBD, patients exhibit a dysbiotic (i.e., altered) microbiota composition during active disease [92]. Exclusive enteral nutrition is one treatment modality used to treat IBD, and it is possible that this therapy exhibits a therapeutic benefit in part due to modulation of the microbiota [93, 94]. A recent study showed that host inflammation, antibiotics, and dietary alterations each independently influence the composition of the dysbiotic microbiome in pediatric Crohn’s disease [89]. It is possible that dietary therapy and/or other diet-related strategies may provide a way to promote restitution of the microbiome and prevent a bloom in more pathogenic species. Results from early evaluation of enhanced recovery after surgery (ERAS) protocols suggest that early enteral feeding may be beneficial for recovery following surgical injury [95]. The microbiota is able to produce SCFA through the fermentation of ingested complex carbohydrates and proteins that reach the colon, which lends credence to the proposal that early enteral feeding may be a strategy to enhance the protective effect of re-establishment of a commensal microbiota following surgical injury.
In response to epithelial injury and mucosal barrier degradation, the host initiates rapid wound healing to reestablish homeostasis. This process—called gastrointestinal epithelial restitution [96]—involves the closure of gaps in injured epithelial surfaces via migration from edges of injured epithelial cells. Restitution is influenced via a variety of factors including GI microenvironment and microbiota [97]. GI restitution is modulated by multiple growth factors, including epidermal growth factor (EGF), transforming growth factor-alpha (TGF-a), and platelet-derived growth factor (PDGF), among others. Likewise, cytokines, SCFAs, bile acids, and the microbiota are all involved in the restitutive response.
Bacterial species interact with Toll-like receptors (TLR) on epithelial cell surfaces; activation of TLR-dependent pathways leads to production of inflammatory cytokines (i.e., IL-6 and heat shock proteins), whereas lack of activation causes a reduction in epithelial cell proliferation [98]. Further, bacterial fermentation of indigestible fiber leads to production of SCFAs, which are known to be the primary energy source for colonocytes and stimulate mucus secretion. Through the production of SCFAs and transformation of bile acids, endogenous bacteria play both a positive and negative role in modulating the GI restitution response to epithelial damage. In a rat model, Bloemen et al. showed that butyrate enemas improve intestinal anastomotic strength [99] by protecting epithelial integrity. In this study, they found that rats treated with butyrate had stronger anastomoses (measured as burst strength) at 7 days compared to placebo and control, and their anastomoses had increased mature-to-immature collagen ratio [99]. It has been hypothesized that SCFAs increase re-epithelialization after injury to the intestinal epithelial layer. A different experimental model using Muc2-deficient mice that lack the ability to produce an outer mucus layer, 20 out of 22 Muc2-deficient mice developed AL, compared with only seven of 22 control mice (p < 0.001) [37], which may be secondary to induced re-epithelialization and support of the functioning mucus layer. Muc2-deficient mice also had significantly higher I-FABP levels than matched controls (Muc2 +/− and +/+), which is released from enterocytes and is a surrogate for cellular damage. This epithelial damage implies that the mucus layer is important for protecting the vulnerable anastomotic site from bacterial encroachment, and thus may be important to the healing process [37].
The multidirectional interactions between the host, the mucus layer, and the microbiota require further elucidation; however, it represents an opportunity for therapeutic dietary interventions, including before or after surgery. One example of this link between diet, the gut microbiota, and intestinal barrier function is the increase in dietary mucus degradation, alteration in microbial community structure, and promotion of colitis following chronic or intermittent dietary fiber deficiency in animal models [100].
Conclusion
While the long-term consequences of surgery on the gut microbiome are not entirely known, the existing literature provides compelling evidence to suggest that the host-microbiota relationship may play a role in causing and/or exacerbating AL after intestinal resection. As such, it is imperative that we further elucidate this complex and dynamic relationship. Likewise, surgeons should strive to minimize excessive perturbations to this equilibrium by decreasing ischemia, eliminating the use of unnecessary medication (e.g., limit opioids as able), and appropriately utilizing oral antibiotics with MBP when indicated.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Paun BC, Cassie S, MacLean AR, et al. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807–18.
Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K. Do we really know why colorectal anastomoses leak? J Gastrointest Surg. 2013;17:1698–707.
Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254–8.
McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–4.
Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg. 2018;105:e131–41.
Snijders HS, Wouters MW, van Leersum NJ, et al. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38:1013–9.
Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A. 2011;108:6252–7.
Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–6.
Ni J, Shen T-CD, Chen EZ, Bittinger K, Bailey A, Roggiani M, et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017;9:eaah6888.
Lagier JC, Dubourg G, Million M, et al. Culturing the human microbiota and culturomics. Nat Rev Microbiol. 2018.
Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2017;14:43–54.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8.
Lagier JC, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203.
• Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14 Seminal work highlighting the structure and function of microbial communities at multiple body sites in a large healthy cohort.
Wu GD, Compher C, Chen EZ, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2014.
Bushman FD, Lewis JD, Wu GD. Diet, gut enterotypes and health: is there a link? Nestle Nutr Inst Workshop Ser. 2013;77:65–73.
• Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8 This study links long-term dietary patterns with distinct gut microbial community structure and shows the resiliency of these communities to short-term dietary interventions.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–99.
Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142:24–31.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36.
El Aidy S, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol. 2015;32:14–20.
Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CCGM, Troost FJ, et al. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–26.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533.
Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48.
van den Bogert B, Erkus O, Boekhorst J, de Goffau M, Smid EJ, Zoetendal EG, et al. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol Ecol. 2013;85:376–88.
Van den Bogert B, Boekhorst J, Herrmann R, et al. Comparative genomics analysis of Streptococcus isolates from the human small intestine reveals their adaptation to a highly dynamic ecosystem. PLoS One. 2013;8:e83418.
Moran C, Sheehan D, Shanahan F. The small bowel microbiota. Curr Opin Gastroenterol. 2015;31:130–6.
Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292.
Roux A, Payne SM, Gilmore MS. Microbial telesensing: probing the environment for friends, foes, and food. Cell Host Microbe. 2009;6:115–24.
Venkataraman A, Rosenbaum MA, Werner JJ, Winans SC, Angenent LT. Metabolite transfer with the fermentation product 2,3-butanediol enhances virulence by Pseudomonas aeruginosa. ISME J. 2014;8:1210–20.
Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30.
• Sommovilla J, Zhou Y, Sun RC, Choi PM, Diaz-Miron J, Shaikh N, et al. Small bowel resection induces long-term changes in the enteric microbiota of mice. J Gastrointest Surg. 2015;19:56–64 discussion 64. This study shows that bowel resection leads to a significant and resilient alteration in the ileal microbiota.
Gibson MK, Pesesky MW, Dantas G. The yin and yang of bacterial resilience in the human gut microbiota. J Mol Biol. 2014;426:3866–76.
Bosmans JW, Jongen AC, Birchenough GM, et al. Functional mucous layer and healing of proximal colonic anastomoses in an experimental model. Br J Surg. 2017;104:619–30.
Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–61.
Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9.
Grootjans J, Hundscheid IH, Lenaerts K, et al. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut. 2013;62:250–8.
Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–24.
Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, Ermund A, Boysen P, Bemark M, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164–77.
Brownlee IA, Havler ME, Dettmar PW, Allen A, Pearson JP. Colonic mucus: secretion and turnover in relation to dietary fibre intake. Proc Nutr Soc. 2003;62:245–9.
Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014;42:49–53.
Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–6.
Png CW, Linden SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–8.
Hoskins LC, Boulding ET. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981;67:163–72.
Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–63 e8.
Friedman ES, Bittinger K, Esipova TV, Hou L, Chau L, Jiang J, et al. Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc Natl Acad Sci U S A. 2018;115:4170–5.
Ringel Y, Maharshak N, Ringel-Kulka T, Wolber EA, Sartor RB, Carroll IM. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes. 2015;6:173–81.
Jalanka J, Salonen A, Salojarvi J, et al. Effects of bowel cleansing on the intestinal microbiota. Gut. 2015;64:1562–8.
Ferrer M, Martins dos Santos VA, Ott SJ, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut Microbes. 2014;5:64–70.
Alverdy J, Zaborina O, Wu L. The impact of stress and nutrition on bacterial-host interactions at the intestinal epithelial surface. Curr Opin Clin Nutr Metab Care. 2005;8:205–9.
•• Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7:286ra68 This study demonstrates that Enterococcus faecalis contributes to the pathogenesis of anastomotic leak in an animal model; additionally, the authors show that the anastomotic tissues of human subjects undergoing colon surgery are colonized with E. faecalis .
Krezalek MA, Skowron KB, Guyton KL, Shakhsheer B, Hyoju S, Alverdy JC. The intestinal microbiome and surgical disease. Curr Probl Surg. 2016;53:257–93.
Lyte M. The effect of stress on microbial growth. Anim Health Res Rev. 2014;15:172–4.
Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–78.
Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2015;6:1543.
Jernberg C, Lofmark S, Edlund C, et al. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66.
Aad G, Abbott B, Abdallah J, et al. Search for new particles in two-jet final states in 7 TeV proton-proton collisions with the ATLAS detector at the LHC. Phys Rev Lett. 2010;105:161801.
Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol. 2017;30:45–53.
Bachmann R, Leonard D, Delzenne N, Kartheuser A, Cani PD. Novel insight into the role of microbiota in colorectal surgery. Gut. 2017;66:738–49.
Rex DK. Optimal bowel preparation--a practical guide for clinicians. Nat Rev Gastroenterol Hepatol. 2014;11:419–25.
Jung B, Matthiessen P, Smedh K, Nilsson E, Ransjö U, Påhlman L. Mechanical bowel preparation does not affect the intramucosal bacterial colony count. Int J Color Dis. 2010;25:439–42.
Harrell L, Wang Y, Antonopoulos D, Young V, Lichtenstein L, Huang Y, et al. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One. 2012;7:e32545.
•• Johansson ME, Gustafsson JK, Holmen-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–91 Demonstration of the breakdown of the mucosal barrier and translocation of bacteria in mice and humans with ulcerative colitis.
Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009;21:1237–44.
Dahabreh IJ, Steele DW, Shah N, Trikalinos TA. Oral mechanical bowel preparation for colorectal surgery: systematic review and meta-analysis. Dis Colon Rectum. 2015;58:698–707.
• Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum. 2016;59:70–8 Summary of relevant literature from RCTs on use of antibiotics with and without mechanical bowel preparation and highlights the difference in effect based on route of administration.
Jung B, Pahlman L, Nystrom PO, et al. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg. 2007;94:689–95.
Contant CM, Hop WC, van’t Sant HP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. 2007;370:2112–7.
Guenaga KF, Matos D, Wille-Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011:CD001544.
Cannon JA, Altom LK, Deierhoi RJ, Morris M, Richman JS, Vick CC, et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum. 2012;55:1160–6.
Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg. 2015;261:1034–40.
Wang F, Li Q, Wang C, Tang C, Li J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS One. 2012;7:e42027.
Cohn I Jr, Rives JD. Antibiotic protection of colon anastomoses. Ann Surg. 1955;141:707–17.
Cohen SR, Cornell CN, Collins MH, Sell JE, Blanc WA, Altman RP. Healing of ischemic colonic anastomoses in the rat: role of antibiotic preparation. Surgery. 1985;97:443–6.
Schardey HM, Kamps T, Rau HG, Gatermann S, Baretton G, Schildberg FW. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother. 1994;38:2564–7.
• Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2014;2:35 Highlights the impact of surgical injury on microbiota structure and function as it relates to development of surgical complications.
Seal JB, Morowitz M, Zaborina O, An G, Alverdy JC. The molecular Koch’s postulates and surgical infection: a view forward. Surgery. 2010;147:757–65.
Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83:461–6.
Fink D, Romanowski K, Valuckaite V, et al. Pseudomonas aeruginosa potentiates the lethal effect of intestinal ischemia-reperfusion injury: the role of in vivo virulence activation. J Trauma. 2011;71:1575–82.
Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–46.
Ferraro FJ, Rush BF Jr, Simonian GT, Bruce CJ, Murphy TF, Hsieh JT, et al. A comparison of survival at different degrees of hemorrhagic shock in germ-free and germ-bearing rats. Shock. 1995;4:117–20.
Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, et al. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg. 2012;255:386–93.
Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch M, et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One. 2012;7:e44326.
•• Alverdy JC, Hyoju SK, Weigerinck M, Gilbert JA. The gut microbiome and the mechanism of surgical infection. Br J Surg. 2017;104:e14–23 Detailed review of the potential mechanisms and relationships between the microbiome as it pertains to the development of surgical complications.
Alverdy JC, Hyman N, Gilbert J, Luo JN, Krezalek M. Preparing the bowel for surgery: learning from the past and planning for the future. J Am Coll Surg. 2017;225:324–32.
Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18:489–500.
Lambert JE, Parnell JA, Eksteen B, Raman M, Bomhof MR, Rioux KP, et al. Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: a randomized controlled trial protocol. BMC Gastroenterol. 2015;15:169.
Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407–12.
Albenberg LG, Lewis JD, Wu GD. Food and the gut microbiota in inflammatory bowel diseases: a critical connection. Curr Opin Gastroenterol. 2012;28:314–20.
Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861–71.
Kaakoush NO, Day AS, Leach ST, Lemberg DA, Nielsen S, Mitchell HM. Effect of exclusive enteral nutrition on the microbiota of children with newly diagnosed Crohn’s disease. Clin Transl Gastroenterol. 2015;6:e71.
Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–50.
Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68–77.
Karrasch T, Jobin C. Wound healing responses at the gastrointestinal epithelium: a close look at novel regulatory factors and investigative approaches. Z Gastroenterol. 2009;47:1221–9.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41.
Bloemen JG, Schreinemacher MH, de Bruine AP, Buurman WA, Bouvy ND, Dejong CH. Butyrate enemas improve intestinal anastomotic strength in a rat model. Dis Colon Rectum. 2010;53:1069–75.
Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–53 e21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition and Obesity
Rights and permissions
About this article
Cite this article
Gershuni, V.M., Friedman, E.S. The Microbiome-Host Interaction as a Potential Driver of Anastomotic Leak. Curr Gastroenterol Rep 21, 4 (2019). https://doi.org/10.1007/s11894-019-0668-7
Published:
DOI: https://doi.org/10.1007/s11894-019-0668-7