Abstract
Purpose of Review
Lymphocytic esophagitis (LE) is an unusual esophageal condition defined by an increased number of lymphocytes in the esophageal epithelium. With few published studies of LE available, it is unclear whether LE is a truly distinct clinical entity or a histological manifestation of other known gastrointestinal disorders. This review summarizes recent studies of lymphocytic esophagitis.
Recent Findings
Studies have suggested that LE may be related to eosinophilic esophagitis (EoE) or a manifestation of gastroesophageal reflux disease (GERD). There is an association between LE and Crohn’s disease in children, but not in adults. Patients with LE frequently report symptoms of dysphagia and GERD. Treatment options for LE are limited and involve symptom management similar to treatment of EoE or GERD, including proton pump inhibitors (PPI), swallowed topical steroids, and endoscopic dilation.
Summary
With no formal definition and a variety of clinical presentations and endoscopic findings, diagnosis and management of symptomatic LE patients is challenging for clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphocytic esophagitis (LE) was first described in 2006 when Rubio et al. identified 20 patients with increased intraepithelial lymphocytes and few intraepithelial granulocytes. The intraepithelial lymphocytosis was confined to the esophagus as no intraepithelial lymphocytes were found in the stomach, small bowel, or colon [1•]. Although 10 years have elapsed since it was first described, the diagnostic criteria and clinical features of LE remain elusive, and it is unclear whether it represents a truly distinct clinical entity or a histological manifestation of other known disorders. This ambiguity may be due in part to the lack of published literature on LE. At the time of this review, there have been 11 published research studies and eight case reports discussing LE. These studies are summarized in Table 1.
Pathogenesis
Part of the difficulty in clarifying the characteristics of LE lies in the rarity of the diagnosis. LE was found in 0.09% of esophageal biopsies obtained by endoscopy in a study of 129,252 adult patients [6•]. In children, the prevalence was 5.7% in a study of 545 patients undergoing endoscopy [8].
The pathogenesis and cause of LE is unclear, and several possible causes of LE have been proposed. LE may be an allergic disorder; Purdy et al. found that LE resembled contact dermatitis with a third of their LE study population reporting atopy, though this prevalence did not differ significantly from that of an unmatched control population [2]. A separate case report identified mast cells on histological examination, suggesting that LE may be due to an allergic or hypersensitivity reaction [17]. Another case report proposed an autoimmune phenomenon when LE was identified in a patient with common variable immune deficiency [19].
It has also been suggested that LE is not an independent clinical entity but rather a histologic process existing along the eosinophilic esophagitis (EoE) or reflux esophagitis spectrum [20]. Lymphocytes are also involved in the pathogenesis of gastroesophageal reflux disease (GERD). In one study of patients with GERD, discontinuation of proton pump inhibitor (PPI) therapy led to lymphocytic infiltration of the esophageal mucosa as GERD symptoms developed [21]. Another study of patients with GERD found 5% of reflux esophagitis specimens met the criteria for LE [3]. Thus, LE may be a manifestation of GERD, related to EoE, or associated with allergic disease.
Diagnostic Criteria
As LE is a histological diagnosis, the gold standard for diagnosis is biopsy of the esophageal mucosa. The changes of LE can be found throughout the esophagus but is identified most frequently in biopsies of the distal esophagus, followed by the proximal and mid esophagus [2, 8]. The pattern of biopsies may impact the diagnosis of LE. In a study of 81 patients, >90% of LE cases were diagnosed through random esophageal biopsies compared to 9% of LE cases diagnosed by targeted biopsies of abnormal mucosa or strictures [5•]. While some commentators have suggested that LE is a transient response to an unknown insult, two studies have found LE to be a chronic process with LE found on biopsies from repeat endoscopies [2, 5•].
Histological changes in LE include an increased number of intraepithelial lymphocytes in the peripapillary areas of the esophageal epithelium with few or no intraepithelial granulocytes. Spongiosis, the dilation of intercellular spaces, has also been reported [6•, 8]. In contrast, lymphocytes are found primarily in the interpapillary area in other causes of esophagitis such as radiation, reflux, and candidiasis. While the normal number of lymphocytes in the esophageal epithelium is 10–12 per high-powered field (HPF), the exact number of lymphocytes required for a diagnosis of LE has not been rigorously defined [9, 22]. Research studies have used a wide range of lymphocyte densities to define LE, most commonly >50 lymphocytes per HPF (range 10–50 lymphocytes per HPF), though some studies only report the lymphocyte counts per HPF without defining a specific cutoff value [1•, 2, 5•, 8, 13, 16, 23]. Haque and Genta advocate against using numeric lymphocyte densities as part of the diagnostic criteria for LE due to its often patchy distribution. Rather, they propose that lymphocytes’ peripapillary location and associated spongiosis are more reliable determinants of the diagnosis [6•]. There is also no consensus regarding the presence of intraepithelial granulocytes in patients with LE. Some investigators have suggested the definition of LE should include the absence of granulocytes, which serves to exclude other conditions such as EoE [18].
Spongiosis is also seen in patients with LE. Rubio et al. examined the intercellular spaces in four LE patients using transmission electron microscopy (TEM). In addition to intraepithelial lymphocytosis, TEM identified marked spongiosis and regressive changes of the squamous cells, ranging from cytoplasmic vacuolization to cell disintegration. In this study, the cause of the spongiosis was unclear, as none of the four types of spongiosis previously described in the pathology literature were identified in the LE patients. Rubio proposed that the combination of hampered cell nutrition due to spongiosis and production of noxious molecules lead to injury of the squamous cells and lymphocyte infiltration [24].
Summarizing the available data, the current diagnostic criteria for LE include an increased number of lymphocytes (≥50 per HPF) in the peripapillary space with associated spongiosis and few to no granulocytes. However, no formal definition for LE has been proposed as the histological criteria are still evolving. Thus, clinicians should consider discussing equivocal cases with a pathologist.
Clinical Characteristics
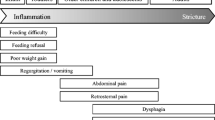
Most published studies of LE are small, the largest to date having included 119 patients [6•]. In terms of demographics, several studies of LE have reported a slight female preponderance with the highest frequency in the fifth and sixth decades of life [5•, 6•, 9, 10]. Common symptoms in LE patients include dysphagia and GERD. Dysphagia has been reported as the predominant complaint in two studies, with a frequency of up to 70% in patients with LE [10, 23]. In the study by Haque and Genta, dysphagia was as frequently reported by LE patients as by those with EoE [6•]. Patients with LE appear to have lower rates of food impaction than EoE patients, however [5•, 25].
GERD symptoms are also frequent, occurring in 20–25% of LE patients [1•, 2, 5•]. Chest and abdominal pain were reported in 44% of LE patients in one study [5•]. Spontaneous esophageal perforation was described in a single case report [14]. Esophageal motility abnormalities, including hypocontractile and hypercontractile patterns, have also been reported in one-third of LE patients. One study examined the association of CD4+ and CD8+ T lymphocytes with esophageal dysmotility and found that patients with CD4+ lymphocyte-predominant LE were twice as likely as patients with CD8+ lymphocyte-predominant LE to have primary motility disorders identified by esophageal manometry or barium esophagram [23].
Numerous studies have searched for an association between LE and other diagnoses. Specifically, there have been several research studies evaluating the association between LE and Crohn’s disease in children and adults. In the initial study of LE by Rubio et al., 40% of LE patients were found to have Crohn’s disease, but almost all patients with both LE and Crohn’s disease were under 17 years of age [1•]. In two further pediatric studies, Ebach et al. found LE in 28% of all children with Crohn’s disease undergoing upper endoscopy as compared with 4.4% without Crohn’s disease, while Sutton et al. found LE in 12% of children with Crohn’s disease compared to 5% without Crohn’s disease. Both studies suggest a significant association between these two processes in the pediatric population [4, 8]. However, subsequent studies in adults have not found any association between Crohn’s disease and LE [2, 5•, 6•, 10, 23]. One study of adults with inflammatory bowel disease (IBD) reported that increased esophageal peripapillary lymphocytosis was associated with higher inflammatory marker levels and an increased IBD disease activity index, suggesting that LE may be a marker of IBD severity in the adult population [13]. No association between celiac disease and LE has been identified [2, 6•].
Based on the available data, LE has a benign clinical course and good overall prognosis. In a study by Cohen et al., 70 of 81 patients with LE were alive after a median follow-up interval of 3.3 years. Among the 29 patients for whom follow-up survey data were obtained, 22 were found to have ER visits in the preceding 5 years, with 41% (9/29) of visits attributable to a gastrointestinal complaint. Despite this finding, 59% of patients reported improvement in their symptoms over time, usually after PPI initiation, with greater than 60% of patients reporting satisfaction with their gastrointestinal health [5•].
Endoscopic Findings
The endoscopic findings reported in patients with LE vary widely by study. Pasricha et al. found that 82% of the 27 LE patients evaluated had abnormal endoscopic findings. In contrast, Purdy et al. found no difference in endoscopic appearance between LE patients and controls [2, 10]. Similarities between the endoscopic appearance of EoE and LE have also been reported. In one study, 34% of LE patients had findings similar to EoE such as rings, furrows, plaques, and strictures [6•]. In another study, LE patients and EoE patients had comparable rates of esophageal rings, but LE patients had lower rates of strictures [5•]. The cause of the endoscopic findings seen in LE is unclear. One study proposed that the endoscopic findings common to EoE and LE may develop due to inflammatory cell infiltration (eosinophils in EoE and lymphocytes in LE), leading to muscularis mucosa contraction and esophageal wall thickening [17]. Endoscopic ultrasound in at least one patient with LE has demonstrated thickening of the esophageal mucosa and submucosa [16]. The endoscopic findings seen in LE have also been evaluated with narrow band imaging (NBI). Using NBI, 90.5% of LE patients were found to have beige discoloration of the mucosa, an increased number of congested intrapapillary capillary loops, and poor visibility of submucosal vessels. However, these findings were not specific for LE. The authors of this study suggested that the presence of all three of the above findings might indicate EoE or LE rather than GERD [7].
Treatment Options
No clear treatment guidelines have been proposed for LE management. As the symptoms of LE are similar to those of EoE, clinicians have attempted to manage LE using similar treatment strategies, including PPI use, swallowed fluticasone, and endoscopic dilation. Several studies of LE patients have demonstrated symptomatic improvement with PPI treatment, which may be due to either the control of acid reflux in cases where LE is a manifestation of GERD or the anti-inflammatory properties of PPIs [5•, 10, 17]. As corticosteroids have been found effective in other gastrointestinal lymphocytic diseases, such as lymphocytic colitis, and in the treatment of EoE, their use has been applied to LE as well. Swallowed fluticasone led to symptomatic improvement of LE symptoms in four studies [10, 11, 14, 15]. One study noted improvement after a 1-month trial. Other studies have not recommended a specific treatment duration when using topical corticosteroids. In the four studies that considered esophageal dilation as therapy for LE, the intervention was targeted to strictures and rings rather than use of empiric dilation when no endoscopic abnormalities were present [12, 15, 16, 18].
At this time, treatment of LE is challenging. Some patients have improvement with PPI treatment, underscoring the hypothesis that LE may be related to GERD or EoE. As some patients with LE have a similar clinical presentation to those with EoE, swallowed topical corticosteroids have also been used with variable success. Symptomatic treatment of dysphagia by dilation of rings and strictures can also be considered.
Conclusions
Awareness of lymphocytic esophagitis by gastroenterologists and pathologists has increased in recent years. At this time, LE should be considered when evaluating patients with dysphagia. When LE is identified on esophageal biopsies, patients can be reassured that it has a typically benign course. A trial of PPI could be considered with escalation to swallowed fluticasone if there is no improvement in symptoms. Patients who have dysphagia as their predominant complaint can be considered for dilation, especially if strictures or rings are present. Additional research is needed to determine whether LE is truly a distinct clinical entity or a manifestation of other GI disorders. Further investigation is also needed to elucidate its pathophysiology and optimal management strategy.
References
Papers of Particular Interest, Published Recently, Have Been Highlighted as: • of Importance
• Rubio CA, Sjödahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432–7. The first study to describe lymphocytic esophagitis.
Purdy JK, Appelman HD, Golembeski CP, McKenna BJ. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. Am J Clin Pathol. 2008;130:508–13.
Basseri B, Levy M, Wang HL, Shaye OA, Pimentel M, Soffer EE, et al. Redefining the role of lymphocytes in gastroesophageal reflux disease and eosinophilic esophagitis. Dis Esophagus. 2010;23:368–76.
Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn’s disease. Inflamm Bowel Dis. 2011;17:45–9.
• Cohen S, Saxena A, Waljee AK, Piraka C, Purdy J, Appelman H, et al. Lymphocytic esophagitis: a diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828–32. Provided data on natural history and quality of life of lymphocytic esophagitis patients after a 3-year follow-up.
• Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61:1108–14. The largest study of patients with lymphocytic esophagitis, identified from a review of a large pathology database. Also proposed criteria for defining lymphocytic esophagitis.
Tanaka K, Rubio CA, Dlugosz A, Truskaite K, Befrits R, Lindberg G, et al. Narrow-band imaging magnifying endoscopy in adult patients with eosinophilic esophagitis/esophageal eosinophilia and lymphocytic esophagitis. GastrointestEndosc. 2013;78:659–64.
Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflamm Bowel Dis. 2014;20:1324–8.
Xue Y, Suriawinata A, Liu X, Li Z, Gabbard S, Rothstein R, et al. Lymphocytic esophagitis with CD4 T-cell-predominant intraepithelial lymphocytes and primary esophageal motility abnormalities: a potential novel clinicopathologic entity. Am J SurgPathol. 2015;39:1558–67.
Pasricha S, Gupta A, Reed CC, Speck O, Woosley JT, Dellon ES. Lymphocytic esophagitis: an emerging Clinicopathologic disease associated with dysphagia. Dig Dis Sci. 2016;61:2935–41.
Kasirye Y, John A, Rall C, Resnick J. Lymphocytic esophagitis presenting as chronic dysphagia. Clin Med Res. 2012;10:83–4.
Mandaliya R, Dimarino AJ, Cohen S. Lymphocytic esophagitis mimicking eosinophilic esophagitis. Ann Gastroenterol. 2012;25:355–7.
Basseri B, Vasiliauskas EA, Chan O, Wang HL, Basseri RJ, Pimentel M, et al. Evaluation of peripapillary lymphocytosis and lymphocytic esophagitis in adult inflammatory bowel disease. GastroenterolHepatol. 2013;9:505–11.
Hendy PJ, Wong DS, Florin TH. Spontaneous oesophageal perforation: an unreported complication of lymphocytic oesophagitis. Gut. 2013;62:1668–9.
Figueiredo PC, Pinto-Marques P, Borralho P, Freitas J. Unusual cause for smoldering dysphagia. Lymphocytic esophagitis. Dysphagia. 2014;29:283–5.
Maejima R, Uno K, Iijima K, Fujishima F, Noguchi T, Ara N, et al. A Japanese case of lymphocytic esophagitis. Dig Endosc. 2016;28:476–80.
Zhang Z, Jain D, Brand M. Ringed esophagus secondary to lymphocytic esophagitis. Gastroenterol Hepatol. 2016;12:237–9.
Niewiarowski TJ, Stoll LM. Recurrent dysphagia in a patient with chronic lymphocytic esophagitis. GastrointestEndosc. 2016; doi:10.1016/j.gie.2016.02.016.
Vangimalla S, Gordon I, Thota PN. Image of the month: lymphocytic esophagitis in common variable immune deficiency. Am J Gastroenterol. 2016;111:170.
Genta RM. Lymphocytic esophagitis. Gastroenterol Hepatol. 2015;11:559–61.
Dunbar KB, Agoston AT, Odze RD, Huo X, Pham TH, Cipher DJ. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–12.
Resnick MB, Finkelstein Y, Weissler A, Levy J, Yakirevich E. Assessment and diagnostic utility of the cytotoxic T-lymphocyte phenotype using the specific markers granzyme-B and TIA-1 in esophageal mucosal biopsies. Hum Pathol. 1999;30:397–402.
Wang HH, Mangano MM, Antonioli DA. Evaluation of T-lymphocytes in esophageal mucosal biopsies. Mod Pathol. 1994;7:55–8.
Rubio CA, Villnow E, Schmidt PT. Lymphocytic oesophagitis preliminary ultrastructural observations. Anticancer Res. 2016;36:2315–22.
Truskaite K, Dlugosz A. Prevalence of eosinophilic esophagitis and lymphocytic esophagitis in adults with esophageal food bolus impaction. Gastroenterol Res Pract. 2016; doi:10.1155/2016/9303858.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Support: This material is the result of work supported with resources at the Dallas VA Medical Center.
VA/US Government disclaimer: The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Additional information
Topical Collection on Esophagus
Rights and permissions
About this article
Cite this article
Nguyen, A.D., Dunbar, K.B. How to Approach Lymphocytic Esophagitis. Curr Gastroenterol Rep 19, 24 (2017). https://doi.org/10.1007/s11894-017-0564-y
Published:
DOI: https://doi.org/10.1007/s11894-017-0564-y