Abstract
Despite decades of obesity research and various public health initiatives, obesity remains a major public health concern. Our most drastic but most effective treatment of obesity is bariatric surgery with weight loss and improvements in co-morbidities, including resolution of type 2 diabetes (T2D). However, the mechanisms by which surgery elicits metabolic benefits are still not well understood. One proposed mechanism is through signals generated by the intestine (nutrients, neuronal, and/or endocrine) that communicate nutrient status to the brain. In this review, we discuss the contributions of gut-brain communication to the physiological regulation of body weight and its impact on the success of bariatric surgery. Advancing our understanding of the mechanisms that drive bariatric surgery-induced metabolic benefits will ultimately lead to the identification of novel, less invasive strategies to treat obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a worldwide epidemic characterized by excessive fat storage due to multiple genetic, environmental, and behavioral factors that drive an imbalance in energy homeostasis. To date, bariatric surgeries that alter gut anatomy remain the most effective treatments for obesity. Aside from weight loss, bariatric surgery improves several co-morbidities and often carries the beneficial side effect of the resolution of type 2 diabetes (T2D) [1, 2].
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are the most common bariatric surgeries worldwide, and both produce substantial reductions in body weight [3]. With RYGB, a small gastric pouch is created and then anastomosed to the jejunum. The remaining stomach and upper GI track remains in the peritoneal cavity but ingested nutrients are rerouted to bypass the stomach, duodenum, and the upper jejunum [4] (Fig. 1). VSG, the most commonly used procedure in the USA, involves an 80% reduction of the stomach along the greater curvature and, unlike RYGB, requires no intestinal rearrangement [4] (Fig. 1). The dogma is that RYGB has greater efficacy in terms of weight loss and resolution of co-morbidities, which is supported by multiple meta-analysis studies [5, 6]. However, a randomized clinical trial found that VSG and RYGB caused similar and sustained weight loss of 61.1% and 68.3%, respectively, in individuals with class 2 obesity 5 years post-surgery [7]. Regardless of whether RYGB is more efficacious than VSG, VSG is still far more effective than currently available pharmaceutical treatments of obesity.
Anatomical and metabolic changes induced by vertical sleeve gastrectomy or Roux-en-Y gastric bypass. A) VSG is a procedure where 80% of the stomach along the greater curvature is removed. RYGB creates a gastric pouch and the intestine is rearranged such that ingested food bypasses 95% of the stomach and the upper intestinal tract. B) While the anatomy of RYGB and VSG surgeries differ, the two share similarities such as increases in sustained weight loss and rapid nutrient entry as well as the resolution of Type 2 Diabetes and some mild differences in gut peptide responses. Created with BioRender.com
Historically, mechanisms responsible for the success of surgery have been proposed based on their respective anatomical rearrangement. Thus, with the construction of a small gastric pouch and the intestinal rearrangement, RYGB has been considered a restrictive (smaller stomach) and malabsorptive (decreased macronutrient absorption due to intestinal rearrangement) procedure, while VSG has been considered only restrictive. However, several studies have shown that malabsorption of macronutrients does not contribute to the weight loss success of RYGB (reviewed in [8]) or VSG [9]. The stomach size reductions with RYGB and VSG have been hypothesized to physically restrict meal size and, thus, overall food intake. However, our lab has evidence in rodent models of VSG that challenge that hypothesis. Food intake is reduced for 2–3 weeks after VSG in mice and rats, but thereafter, 24-h food intake is similar between sham and VSG animals [10, 11]. Despite the similar daily food intake, VSG animals maintain a lower body weight and a different meal pattern by ingesting smaller but more frequent meals [10]. Although total food intake becomes similar, it is important to note that it never exceeds the levels seen in sham animals. Because body mass is a highly regulated variable, non-surgical animals that undergo a period of food restriction ingest more calories than control animals when returned to ad lib feeding in an effort to return to their pre-restricted bodyweight trajectory. If VSG was a restrictive procedure, animals would not be able to become hyperphagic, even after a period of restriction. Similarly, lactation drives an increase in feeding in mammals, and the degree of hyperphagia that occurs during lactation is similar between sham and VSG animals [12]. Thus, when the physiology demands it, VSG animals can become hyperphagic, indicating that mechanical restriction of meal size is not the major driver of reduced feeding and consequent weight loss. This interesting finding suggests there are two major energy homeostatic phases to bariatric surgery. First, there is the early reductions in feeding and immediate weight loss, and then there is a weight-maintenance phase driven by the prevention of hyperphagia (Fig. 2). An important question surrounds understanding what signals drive these two phases of surgery.
Changes in food intake and body weight over time in rodent models of bariatric surgery. Food intake after bariatric surgery in rodents is reduced during the first two–three weeks post-operatively, and then returns to, but never exceeds, the level of food intake seen in the sham animals [10, 11]. If an animal is food restricted (FR) for a period of time and body weight is lost, once returned to ad lib feeding, the animal will ingest more food than unrestricted animals until body weight returns to what it would have been had the animal never been restricted, something that does not occur with VSG [10]. Overall, this suggests that there are two phases to bariatric surgery, the initial weight loss phase with reduced food intake, and a second phase where hyperphagia is prevented and the weight loss is sustained
Thus, we contend that the success of bariatric surgery goes beyond mechanical restriction and malabsorption. So exactly how does bariatric surgery cause weight loss? Decades of preclinical and clinical research have failed to identify one sole mechanism for the weight loss success of bariatric surgery. However, one of the leading candidates is surgery-induced increases in gut signals that regulate feeding and metabolism. Here, we review the most commonly discussed gut signals and highlight recent advances in our technical repertoire that we believe will advance our understanding of the role of gut-derived factors on the metabolic success of bariatric surgery in the future.
Gut-Brain Communication: the Machinery
Signals and Cues
Once nutrients enter the mouth and pass through the stomach into the intestine, there is a cascade of physiological events. Specifically, the gastrointestinal (GI) tract secretes a variety of neuropeptides and hormones, initiates the mechanical breakdown of nutrients, regulates gastric emptying rate, and increases small intestinal motor activity, all of which are critical processes in nutrient assimilation and regulation of feeding behavior.
The lumen of the intestine is lined with villi that are comprised of multiple cell types including enterocytes (absorptive cells), goblet cells (mucin secreting cells), Tuft and Paneth cells (immune cells), and enteroendocrine cells (EECs; endocrine cells). Enterocytes function in the absorption of both macro- and micronutrients as well as ions and water, and comprise much of the cell population within the intestinal epithelium. On the other hand, EECs comprise only 1% of the total epithelial cell population but are very diverse and capable of synthesizing and secreting various hormones in response to changes in the intestinal milieu [13]. Conventionally, EEC types were characterized by the distinct hormones in which they secrete [14]. For instance, L cells were characterized as an EEC population that specifically secreted preproglucagon-derived peptides (i.e., glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), and oxyntomodulin), as well as peptide YY (PYY). Conversely, K-cells were characterized as an EEC population that specifically secreted glucose-dependent insulinotropic peptide (GIP) and I-cells secreted cholecystokinin (CCK) [15,16,17]. However, multiple studies have demonstrated that this simplistic EEC characterization is inadequate and that, instead, there is more complex co-expression of hormones within the EECs [18]. Regardless of peptide composition, all EEC types respond to nutrients within the intestine, and the peptides these cells secrete influence postprandial and intestinal physiology. Many of these gut-derived hormones promote satiation, regulate glucose homeostasis, intestinal nutrient digestion and processing, and even gastrointestinal motility. Regulation of the secretion of these various gut peptides is still being actively studied but the thinking, thus far, is that various sensory processes are initiated with nutrient transport and/or that there are nutrient-sensing receptors expressed on EECs that induces secretory processes [19]. These gut peptides also interact with the enteric nervous system (ENS) or with the vagal innervation of the gut and portal vein (see below for more details). Some of these peptides are rapidly degraded, limiting endocrine action (i.e., GLP-1). However, others enter the systemic circulation and act as hormones. Although there is much to be learned about the differentiation, anatomy, and regulation of EEC’s and their peptide secretions, there is no doubt that gut peptides have an important role to play in the physiological regulation of feeding and nutrient processing.
GI Tract Innervation
The ENS, referred to as the “brain” of the gastrointestinal (GI) tract, integrates information from intestinal signals to induce appropriate changes in gut motility, mucosal barrier function, blood flow to the intestine, and gastric acid secretion [20, 21], all processes important for nutrient processing. The neurons of the ENS within either the myenteric plexus or the submucosal plexus [22] transmit messages amongst themselves as well as to other integral players of the gut-brain axis [23]. Not much is known about the organizational hierarchy of the ENS. However, recent studies have sought to identify ENS anatomy and cell diversity [24, 25]. In addition to neurotransmitters, one study demonstrated that the ENS expressed a variety of neuropeptides, including cholecystokinin (CCK), traditionally thought to be only expressed in EECs [25]. Another showed that intestinal locations, circadian rhythm, and age are all factors that alter ENS gene expression. Further, Drokhlyansky et al. found a set of genes that are associated with a high risk of inflammatory bowel disease enriched in human ENS cells [24]. Because the gene expression profiles indicate putative interactions between the ENS and immune function, the authors speculated that ENS disruptions could potentially indirectly impact CNS functions via modulation of the gut microbiome. These studies illustrate how understanding the spatial and transcriptomic makeup of the ENS has great potential to advance our knowledge of gut-brain communications.
On the other hand, the intestine is also richly innervated by the vagus nerve, which has the molecular machinery necessary to directly sense ingested nutrients, respond to, and control mechanical function (i.e., stomach and intestinal distention), and also express receptors for several EEC-secreted peptides [26]. Vagal afferent neurons have axons that transmit messages from peripheral organs, like the stomach and intestine, to the brain. Vagal afferent neurons terminate in the nucleus of the solitary tract (NTS) of the brainstem, a brain center necessary for integrating information from diverse peripheral signals [27]. Vagal afferent neurons innervate three distinct layers within the gastrointestinal tract: the muscle layer, the myenteric layer, and the intraganglionic laminar endings. Vagal afferents that innervate the intraganglionic laminar endings are thought to be mechanoreceptors that detect stomach and intestinal distension [28]. On the other hand, the afferent neurons that innervate the muscle and myenteric layers are thought to be chemoreceptors that are activated by nutrients as well as gut-derived peptides [28]. Overall, communication between the gut and the brain is a tightly regulated, complex, and intricate network. In the following sections, we will discuss to the impact of obesity and bariatric surgery on gut-brain communications.
Gut-Brain Communication in Obesity and T2D
If the gut-brain axis is so critical for regulating body mass, one would predict that the processes described above would be altered by obesity. Animal models have been utilized to determine the necessity of these peptides in physiological regulation of body weight, yet many of these models have a modest phenotype. Ghrelin deficient mice have normal, rather than the predicted resistance to weight gain in response to dietary-induced obesity [29]. The GLP-1R knockout mouse that would be predicted to be obese is actually resistant to dietary-induced obesity [30]. Conversely, the PYY knockout mouse has a modest increase in body mass with a prolonged exposure to high fat diet relative to its wild-type control [31]. Perhaps one of the more striking phenotypes is rats devoid of the CCK-A receptors (Otsuka Long-Evans Tokushima Fatty rats). These rats are obese and hyperphagic [32]. Mice lacking CCK receptors have increased meal size, but they compensate for this by decreasing meal frequency such that long-term food intake, and consequently body weight, is similar between control and knockout animals [33].
In patients, there is even less clarity of the role of individual gut-peptides in the pathophysiology of obesity. For example, most studies suggest minimal differences in ghrelin levels between obese and normal-weight individuals, but postprandial ghrelin is clearly not suppressed in Prader Willi syndrome in which patients have extreme hyperphagia [29]. Circulating plasma levels of peptide YY (PYY), on the other hand, a hormone that increases satiety and reduces food intake, were found to be significantly reduced in obese individuals [34] and rodents [35]. The data surrounding GLP-1 levels in obese individuals are conflicting. One study has shown that the postprandial GLP-1 response is markedly blunted in obese vs. normal-weight individuals [36]. Other studies have demonstrated that postprandial levels of GLP-1 are similar in obese vs. non-obese patients [37, 38]. The timing of GLP-1 assessment and the quality and type (total vs. active GLP-1) of assays used to assess GLP-1, as well as diet are all factors that likely contribute to the discordance between these studies. These issues with assays and timing of assessment are a consistent problem with measurement of all the gut peptides. Furthermore, since some of these peptides are likely signaling locally within the intestine, plasma levels do not necessarily reflect action. Lastly, the levels of the peptides may be similar, but the responses to the same level of peptide could be reduced with obesity or T2D.
In addition to differences in gut peptide levels and/or signaling, alterations in neuronal activity have also been observed in obesity. Several studies have demonstrated that cFOS, a marker of neuronal activation, is significantly reduced within the NTS of obese animals compared to lean animals after a meal [39]. Whether this is due to impairments of direct nutrient and/or hormonal sensing within the NTS or via vagal signaling is unclear. Interestingly, vagal afferent activity is blunted in high-fat diet mice [40], suggesting that the vagus is at least partially involved. Collectively, these data suggest that there is a strong correlation between obesity and dysfunctional gut-brain communications. However, it will be critical to discern whether disruptions in gut-brain communications are consequences of obesity or whether they contribute to obesity pathophysiology.
Gut-Brain Communication in Bariatric Surgery
The question now becomes, does bariatric surgery reverse gut-brain processes impaired by obesity, or do they work via distinct overriding mechanisms? A recent commentary posed the question, “Is bariatric surgery brain surgery” [41]? This commentary focused on a study that used multimodal neuroimaging to demonstrate that RYGB patients undergo brain adaptations (i.e., changes in cerebral blood flow and glucose uptake) to maintain normoglycemia [42]. However, the concept of bariatric surgery modulating the brain is not new. The CNS tightly regulates body mass by responding to peripheral signals and altering aspects of energy homeostasis, including feeding behavior [43,44,45]. As bariatric surgery alters feeding behaviors in both humans and rodents, and as we discussed above, this is not simply due to mechanical restriction, it follows that surgery impacts the way the brain controls feeding behavior. In humans and rodents, feeding patterns are altered such that smaller, more frequent meals are consumed post-surgery [46,47,48,49,50,51,52]. Additionally, bariatric surgery also shifts what humans and rodents want to eat. For example, when given a choice between macronutrients, preference shifts from fats to carbohydrates [10, 11, 53, 54]. Although it is logical to hypothesize that bariatric surgery is mediating these effects via the CNS, exactly what signals drive the CNS to induce these changes are unknown. Because of the drastic changes in GI anatomy and physiology induced by bariatric surgery, here, we contend that these signals are gut-driven. A summary of the gut-signals we discussed is illustrated in Fig. 3. Below, we will discuss some of the potential gut-brain signals that are altered by, and are implicated as, mechanisms for bariatric surgery.
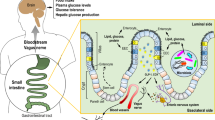
Summary of intestinal signals and cues that are altered after bariatric surgery. 1) After VSG and RYGB there are changes in the flow of nutrients, gut-generated peptides, and neuronal activation. 2) Increases in nutrient flow along with alterations in nutrient handling (i.e., increased glucose rate of appearance) can lead to the increased secretion of gut signals such as hormones and bile acids. Specific regions of the CNS (NTS and AP) are also activated after a meal. This increased activation may be due to direct neural innervation, the altered nutrient flow 3) or increases in gut-generated signals, such as 4) gut peptides, FGF19/15, bile acids, and/or the microbiome. Created with BioRender.com
Nutrients
Nutrients entering the intestine after gastric emptying provide nourishment to the body and serve as signaling molecules on local neurons to initiate many GI mechanical responses (e.g., changing the rate of gastric emptying and/or intestinal motility). These nutrients also enter the circulation and act directly on the CNS to regulate feeding, energy expenditure, and glucose homeostasis [45]. An obvious impact of bariatric surgery on nutrient processing is the rapid pouch or sleeve emptying of nutrients into the intestine [55,56,57]. In RYGB, this rapid nutrient entry is because there is no pylorus to gate nutrient entry from the pouch into the intestine. In VSG, there is an increase in gastric pressure that likely drives an increase in gastric emptying even though the pylorus is still intact [58]. In humans, while both RYGB and VSG increase the “gastric” emptying rate, this is more rapid after RYGB [59]. This rapid nutrient entry into the system with both surgeries likely influences nutrient handling and has been hypothesized to drive the increases in gut peptide secretion and intestinal adaptation to surgery. Along with the increased gastric emptying rate, the glucose rate of appearance into the system is higher with RYGB vs. VSG, but VSG patients still have a greater glucose rate of appearance compared to controls [59, 60]. The physiological relevance of these quantitative differences between RYGB and VSG is unknown. It is possible that the rapid nutrient entry induces morphological differences within the intestine. There are reports of intestinal hypertrophy with RYBG but not VSG, including increased bowel width, villus height, and crypt depth [61]. However, it is unclear whether this surgical difference is driven by the intestinal re-routing or the higher nutrient entry into the intestine.
Solid food emptying has also been shown to be enhanced by VSG in rodents [62], suggesting that fat and protein processing is also impacted by surgery. Patients and rodents with RYGB demonstrated significant enhancements in protein absorption when compared to VSG [59, 63]. Two rodent studies have also demonstrated that VSG reduced chylomicron production [64] and decreased lipid absorption [65] indicating the surgery also impacts lipid processing. Collectively, these data highlight differences in postprandial macronutrient absorption between RYGB and VSG, but whether this accounts for differences in efficacies between the surgeries and/or suggests distinct mechanisms in terms of their overall success remains unknown.
Gut Peptides
One of the most reliable markers of changes in gut physiology with bariatric surgery is that both RYGB and VSG cause robust changes in postprandial GI hormones in rodents and humans. Namely, postprandial increases of PYY and GLP-1 are found in both rodents and humans after VSG and RYGB [60, 66,67,68,69]. Because RYGB excludes nutrient exposure to 90% of the stomach and the upper GI tract, duodenally produced (gastric inhibitory peptide, GIP, cholecystokinin, CCK) gut peptides are not altered in this surgery. Conversely, CCK and GIP have been found to be significantly increased in humans after VSG [70]. These surgery-induced changes in postprandial levels of gut peptides have been extensively reviewed elsewhere [71,72,73,74]. Therefore, here, we will briefly highlight the physiology of key gut-derived hormones and their contributions to the success of bariatric surgery.
Mechanisms for Increases in Gut Peptides
A critical question is what are the mechanisms that drive bariatric surgery-induced changes in postprandial gut hormones? Rapid nutrient entry into the intestine has been implicated in this process. We have found that a barium plus nutrient mixture gavaged into rats that have had RYGB or VSG was well into the distal intestine within 5 min of the nutrient gavage [57] indicating extraordinarily high gastric emptying rate. To determine whether this rapid nutrient entry is what drives the increase in GLP-1, specifically, we then infused dextrose directly into the intestine at the same rate of infusion between VSG vs. sham rats. Despite the similar rate of nutrient into the intestine, there was still significantly higher levels of nutrient-stimulated GLP-1 with VSG [57]. We speculated that the intestine adapts to the continual rapid influx of nutrients into the intestine with each meal by increasing production of GLP-1. One potential adaptation that would explain this is the observed increase in EEC number after VSG [75,76,77]. Moreover, recent data from our lab has suggested that VSG drives differentiation of the intestinal stem cell population towards EECs [78]. Thus, our hypothesized model is that increased nutrient entry into the system with every meal pushes the intestine to adapt to be able to absorb nutrients under these conditions. One way it does this is to drive increased EEC differentiation and consequently increased postprandial gut peptide secretion.
The next question is whether the changes in postprandial gut peptides is necessary for the success of bariatric surgery. Administration of a GLP-1 antagonist impairs acute postprandial fluctuations in glucose in both rodents [79] and humans [80] suggesting an important role for GLP-1 in the surgery-induced improvements in glucose homeostasis. However, these results are all complicated by the fact that the GLP-1 antagonist impairs glucose tolerance and insulin secretion in both controls and surgery groups equally. In rodents, multiple genetic mouse studies that ablate either GLP-1 receptor signaling [81] or GLP-1 production [82, 83] demonstrate that these mice lose weight and improve glucose tolerance similar to controls suggesting that GLP-1 alone is not necessary for the success of the surgery. While it is clear that GLP-1 is effectively targeted for both weight loss and improvements in glucose control in T2D, and the tenfold increase in plasma GLP-1 with surgery might be viewed as important, these pharmacological agents have a stronger affinity for the receptor than endogenous GLP-1.
Rodents devoid of either CCK receptors [84] or ghrelin [85] have also been found to respond normally to bariatric surgery. However, mice deficient in PYY have been found to have blunted weight loss in response to a particular surgery where the upper GI tract was excluded from nutrient access while the stomach remained intact [86]. These data bring into question whether differences in species, assays, or experimental conditions contribute to the mechanistic differences (or lack thereof) exhibited with the assessment of the role of individual gut peptides on the success of surgery in mice.
Gut-Hormone Cocktail: Is More Better?
While individual gut peptides may not be necessary for the metabolic success of bariatric surgery, given the increase in multiple gut peptides, it is possible the increase in the whole cocktail of gut peptides is important for surgical success. This idea was the impetus for the multiple dual and tri-agonist drugs in the pipeline for the treatment of obesity [87]. To directly test this hypothesis, a recent study sought to mimic the postprandial increases of gut hormones after RYGB (GLP-1, oxyntomodulin, and PYY) via combinatorial infusions that were administered 12 h per day for 28 days [88]. This study demonstrated that infusion of the tripeptide cocktail offered significant enhancements in glucose homeostasis that surpassed both RYGB and very low-calorie diet groups. However, weight loss in the tripeptide group was inferior to that exhibited in the RYGB and very low-calorie diet groups, suggesting additional mechanisms beyond physiological hormone secretion contribute to the RYGB-induced weight loss [88].
Thus, while GLP-1, GIP, oxyntomodulin, PYY, and ghrelin have been demonstrated to be mediators of glucose-stimulated insulin secretion and/or feeding [89], studying their mechanistic role after surgery is complicated. Despite this, the pharmacological implications of administering multiple peptides at once to treat obesity offer promise for surgery-like induced weight loss.
Bile Acids
Bile acids made in the liver are known for their fat-emulsifying properties and, as of late, their critical roles as signaling molecules that regulate metabolism. In both humans and rodents, VSG and RYGB increase circulating bile acids and alter the composition of bile acid species and does so in a weight-loss independent fashion [90,91,92]. The increase in circulating bile acids, at least after VSG, is due to changes in enterohepatic circulation. Namely, there is increased bile acid intestinal resorption and decreased hepatic reuptake of bile acids[92] leading to increased circulating bile acids that are then available to act as signaling molecules.
Bile acids signal through several different receptors, but the most widely studied in terms of bile acid-induced regulation of metabolism are the G-protein-coupled bile acid receptor (TGR5) and farnesoid X receptor (FXR). FXRs are expressed in the intestine, liver, and kidney [93], while TGR5 receptors are expressed in the gut, adipose tissue, skeletal muscle, pancreas, and throughout the CNS [94]. Recent work demonstrates that CNS TGR5 signaling is at least partially required for the anti-obesity effects of peripheral bile acid supplementation [95]. However, the role of TGR5 in the success of bariatric surgery remains controversial. Using a total TGR5 knockout mouse, one study demonstrated that TGR5 was necessary for VSG-induced weight loss [96], while another demonstrated that TGR5 was not necessary for weight loss but was required for the VSG-induced improvements in glucose homeostasis [97]. TGR5 also regulates GLP-1 secretion, and only the latter study found that it was necessary for the increase in GLP-1 with VSG. It is not clear why the results of these studies differ but given the potential role of CNS TGR5 signaling in body weight regulation, the use of tissue-specific knockouts of TGR5 may help to clarify the role of this receptor in the success of bariatric surgery.
While tissue-specific manipulations of TGR5 may help to clarify the role of TGR5 with surgery, tissue-specific manipulations of FXR and its downstream targets have revealed more complexity to the system. FXR is a nuclear transcription factor that has been shown to be a critical mediator of bile acid, cholesterol, lipid, and glucose metabolism [93]. Whole-body FXR knockout mice fail to lose weight or improve glucose tolerance and have different surgery-induced shifts in the microbiome [98], suggesting that FXR is necessary for the metabolic success of surgery. However, one study demonstrated that neither intestinal nor hepatic FXR is necessary for weight loss or improvements in glucose tolerance after VSG in mice [65]. There are two possibilities that can explain these seemingly discordant data. First, the FXR knockout mice may have a distinct metabolic impairment that outweighs the pathway corrected by VSG. Second, as has been recently proposed in the regulation of hepatic lipid metabolism [99], intestinal and hepatic FXR may be integrated such that changes in both are required to regulate the metabolic improvements seen with VSG.
Downstream of hepatic FXR activation is the activation of a small heterodimer partner (SHP). However, viral knockdown of SHP in obese mice had no impact on their ability to lose weight or even to reduce hepatic triglycerides after VSG [100]. Downstream of intestinal FXR activation is fibroblast growth factor 15 (FGF15 in mice and FGF19 in humans), a gut hormone that has a variety of physiological actions, including suppression of food intake and regulation of lipid and glucose metabolism and suppression of bile acid synthesis [101]. It is interesting to note that FGF19 (or FGF15 in mice) serum levels are elevated after VSG along with bile acids in humans and mice [90, 102,103,104]. Given that the pharmacological benefits of FGF19/15 include weight loss and enhancements in glycemia in rodents [105] as well as reductions in gluconeogenesis and liver triglycerides in humans [106, 107], it was hypothesized to play a role in mediating the success of bariatric surgery. However, a recent study on intestinal-specific FGF15 knockout mice found that these animals lost more, not less, weight after VSG compared to wildtype VSG mice due primarily to losses in lean and bone mass [108]. Despite the greater weight loss, the VSG-induced improvement in glucose tolerance was absent in the intestinal FGF15 KO mice. They also had even greater increases in bile acids than the control VSG mice [108, 109]. On the one hand, these data suggest a role for FGF15 in the improvements in glucose homeostasis after VSG, but this interpretation is confounded by the complicated phenotype of the animals. One possibility is that the function of the increase in FGF15 may serve as a protective mechanism to prevent muscle and bone mass loss and/or excessively high bile acid levels after surgery. Certainly, some patients do require cholecystectomy after bariatric surgery to remedy the accumulation of bile acids in the gallbladder. Furthermore, FGF19 has also been found to be higher in patients that experience a high incidence of hypoglycemia (post-bariatric hypoglycemia) [110] linking FGF19 to another complication of surgery.
Neural
Because bariatric surgery increases nutrient and gut peptides throughout the GI tract, it is possible that these nutrients and hormones act on the local sensory and/or enteric innervation of the gut and/or enter the circulation to directly provide feedback to the CNS. Many nutrients and hormones have direct effects on regions of the CNS to regulate feeding and/or glucose homeostasis (see [44, 111, 112] for review). Data from our own lab have demonstrated enriched cFOS immunoreactivity, a marker of neuronal activation, within the NTS after sucrose or lipid gavage in male rats with VSG compared to their sham counterparts [113]. However, these data do not indicate whether the nutrients were acting directly on the CNS, via hormonal, or neuronal, feedback from the periphery.
Intestinal delivery of nutrients has demonstrated effects on modulating CNS signaling. For example, in mice, intraduodenal infusion of nutrients suppressed agouti-related protein (AgRP) neurons, a neuronal population known to increase appetite [114, 115]. Additionally, another study demonstrated that different macronutrients activate AgRP neurons via distinct pathways. Specifically, Goldstein et al. showed that fat in the intestine inhibits AgRP neurons via vagal afferents while glucose inhibits AgRP neuronal activity through spinal afferent signaling [116].
Given the above, the question has been asked whether GI vagal innervation is necessary for the success of surgery. However, generally, studies have shown very little necessity of the vagus in the success of surgery. While subdiaphragmatic vagotomy in rodents prevented shifts in macronutrient preference with a RYGB-like surgery, it did not prevent weight loss [117]. In addition, vagal innervation of the portal vein and liver is not necessary for weight loss in rats [118]. In humans, one study demonstrated that RYGB patients subjected to vagotomy had similar weight loss to those without vagotomy [119]. While these data might suggest that vagal innervation is not necessary for the metabolic success of surgery, total vagotomy is a sledgehammer approach that likely drives compensatory responses. In support of this, a more targeted approach of specific ligation of the celiac vagal branches, which innervate the intestine, did blunt the RYBG-induced weight loss and hypophagia in rodents [120]. These data suggest that more specific targeting of the vagus is necessary to understand its role in mediating the effects of surgery.
In fact, like any nucleus within the CNS, the vagus is comprised of many different neuronal cell types with differential innervation and sensory patterns [121, 122]. Although not yet utilized in the context of bariatric surgery, the generation of new genetic tools that allow manipulation of these specific vagal neurons offer promise. For instance, chemogenetic acute activation of vagal afferent neurons that express the oxytocin receptor (Oxtr) [121] or the GLP-1R [123] both induced a reduction in food intake in mice. While there are conflicting data [124], using a different genetic strategy, a recent study has found that GLP-1R vagal afferent neurons are required for normal glucose homeostasis [125]. Applying these more complex genetic strategies, rather than blunt surgical dissection, to the role of the vagus will advance our knowledge of the contribution of the vagus to bariatric surgery success.
Conclusion and Future Perspectives
Here, we have discussed the signals that relay the nutrient status from the intestine to the brain and how these signals impact the metabolic success of bariatric surgery (Fig. 3). Despite numerous studies that have attempted to identify mechanisms that contribute to the metabolic success of bariatric surgery, we know more about what is not necessary, rather than what is necessary for the success of surgery. While individual gut peptide secretions have long been touted as playing a mechanistic role in surgery, mouse studies suggest a very limited mechanistic role for individual gut peptides. Multiple studies demonstrate intestinal adaptations to bariatric surgery, but whether these adaptations are simply markers of intestinal physiology or as mechanisms underlying the success of surgery remains to be determined. Lastly, new methodological tools reveal that we have only scratched the surface when it comes to fully assessing gut-brain axis communication and its role in regulating homeostasis. Utilizing these tools to assess how chronic manipulation of vagal subtypes in the context of bariatric surgery impacts food preference, food intake, glucose homeostasis, and body weight is an exciting opportunity moving forward.
References
Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009; 122:248–256 e5. https://doi.org/10.1016/j.amjmed.2008.09.041
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76. https://doi.org/10.1056/NEJMoa1200225
Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg Obes Surg. 2019;29:782–95. https://doi.org/10.1007/S11695-018-3593-1.
Sandoval DA. Mechanisms for the metabolic success of bariatric surgery. J Neuroendocr. 2019; 31:e12708. https://doi.org/10.1111/jne.12708
Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg Springer; 2016. p. 429–42. Doi: https://doi.org/10.1007/s11695-015-1996-9
Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis Elsevier. 2017;13:170–80. https://doi.org/10.1016/j.soard.2016.08.011.
Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kroll D, Borbely Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss in patients with morbid obesity the sm-boss randomized clinical trial. JAMA - J Am Med Assoc. 2018;319:255–65. https://doi.org/10.1001/jama.2017.20897
Mahawar KK, Sharples AJ. Contribution of malabsorption to weight loss after Roux-en-Y Gastric Bypass: a systematic review. Obes. Surg. Springer New York LLC; 2017. p. 2194–206. https://doi.org/10.1007/s11695-017-2762-y
Evers SS, Sandoval DA, Seeley RJ. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev Physiol Annu Rev Physiol. 2017;79:313–34. https://doi.org/10.1146/annurev-physiol-022516-034423.
Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–36, 2436 e1–3. https://doi.org/10.1053/j.gastro.2010.02.059
Wilson-Perez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes. 2013; 37:288–95. https://doi.org/10.1038/ijo.2012.18
Grayson BE, Schneider KM, Woods SC, Seeley RJ. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci Transl Med. 2013;5. https://doi.org/10.1126/scitranslmed.3006505
Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol. Elsevier; 2001;12.
Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30.
Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–95. https://doi.org/10.1210/en.2012-1595.
Grunddal KV, Ratner CF, Svendsen B, Sommer F, Engelstoft MS, Madsen AN, et al. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology. 2016;157:176–94. https://doi.org/10.1210/en.2015-1600.
Sykaras AG, Demenis C, Cheng L, Pisitkun T, McLaughlin JT, Fenton RA, et al. Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinol (United States). 2014;155:3339–51. https://doi.org/10.1210/en.2013-2165.
Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB. Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology Oxford Academic. 2017;158:2113–23. https://doi.org/10.1210/en.2017-00243.
Duca FA, Waise TMZ, Peppler WT, Lam TKT. The metabolic impact of small intestinal nutrient sensing. Nat Commun Nature Publishing Group; 2021. p. 1–12. https://doi.org/10.1038/s41467-021-21235-y
Furness JB. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012. p. 286–94. https://doi.org/10.1038/nrgastro.2012.32
Yoo BB, Mazmanian SK. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity. 2017. p. 910–26. https://doi.org/10.1016/j.immuni.2017.05.011
Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. https://doi.org/10.1016/S0165-1838(00)00127-2.
Powley TL. Vagal input to the enteric nervous system. Gut Gut; 2000. https://doi.org/10.1136/gut.47.suppl_4.iv30
Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, et al. The human and mouse enteric nervous system at single-cell resolution cell. Cell Press; 2020;182:1606–1622.e23. https://doi.org/10.1016/J.CELL.2020.08.003
Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, et al. Molecular architecture of the mouse nervous system. Cell Cell. 2018;174:999-1014.e22. https://doi.org/10.1016/j.cell.2018.06.021.
Wang YB, de Lartigue G, Page AJ. Dissecting the role of subtypes of gastrointestinal vagal afferents. Front Physiol Front; 2020. p. 643. https://doi.org/10.3389/fphys.2020.00643
Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Aut Neurosci. 2000;85:1–17. https://doi.org/10.1016/S1566-0702(00)00215-0
Berthoud HR, Blackshaw LA, Brookes SJH, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. John Wiley & Sons, Ltd; 2004. p. 28–33. https://doi.org/10.1111/j.1743-3150.2004.00471.x
Peris-Sampedro F, Le May M V., Stoltenborg I, Schéle E, Dickson SL. A skeleton in the cupboard in ghrelin research: Where are the skinny dwarfs? J Neuroendocrinol J Neuroendocrinol; 2021. https://doi.org/10.1111/jne.13025
Ayala JE, Bracy DP, James FD, Burmeister MA, Wasserman DH, Drucker DJ. Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology The Endocrine Society. 2010;151:4678–87. https://doi.org/10.1210/en.2010-0289.
Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia Diabetologia. 2006;49:1360–70. https://doi.org/10.1007/s00125-006-0237-0.
Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol - Regul Integr Comp Physiol Am J Physiol; 1998;274. https://doi.org/10.1152/ajpregu.1998.274.3.r618
Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav Physiol Behav. 2007;92:969–74. https://doi.org/10.1016/J.PHYSBEH.2007.07.003.
Guida C, Stephen SD, Watson M, Dempster N, Larraufie P, Marjot T, et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine. Elsevier; 2019;40. https://doi.org/10.1016/J.EBIOM.2018.12.040
Le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology Endocrinology. 2006;147:3–8. https://doi.org/10.1210/en.2005-0972.
Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety - Effect of obesity and weight reduction. Int J Obes Nature Publishing Group. 2001;25:1206–14. https://doi.org/10.1038/sj.ijo.0801655.
Knop FK, Aaboe K, Vilsbøll T, Vølund A, Holst JJ, Krarup T, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes, Obes Metab. Diabetes Obes Metab; 2012;14:500–10. https://doi.org/10.1111/j.1463-1326.2011.01549.x
Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. J Clin Endocrinol Metab; 2003;88:2706–13. https://doi.org/10.1210/jc.2002-021873
Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86:83–8. https://doi.org/10.1016/s0167-0115(99)00084-1
Page AJ, Kentish SJ. Plasticity of gastrointestinal vagal afferent satiety signals. Neurogastroenterol. Motil. Neurogastroenterol Motil; 2017. https://doi.org/10.1111/nmo.12973
Sewaybricker LE, Schur EA. Is Bariatric Surgery Brain Surgery? Diabetes American Diabetes Association. 2021;70:1244–6. https://doi.org/10.2337/DBI21-0022.
Almby KE, Lundqvist MH, Abrahamsson N, Kvernby S, Fahlström M, Pereira MJ, et al. Effects of gastric bypass surgery on the brain: simultaneous assessment of glucose uptake, blood flow, neural activity, and cognitive function during normo- and hypoglycemia. Diabetes American Diabetes Association. 2021;70:1265–77. https://doi.org/10.2337/DB20-1172.
Zimmerman CA, Knight ZA. Layers of signals that regulate appetite. Curr Opin Neurobiol Curr Opin Neurobiol. 2020;64:79–88. https://doi.org/10.1016/j.conb.2020.03.007.
Moura-Assis A, Friedman JM, Velloso LA. Gut-to-brain signals in feeding control. Am J Physiol - Endocrinol Metab, 2021 p. E326–32. https://doi.org/10.1152/AJPENDO.00388.2020
Kim KS, Seeley RJ, Sandoval DA. Signalling from the periphery to the brain that regulates energy homeostasis. Nat Rev Neurosci Nat Rev Neurosci; 2018. p. 185–96. https://doi.org/10.1038/nrn.2018.8
Bobbioni-Harsch E, Huber O, Morel P, Chassot G, Lehmann T, Volery M, et al. Factors influencing energy intake and body weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–6. https://doi.org/10.1038/sj.ejcn.1601357
Dias MC, Ribeiro AG, Scabim VM, Faintuch J, Zilberstein B, Gama-Rodrigues JJ. Dietary intake of female bariatric patients after anti-obesity gastroplasty. Clin (Sao Paulo). 2006;61:93–8. https://doi.org/10.1590/s1807-59322006000200002
Warde-Kamar J, Rogers M, Flancbaum L, Laferrere B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14:1070–9. https://doi.org/10.1381/0960892041975668
Moize V, Geliebter A, Gluck ME, Yahav E, Lorence M, Colarusso T, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003;13:23–8. Doi: https://doi.org/10.1381/096089203321136548
Trostler N, Mann A, Zilberbush N, Charuzi II, Avinoach E. Nutrient intake following vertical banded gastroplasty or gastric bypass. Obes Surg. 1995; 5:403–10. https://doi.org/10.1381/096089295765557502
Naslund I, Jarnmark I, Andersson H. Dietary intake before and after gastric bypass and gastroplasty for morbid obesity in women. Int J Obes. 1988;12:503–13.
Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220:782–90. https://doi.org/10.1097/00000658-199412000-00012
Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009; 297:R1273–82. https://doi.org/10.1152/ajpregu.00343.2009
Ullrich J, Ernst B, Wilms B, Thurnheer M, Schultes B. Roux-en y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes Surg Obes Surg. 2013;23:50–5. https://doi.org/10.1007/s11695-012-0754-5.
Sista F, Abruzzese V, Clementi M, Guadagni S, Montana L, Carandina S. Resolution of type 2 diabetes after sleeve gastrectomy: a 2-step hypothesis. Surg Obes Relat Dis. Surg Obes Relat Dis; 2018;14:284–90. https://doi.org/10.1016/j.soard.2017.12.009
Braghetto I, Lanzarini E, Korn O, Valladares H, Molina JC, Henriquez A. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg Obes Surg. 2010;20:357–62. https://doi.org/10.1007/s11695-009-0040-3.
Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014; 306:E424–32. https://doi.org/10.1152/ajpendo.00469.2013
Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim KS, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. Cell Press; 2017;25:927–934 e3. https://doi.org/10.1016/j.cmet.2017.02.008
Svane MS, Bojsen-Møller KN, Martinussen C, Dirksen C, Madsen JL, Reitelseder S, et al. Postprandial nutrient handling and gastrointestinal hormone secretion after roux-en-y gastric bypass vs sleeve gastrectomy. Gastroenterology. Elsevier; 2019;156.
Dirksen C, Bojsen-Møller KN, Jørgensen NB, Jacobsen SH, Kristiansen VB, Naver LS, et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia Diabetologia. 2013;56:2679–87. https://doi.org/10.1007/s00125-013-3055-1.
Le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252:50–6. https://doi.org/10.1097/SLA.0b013e3181d3d21f.
Kulkarni B V., Lasance K, Sorrell JE, Lemen L, Woods SC, Seeley RJ, et al. The role of proximal versus distal stomach resection in the weight loss seen after vertical sleeve gastrectomy. Am J Physiol - Regul Integr Comp Physiol. Am J Physiol Regul Integr Comp Physiol; 2016;311:R979–87. https://doi.org/10.1152/ajpregu.00125.2016
Tessier R, Ribeiro-Parenti L, Bruneau O, Khodorova N, Cavin J-B, Bado A, et al. Effect of different bariatric surgeries on dietary protein bioavailability in rats. American Physiological Society Bethesda, MD ; 2019;317:G592–601. https://doi.org/10.1152/AJPGI.00142.2019
Stefater MA, Sandoval DA, Chambers AP, Wilsonpérez HE, Hofmann SM, Jandacek R, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. W.B. Saunders; 2011;141:939–949.e4. https://doi.org/10.1053/J.GASTRO.2011.05.008
Ding L, Zhang E, Yang Q, Jin L, Sousa KM, Dong B, et al. Vertical sleeve gastrectomy confers metabolic improvements by reducing intestinal bile acids and lipid absorption in mice. Proc Natl Acad Sci. National Academy of Sciences; 2021;118:e2019388118. https://doi.org/10.1073/pnas.2019388118
Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity Obesity (Silver Spring). 2006;14:1553–61. https://doi.org/10.1038/oby.2006.179.
Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of roux-en-Y gastric bypass surgery. Endocrinology Endocrinology. 2010;151:1588–97. https://doi.org/10.1210/en.2009-1332.
Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg Obes Surg. 2014;24:241–52. https://doi.org/10.1007/s11695-013-1066-0.
Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. J Clin Endocrinol Metab; 2013;98:4391–9. https://doi.org/10.1210/jc.2013-2538
Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg Springer. 2012;22:1084–96. https://doi.org/10.1007/s11695-012-0621-4.
Papamargaritis D, Le Roux CW. Do gut hormones contribute to weight loss and glycaemic outcomes after bariatric surgery? Nutrients. Multidisciplinary Digital Publishing Institute; 2020. p. 1–28. https://doi.org/10.3390/nu13030762
Hutch CR, Sandoval DA. Physiological and molecular responses to bariatric surgery: markers or mechanisms underlying T2DM resolution? Ann. N. Y. Acad. Sci. Ann N Y Acad Sci; 2017. p. 5–19. https://doi.org/10.1111/nyas.13194
Kim KS, Sandoval DA. Endocrine function after bariatric surgery. Compr Physiol Compr Physiol. 2017;7:783–98. https://doi.org/10.1002/cphy.c160019.
Pucci A, Cheung WH, Jones J, Manning S, Kingett H, Adamo M, et al. A case of severe anorexia, excessive weight loss and high peptide YY levels after sleeve gastrectomy. Endocrinol Diabetes Metab Case Reports. Endocrinol Diabetes Metab Case Rep; 2015; 2015. https://doi.org/10.1530/edm-15-0020
Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, et al. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2016;150:454-464.e9. https://doi.org/10.1053/j.gastro.2015.10.009.
Nausheen S, Shah IH, Pezeshki A, Sigalet DL, Chelikani PK. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol - Endocrinol Metab. 2013;305:507–18. https://doi.org/10.1152/ajpendo.00130.2013.
Li F, Peng Y, Zhang M, Yang P, Qu S. Sleeve gastrectomy activates the GLP-1 pathway in pancreatic β cells and promotes GLP-1-expressing cells differentiation in the intestinal tract. Mol Cell Endocrinol. 2016;436:33–40. https://doi.org/10.1016/j.mce.2016.07.019.
Kim K-S, E Peck BC, Hung Y-H, Koch-Laskowski K, Wood L, Spence JR, et al. Vertical sleeve gastrectomy induces enteroendocrine cell differentiation of intestinal stem cells through farnesoid x receptor activation. bioRxiv. Cold Spring Harbor Laboratory; 2021;2021.04.22.440705. https://doi.org/10.1101/2021.04.22.440705
Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. NIH Public Access; 2011;141:950. https://doi.org/10.1053/J.GASTRO.2011.05.050
Hindsø M, MS S, N H, JJ H, S M, KN B-M, et al. The role of GLP-1 in postprandial glucose metabolism after bariatric surgery: a narrative review of human GLP-1 receptor antagonist studies. Surg Obes Relat Dis. Surg Obes Relat Dis; 2021;17:1383–91. https://doi.org/10.1016/J.SOARD.2021.01.041
Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. Diabetes; 2013;62:2380–5. https://doi.org/10.2337/db12-1498
Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2014; 3:191–201. https://doi.org/10.1016/j.molmet.2013.11.010
Kim K-S, Hutch CR, Wood L, Magrisso IJ, Seeley RJ, Sandoval DA. Glycemic effect of pancreatic preproglucagon in mouse sleeve gastrectomy.https://doi.org/10.1172/jci.insight.129452
Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol - Gastrointest Liver Physiol. American Physiological Society; 2010;299:G967. https://doi.org/10.1152/ajpgi.00070.2010
CHAMBERS AP, KIRCHNER H, WILSON–PEREZ HE, WILLENCY JA, HALE JE, GAYLINN BD, et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. NIH Public Access; 2013;144:50. https://doi.org/10.1053/J.GASTRO.2012.09.009
Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes Diabetes. 2011;60:810–8. https://doi.org/10.2337/DB10-0566.
Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med Nature Publishing Group. 2015;21:27–36. https://doi.org/10.1038/nm.3761.
Jones B, Sands C, Alexiadou K, Minnion J, Tharakan G, Behary P, et al. The metabolomic effects of tripeptide gut hormone infusion compared to Roux-en-Y gastric bypass and caloric restriction. J Clin Endocrinol Metab. 2021;XX:1–16. https://doi.org/10.1210/clinem/dgab608
Gimeno RE, Briere DA, Seeley RJ. Leveraging the gut to treat metabolic disease. Cell Metab. 2020. p. 679–98. https://doi.org/10.1016/j.cmet.2020.02.014
Sachdev S, Wang Q, Billington C, Connett J, Ahmed L, Inabnet W, et al. FGF 19 and Bile acids increase following Roux-en-Y gastric bypass but not after medical management in patients with type 2 diabetes. Obes Surg Springer. 2016;26:957–65. https://doi.org/10.1007/s11695-015-1834-0.
Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. John Wiley & Sons, Ltd; 2009;17:1671–7. https://doi.org/10.1038/oby.2009.102
Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. John Wiley & Sons, Ltd; 2014;22:390–400. https://doi.org/10.1002/oby.20548
Stofan M, Guo GL. Bile Acids and FXR: Novel Targets for Liver Diseases. Front. Med. Frontiers; 2020. p. 544. https://doi.org/10.3389/fmed.2020.00544
Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. Elsevier; 2014. p. 302–12. https://doi.org/10.1016/j.dld.2013.10.021
Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fénelon VS, Zizzari P, Quarta C, Bellocchio L, et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab Elsevier. 2021;33:1483-1492.e10. https://doi.org/10.1016/j.cmet.2021.04.009.
Ding L, Sousa KM, Jin L, Dong B, Kim B-WW, Ramirez R, et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. Hepatology; 2016;64:760–73. https://doi.org/10.1002/HEP.28689
McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut Gut. 2017;66:226–34. https://doi.org/10.1136/GUTJNL-2015-309871.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature NIH Public Access. 2014;509:183–8. https://doi.org/10.1038/nature13135.
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab Cell Metab. 2021;33:1671-1684.e4. https://doi.org/10.1016/J.CMET.2021.06.012.
Myronovych A, Salazar-Gonzalez R-MM, Ryan KK, Miles L, Zhang W, Jha P, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity. Obesity (Silver Spring); 2014;22:2301–11. https://doi.org/10.1002/OBY.20890
Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab Elsevier. 2005;2:217–25. https://doi.org/10.1016/j.cmet.2005.09.001.
DePaoli AM, Zhou M, Kaplan DD, Hunt SC, Adams TD, Marc Learned R, et al. FGF19 analog as a surgical factor mimetic that contributes to metabolic effects beyond glucose homeostasis. Diabetes American Diabetes Association. 2019;68:1315–28. https://doi.org/10.2337/db18-1305.
Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr Elsevier. 2017;36:861–8. https://doi.org/10.1016/j.clnu.2016.04.027.
Haluzíková D, Lacinová Z, Kaválková P, Drápalová J, Křížová J, Bártlová M, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. John Wiley & Sons, Ltd; 2013;21:1335–42. https://doi.org/10.1002/oby.20208
Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, et al. FGF19, FGF21, and an FGFR1/β-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab Cell Metab. 2017;26:709-718.e3. https://doi.org/10.1016/j.cmet.2017.09.005.
Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science (80- ). Science; 2011;331:1621–4. https://doi.org/10.1126/science.1198363
Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab Cell Metab. 2011;13:729–38. https://doi.org/10.1016/j.cmet.2011.03.019.
Bozadjieva-Kramer N, Shin JH, Shao Y, Gutierrez-Aguilar R, Li Z, Heppner KM, et al. Intestinal-derived FGF15 protects against deleterious effects of vertical sleeve gastrectomy in mice. Nat Commun Nature Publishing Group. 2021;12:1–19. https://doi.org/10.1038/s41467-021-24914-y.
Myronovych A, Bhattacharjee J, Salazar-Gonzalez RM, Tan B, Mowery S, Ferguson D, et al. Assessment of the role of FGF15 in mediating the metabolic outcomes of murine vertical sleeve gastrectomy. Am J Physiol - Gastrointest Liver Physiol. 2020;319:G669–84. https://doi.org/10.1152/AJPGI.00175.2020.
Mulla C, Goldfine M, Dreyfuss J, Houten S, Pan H, Pober, et al. Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. 2019;29:2092–9. https://doi.org/10.1007/S11695-019-03845-0
Lundqvist MH, Almby K, Abrahamsson N, Eriksson JW. Is the brain a key player in glucose regulation and development of type 2 diabetes? Front. Physiol. Frontiers; 2019. p. 457. https://doi.org/10.3389/fphys.2019.00457
Nogueiras R. The gut-brain axis: regulating energy balance independent of food intake. Eur. J. Endocrinol. Bioscientifica Ltd; 2021. p. R75–91. https://doi.org/10.1530/EJE-21-0277
Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D’Alessio DA, et al. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012/08/31. 2012;303:E1076–84. https://doi.org/10.1152/ajpendo.00211.2012
Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA, Knight ZA. Dynamics of gut-brain communication underlying hunger. Neuron Cell Press. 2017;96:461-475.e5. https://doi.org/10.1016/j.neuron.2017.09.043.
Su Z, Alhadeff AL, Betley JN. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep Cell Press. 2017;21:2724–36. https://doi.org/10.1016/j.celrep.2017.11.036.
Goldstein N, McKnight AD, Carty JRE, Arnold M, Betley JN, Alhadeff AL. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021;33:676-687.e5.
Hankir MK, Seyfried F, Hintschich CA, Diep T-A, Kleberg K, Kranz M, et al. Gastric bypass surgery recruits a Gut PPAR-α-Striatal D1R Pathway to reduce fat appetite in obese rats. 2017; 25:335–44. https://doi.org/10.1016/J.CMET.2016.12.006
Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg. 2012;255:294–301.
Okafor PN, Lien C, Bairdain S, Simonson DC, Halperin F, Vernon AH, et al. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes Res Clin Pract. 2015;9:274–80. https://doi.org/10.1016/j.orcp.2014.09.005.
Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes Surg. 2014;24:2145–51. https://doi.org/10.1007/s11695-014-1338-3
Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179:1129–1143 e23. https://doi.org/10.1016/j.cell.2019.10.031
Kupari J, Haring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019; 27:2508–2523 e4. https://doi.org/10.1016/j.celrep.2019.04.096
Brierley DI, Holt MK, Singh A, de Araujo A, McDougle M, Vergara M, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab 2021 32. Nature Publishing Group; 2021;3:258–73. https://doi.org/10.1038/s42255-021-00344-4
Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ, et al. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. American Society for Clinical Investigation; 2014;124:2456–63. https://doi.org/10.1172/JCI72434
Borgmann D, Ciglieri E, Biglari N, Brandt C, Cremer AL, Backes H, et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab. 2021;1466–1482.e7. https://doi.org/10.1016/j.cmet.2021.05.002
Funding
This work is supported by NIH R01DK121995, NIH R01DK107282, and by an American Diabetes Association grant (1–19-IBS-252) to DAS and institutional NIH Training grant NIH T32DK120521 to MB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Bethea, M., Sandoval, D.A. Gut Factors Mediating the Physiological Impact of Bariatric Surgery. Curr Diab Rep 22, 371–383 (2022). https://doi.org/10.1007/s11892-022-01478-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-022-01478-9