Abstract
Purpose of Review
Engineering endocrine pancreatic tissue is an emerging topic in type 1 diabetes with the intent to overcome the current limitation of β cell transplantation. During islet isolation, the vascularized structure and surrounding extracellular matrix (ECM) are completely disrupted. Once implanted, islets slowly engraft and mostly are lost for the initial avascular phase. This review discusses the main building blocks required to engineer the endocrine pancreas: (i) islet niche ECM, (ii) islet niche vascular network, and (iii) new available sources of endocrine cells.
Recent Findings
Current approaches include the following: tissue engineering of endocrine grafts by seeding of native or synthetic ECM scaffolds with human islets, vascularization of native or synthetic ECM prior to implantation, vascular functionalization of ECM structures to enhance angiogenesis after implantation, generation of engineered animals as human organ donors, and embryonic and pluripotent stem cell-derived endocrine cells that may be encapsulated or genetically engineered to be immunotolerated.
Summary
Substantial technological improvements have been made to regenerate or engineer endocrine pancreatic tissue; however, significant hurdles remain, and more research is needed to develop a technology to integrate all components of viable endocrine tissue for clinical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
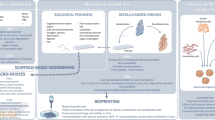
Approximately 20 million people worldwide are affected by T1D. Although exogenous insulin administration is a valuable therapy, most patients would benefit from engineered therapeutics providing a living, functional beta cell replacement [1, 2]. The human pancreas is a 13–15-cm-long soft lobulated organ composed of endocrine and exocrine compartments. The endocrine pancreas is organized into hierarchically distributed building blocks such as vasculature and endocrine cell niches that enable spatial distribution and function of various endocrine cells. The endocrine macro- and microenvironments are structured into vascularized independent units, called niches. In their specific niche, islets are embedded within extracellular matrix (ECM), composed of interstitial matrix (IM) and basement membrane (BM) proteins predominated by collagen type IV, laminin, and fibronectin [3]. Pancreatic islets represent approximately 1% of the overall pancreatic tissue but they receive more than 15% of the entire pancreatic blood flow to perform the endocrine functions [4, 5]. The blood supply of the endocrine pancreatic niche is provided by one to five arterioles per islet, branching into capillaries and forming a spherical framework with structural and functional similarities to a glomerulus [6, 7]. Endocrine cells within the pancreatic islet niche reside within this framework, all closely associated with the vasculature. Thanks to this vascular distribution, the islet niche receives oxygen and nutrient supply, and hormonal signals across the capillary endothelium connected to the arterial circulation, ensuring that they are all simultaneously exposed to the same hormonal and nutrient stimuli [8]. The islet itself is a highly specialized independent organ composed of single cells in a specific distribution: as parenchymal cells, α, β, and δ, and PP cells are the main components distributed in a 3D spheroid structure [9] interconnected with specific islet vascular endothelial cells (VECs) [10]. VECs not only make up a significant part of the islet volume but also sustain islet function. Any effort to re-engineer the endocrine pancreas will require careful consideration of all these pivotal components of the islet niche and the endocrine cells within. Type 1 diabetes (T1D) is the most relevant disease that afflicts the endocrine pancreas [1]. T1D is an autoimmune disease in which β cells are selectively destroyed. β cell loss results in permanent endocrine deficiency that makes daily insulin injections necessary to sustain normoglycemia [2]. As of now, islet transplantation is the only available cell therapy that can provide at least temporary insulin independence [11]. However, during islet isolation, the pancreatic endocrine niche is completely disrupted. Once implanted, islets slowly engraft while a large number is lost during the initial avascular phase, mainly due to ischemia and inflammation [12,13,14]. This review will discuss currently available technologies to recreate the main components needed to synthetically or naturally re-engineer the endocrine pancreas (Fig. 1).
Minimal building blocks required to engineer the endocrine pancreas. The pancreas is composed of independent endocrine units, connected by an extensive and highly specialized vascular network. The ECM (BM and IM) as a unique pancreatic islet niche, its vascular network, and the endocrine cells are the three minimum building blocks required to engineer a bioartificial endocrine pancreas
Engineering the Endocrine Pancreas: the Importance of the Islet ECM Niche
The islet ECM plays a pivotal role in spatial organization and function of islet cells. The IM confers flexibility and elasticity whereas the BM acts as a barrier limiting the movement of both soluble molecules and cells [15]. The BM provides attachment sites to epithelium and endothelium and consists of different layers of laminin [16, 17], collagen IV [17, 18], collagen I [19], and fibronectin [16, 17]. Mature islet interaction with native or similar ECM material regulates survival [20,21,22,23], insulin secretion [24], and proliferation and morphology preservation [25, 26]. Moreover, isolated islets with retained native ECM (via an incomplete isolation process) showed a reduction of apoptosis and a significant improvement of function [17, 27, 28]. Glycosaminoglycans such as heparan sulfate are involved in the regulation of postnatal islet growth and insulin secretion [29]. Moreover, laminins α4 and α5 are essential for physiological beta cell adhesion and hormone secretion [30]. These datapoints highlight the importance of a defined ECM niche in islet development and function.
Recognizing the essential role of the ECM in islet survival and endocrine function, most engineering approaches aim to utilize natural or synthetic ECM components to mimic the native ECM. The ultimate goal is to define and replicate the minimal ECM niche requirement (collagen, laminin, and fibronectin) to create an adequate microenvironment for islets. One of the most recent technologies enabling the isolation of native extracellular matrix is organ decellularization [31,32,33]. Various decellularization techniques preserve organ architecture including the native vascular network [34••]. To date, several protocols have been developed to decellularize native organs including the pancreas [35••]. Human, pig, and rodent pancreata have been successfully decellularized showing [35••, 36,37,38] that decellularized native pancreatic ECM preserves micro- and macrostructure and most importantly the islet niche. Moreover, such native scaffolds have been consistently produced from human pancreata maintaining their innate molecular and spatial framework and stiffness and growth factors. Rodent pancreatic ECM were successfully repopulated with rodent islets and support islet function in vitro and after transplantation in a diabetic mouse model in vivo [39]. Peloso et al. first repopulated a human decellularized pancreas with endothelial cells and islets, showing the preserved ability to sustain the endocrine function of islets in this 3D context ex vivo in the presence of different stimuli [35••]. More recently, pancreatic ECM hydrogels have been reported to support cell survival and engraftment [40]. On the other hand, several non-pancreas-derived decellularized matrix products have been used to treat type 1 diabetes in preclinical models. Decellularized porcine lung slices (300 μm) have been employed as mechanical support for islet seeding prior to intraperitoneal transplantation. In this report, authors showed long-term follow-up with preserved function with a consistent initial engraftment gap compared to an internal control; indeed, a de novo vascular network was established 60 days post transplantation [41]. Wang et al. have used a composite decellularized scaffold, based on decellularized pericardium and collagen powder. In this report, a composite of native and artificial ECM, used as support for islet transplantation, showed successful reversal of diabetes in a syngeneic transplantation model of type 1 diabetes [42]. While the response was reported in correlation with the number of equivalent transplanted islets, no comparison with preclinical gold standard techniques, such as intraportal islet transplantation or kidney capsule, was performed [42]. Extensive research has been performed to explore pure synthetic ECM scaffolds for beta cell replacement. These technologies are more amenable to controlled scaffold architecture, reproducible matrix functionalization, and scalable production. Several reports attempted to define minimum requirements of ECM components to generate biologically relevant islet scaffolds. Alginate capsules are among the oldest and most commonly used synthetic scaffolds. Recent developments in the islet encapsulation field provided improvements in the capsule structure and supplementation with biologically relevant ECM components to improve islet survival and function in vivo. Although encapsulation is able to immune-protect pancreatic islet, the major obstacle is the drastic loss of islet survival within the first 2 weeks after transplantation [43, 44]. To overcome this limitation, recent studies have evaluated alginate capsules that can be supplemented with ECM component to improver islet survival and function [45]. Of note, recent encapsulation technologies were used to generate alginate capsule based on laminin and collagen IV [46]. Moreover, Llacua et al. improved ECM encapsulation technologies by integrating bioactive sequences of ECM molecules instead of full proteins. These bioactive sequences were synthetically produced reducing the variability of ECM components in comparison with those from biological origin. Interestingly, not all ECM components had beneficial effects and graft performance varied depending on their concentration. Different concentrations of collagen IV did improve insulin secretion when used in a physiological range. However, when used at high concentration, it had a detrimental effect on glucose-stimulated insulin secretion [45, 47, 48]. Other approaches combined decellularized ECM tissues as cell-instructive component within a hydrogel carrier. This interesting approach combines the flexibility of a hydrogel with the biological activity of ECM proteins that can be tuned to generate the desired cellular response [49]. All these reports emphasize the urgent need to further improve the cell-scaffold interaction and to develop biocompatible synthetic scaffolds to optimize islet survival, engraftment, integration, and function.
Engineering the Endocrine Pancreas: the Importance of the Islet Vascular Network
One of the major obstacles towards manufacturing of a valuable scaffold for endocrine replacement is the lack of a physiologic islet vascular network to allow nutrient distribution and oxygen diffusion. Pancreatic islets depend on substantial blood flow even if they represent only a small percentage of the overall pancreatic tissue [4, 5]. Pancreatic islets have a high metabolic rate and are susceptible to functional impairment even at moderate decreases of oxygen tension. In transplantation, adequate oxygenation during the early engraftment phase plays a critical role in the preservation of the transplanted islet mass [50]. After isolation, the lack of a proper vascular system and perfusion limits oxygen supply to diffusion from the surrounding media. Timely revascularization represents a key strategy in preserving beta cell mass during the early engraftment phase. Functional revascularization re-establishes the dynamic interplay between the islet cell core and recipient system metabolism. Thus, prompt islet revascularization after transplantation is crucial for survival and sustained function. To this date, revascularization is the most challenging limitation an engineered endocrine pancreas has to overcome. Current approaches can be divided into two categories: vascularization ex vivo before implantation, or in vivo after implantation. While work in this field is ongoing, no reports to date have shown a prevascularized endocrine device before implantation. Recently, decellularized rat pancreata were re-endothelialized with EPC (endothelial progenitor cells) [51]. In this model, the newly formed EPC vessels were able to promote new vascular formation in the subcutaneous site compared to the empty scaffold. Although this study did not show evidence of functional vascular anastomosis or perfusion, it demonstrated that an empty scaffold can recruit vascular ingrowth, emphasizing the idea that a prevascularized construct will improve scaffold integration after transplantation [51]. On the other hand, revascularization post implantation is the most commonly used approach. Indeed, natural and synthetic scaffolds can be engineered to improve neo-vasculogenesis derived from the recipient. Native decellularized pancreatic scaffold was recently edited to enhance the angiogenic properties. The immobilization of heparin was performed through 1-ethyl-3–(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide. In vitro data showed increased endothelial cell adhesion, proliferation, and angiogenesis on heparin-enhanced decellularized pancreatic scaffolds [52, 53].
A similar strategy was developed by Kim et al. using a layer-by-layer approach to shield non-human primate islets with polyethylene glycol plus heparin reducing the instant blood mediated inflammatory reaction (IBMIR) compared to the control group without heparin [54]. In another approach, scaffolds have been functionalized via incorporation of vascular endothelial growth factor (VEGF). Indeed, large-pore polymeric sponge scaffolds were loaded with peripheral cavities that contained islets suspended in a collagen hydrogel while a central cavity contained an alginate sphere for controlled release of pro-angiogenic VEGF. The functionalized scaffold was able to restore normoglycemia in a preclinical model of diabetes, 30 days after subcutaneous implantation [55]. Although scaffold functionalization can enhance vascular integration and invasion from the recipient, it is not able to recapitulate native vascular structure of islet niche, and most importantly, requires time for cell recruitment and structure generation after implantation.
Engineering the Cellular Building Blocks: Strategies to Produce Endocrine Cells
Thus, successful endocrine pancreatic engineering depends not only on innovative scaffolds but most importantly on cellular building blocks committed to beta cell phenotype and function. Several approaches have been developed to generate a stable and reproducible source of human beta/islet cells. One possibility is to obtain both vascular and islet cells from genetically engineered animals. The first exploratory studies in xenotransplantation were performed decades ago [56,57,58]. In more recent years, genetic engineering has improved the translational potential of porcine cell sources. Major obstacles to the clinical translation of xenotransplants are rejection by the human immune system, and the presence of large numbers of pig retroviruses that may jump across species. Thanks to CRISPR–Cas9 technology, several retroviruses have been cleaned from pig skin cells showing the possibility to use those cells to make iPSC (induced pluripotent stem cells) and clean islets [59, 60]. Moreover, a recent report demonstrated that genetically engineered neonatal pig islets, when induced to overexpress LEA29Y (a high-affinity variant of the T cell costimulation inhibitor CTLA-4Ig), were able to engraft and restore normoglycemia after transplantation into streptozotocin-diabetic humanized mice. This report provided the first evidence that beta cell-specific LEA29Y expression is effective for pig islet engraftment in the presence of a humanized immune system and it has a long-lasting protective effect on inhibition of human anti-pig xenoimmunity showing a possible clinical application [61]. A recent report demonstrated further proof-of-concept that the injection of mouse PSC in rat PDX1−/− blastocysts enables the generation of rat-sized pancreata composed of mouse PSC-derived cells. In addition, these mouse pancreatic islets could be isolated, harvested, and safely implanted in diabetic recipient mice to restore normoglycemia showing the feasibility and therapeutic potential of PSC-derived islets generated by blastocyst complementation in a xenogeneic host [62••]. Although this approach represents a promising strategy, several limitations need to be addressed to fully understand the clinical potential. A more robust and already tested approach is the use of PSC, in particular embryonic stem cells (ESC) and iPSC, generated from the reprogramming of somatic cells with defined factors [63]. In 2006, Novocell (known as ViaCyte, Inc.) generated an efficient in vitro protocol for ESC mimicking pancreatic organogenesis for the generation of beta cells [64]. Two years later, the same group successfully demonstrated the spontaneous in vivo differentiation of ESC-derived pancreatic endoderm cells into glucose-responsive endocrine cells 3 months after implantation [65]. These findings paved the way for the first clinical trial in phase I/II started in 2014 (ClinicalTrials.gov identifier: Nbib2239354) by ViaCyte. Over the last 10 years, several modifications have been made to the ViaCyte original protocol to finally enrich the pancreatic endocrine end products from in vitro differentiation. In 2014, promising protocols were published showing improvements in insulin secretion of PSC-derived pancreatic cells [66, 67]; Rezania et al. reported the seven-stage in vitro differentiation protocol that led to efficient ESC conversion into glucose-responsive insulin-producing cells with an effective role in reversing diabetes in 2 months after transplantation in diabetic mice [66]. On the other hand, Melton’s group developed an alternative strategy using a three-dimensional cell culture system obtaining mature, mono-hormonal, and functional stem cell-derived β cells showing their efficacy in hyperglycemic mice [67]. The first successful report of differentiation of human iPSC into insulin-secreting cells dates back to 2008 [68]. Although this was the first proof of concept, the differentiation process struggled with very low efficiency [69]. Later protocols focused on the differentiation successful rate. Yupo Ma et al. were able to obtain up to 50% of beta cells capable of secreting insulin in response to glucose stimulus from murine iPSC with in vivo successful ability to restore normoglycemia in diabetic recipient mice [70, 71]. Last updates were performed by Melton’s group. They defined a 5-week in vitro differentiation protocol that led to the generation of ~ 50% of C-peptide and Nkx6.1 double-positive cells from human iPSC [67]. Moreover, iPSC from patients affected by T1D were also differentiated into beta-like cells, as for a new concept of autologous therapy [72, 73].
Beta cell replacement in T1D is not only limited by shortage of islet donors, but also by the immunological aspect related to the allo and autoimmune response after transplantation. To overcome this limitation, gene editing was recently applied also to the PSC technology. Indeed, Russel’s group demonstrates that HLA-engineered PSCs and their differentiated derivatives are not recognized by CD8+ T cells, do not bind anti-HLA antibodies, and are resistant to NK-mediated citotoxicity, opening to the use of these cells as universal donor cells [74]. This approach will extend the use of cell therapy and reduce the abuse of immunosuppression to fight against allo-autoimmune responses.
Conclusion
In this review, we have discussed the minimum required building blocks needed to engineer endocrine pancreas grafts. The islet ECM plays a pivotal role in preserving islet morphology, survival, and function. Moreover, the islet vascular network is necessary to control glucose homeostasis and nutrient distribution. The most important component remains to be endocrine cells; to date, several approaches have been developed to engineer alternative sources of beta cells.
As of now, substantial technological improvements have been made to outline viable strategies towards the development of bioartificial pancreas grafts. However, more research is needed to generate fully functional and translatable grafts ready for clinical application.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–300.
Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, et al. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60:1370–9.
Cheng JY, Raghunath M, Whitelock J, Poole-Warren L. Matrix components and scaffolds for sustained islet function. Tissue Eng B Rev. 2011;17:235–47.
Lifson N, Lassa CV, Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Phys. 1985;249:E43–8.
Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci. 2016;121:81–95.
Zanone MM, Favaro E, Doublier S, Lozanoska-Ochser B, Deregibus MC, Greening J, et al. Expression of nephrin by human pancreatic islet endothelial cells. Diabetologia. 2005;48:1789–97.
Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749–63.
Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31:343–63.
Brereton MF, Vergari E, Zhang Q, Clark A. Alpha-, delta- and PP-cells: are they the architectural cornerstones of islet structure and co-ordination? J Histochem Cytochem. 2015;63:575–91.
Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–14.
Citro A, Cantarelli E, Piemonti L. Anti-inflammatory strategies to enhance islet engraftment and survival. Current Diabetes Reports. 2013;13:733–44.
Cantarelli E, Citro A, Pellegrini S, Mercalli A, Melzi R, Dugnani E, et al. Transplant site influences the immune response after islet transplantation: bone marrow versus liver. Transplantation. 2017;101:1046–55.
Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122:3647–51.
Citro A, Cantarelli E, Pellegrini S, Dugnani E, Piemonti L. Anti-inflammatory strategies in intrahepatic islet transplantation: a comparative study in preclinical models. Transplantation. 2018;102:240–8.
Korpos E, Kadri N, Kappelhoff R, Wegner J, Overall CM, Weber E, et al. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes. 2013;62:531–42.
Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. Blockade of beta1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes. 2006;55:1413–20.
Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12.
van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267:139–46.
Van Deijnen JH, Van Suylichem PT, Wolters GH, Van Schilfgaarde R. Distribution of collagens type I, type III and type V in the pancreas of rat, dog, pig and man. Cell Tissue Res. 1994;277:115–21.
Nagata NA, Inoue K, Tabata Y. Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polym Ed. 2002;13:579–90.
Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, et al. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant. 2008;17:111–9.
Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, et al. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53:2034–41.
Ris F, Hammar E, Bosco D, Pilloud C, Maedler K, Donath M, et al. Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human beta-cells in vitro. Diabetologia. 2002;45:841–50.
Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human beta-cells: effect on insulin content, secretion, and gene transcription. Diabetes. 2006;55:2723–9.
Beattie GM, Montgomery AMP, Lopez AD, Hao E, Perez B, Just ML, et al. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes. 2002;51:3435–9.
Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9–20.
Ricordi C, et al. Long-term in vivo function of human mantled islets obtained by incomplete pancreatic dissociation and purification. Transplant Proc. 1995;27:3382.
Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126:299–304.
Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse beta cell survival and autoimmune diabetes. J Clin Invest. 2012;122:132–41.
Meda P. Protein-mediated interactions of pancreatic islet cells. Scientifica (Cairo). 2013;2013:621249.
Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21.
Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33.
Guyette JP, Gilpin SE, Charest JM, Tapias LF, Ren X, Ott HC. Perfusion decellularization of whole organs. Nat Protoc. 2014;9:1451–68.
•• Ren X, et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol. 2015;33:1097–102. This study provides a technique to functionally revascularize a native decellularized organ.
•• Peloso A, et al. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann Surg. 2016;264:169–79. This study describes a technique to use a decellularized human pancreas as native scaffold for bioengineering endocrine organ.
Katsuki Y, Yagi H, Okitsu T, Kitago M, Tajima K, Kadota Y, et al. Endocrine pancreas engineered using porcine islets and partial pancreatic scaffolds. Pancreatology. 2016;16:922–30.
Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34:5488–95.
Yu H, Chen Y, Kong H, He Q, Sun H, Bhugul PA, et al. The rat pancreatic body tail as a source of a novel extracellular matrix scaffold for endocrine pancreas bioengineering. J Biol Eng. 2018;12:6.
Claudius C, et al. Bio-engineered endocrine pancreas based on decellularized pancreatic matrix and mesenchymal stem cell/islet cell coculture. J Am Coll Surg. 2010;211:S62.
Sackett SD, Tremmel DM, Ma F, Feeney AK, Maguire RM, Brown ME, et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep. 2018;8:10452.
Abualhassan N, Sapozhnikov L, Pawlick RL, Kahana M, Pepper AR, Bruni A, et al. Lung-derived microscaffolds facilitate diabetes reversal after mouse and human intraperitoneal islet transplantation. PLoS One. 2016;11:e0156053.
Wang X, Wang K, Zhang W, Qiang M, Luo Y. A bilaminated decellularized scaffold for islet transplantation: structure, properties and functions in diabetic mice. Biomaterials. 2017;138:80–90.
de Vos P, van Hoogmoed CG, van Zanten J, Netter S, Strubbe JH, Busscher HJ. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials. 2003;24:305–12.
de Vos P, de Haan BJ, de Haan A, van Zanten J, Faas MM. Factors influencing functional survival of microencapsulated islet grafts. Cell Transplant. 2004;13:515–24.
Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61:1261–72.
Llacua LA, de Haan BJ, de Vos P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J Tissue Eng Regen Med. 2018;12:460–7.
Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng A. 2008;14:1959–68.
Llacua A, de Haan BJ, Smink SA, de Vos P. Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J Biomed Mater Res A. 2016;104:1788–96.
Shridhar A, Gillies E, Amsden BG, Flynn LE. Composite bioscaffolds incorporating decellularized ECM as a cell-instructive component within hydrogels as in vitro models and cell delivery systems. In: Methods in Molecular Biology. Humana Press; 2017.
Coronel MM, Stabler CL. Engineering a local microenvironment for pancreatic islet replacement. Curr Opin Biotechnol. 2013;24:900–8.
Guo Y, Wu C, Xu L, Xu Y, Xiaohong L, Hui Z, et al. Vascularization of pancreatic decellularized scaffold with endothelial progenitor cells. J Artif Organs. 2018;21(2):230–7.
Xu L, Guo Y, Huang Y, Xiong Y, Xu Y, Li X, et al. Constructing heparin-modified pancreatic decellularized scaffold to improve its re-endothelialization. J Biomater Appl. 2018;32:1063–70.
Ren X, Feng Y, Guo J, Wang H, Li Q, Yang J, et al. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem Soc Rev. 2015;44:5680–742.
Park H, Haque MR, Park JB, Lee KW, Lee S, Kwon Y, et al. Polymeric nano-shielded islets with heparin-polyethylene glycol in a non-human primate model. Biomaterials. 2018;171:164–77.
Gebe JA, Preisinger A, Gooden MD, D'Amico LA, Vernon RB. Local, controlled release in vivo of vascular endothelial growth factor within a subcutaneous scaffolded islet implant reduces early islet necrosis and improves performance of the graft. Cell Transplant, 963689718754562. 2018;27:531–41.
Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98:1417–22.
Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006;81:1345–53.
Elliott RB. Towards xenotransplantation of pig islets in the clinic. Curr Opin Organ Transplant. 2011;16(2):195–200.
Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357:1303–7.
Yang L, Guell M, Niu D, George H, Lesha E, Grishin D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350:1101–4.
Buerck LW, Schuster M, Oduncu FS, Baehr A, Mayr T, Guethoff S, et al. LEA29Y expression in transgenic neonatal porcine islet-like cluster promotes long-lasting xenograft survival in humanized mice without immunosuppressive therapy. Sci Rep. 2017;7:3572.
•• Yamaguchi T, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542:191–6. This study describes successful blastocyst complementation as alternative strategy to generate species-specific beta cells with therapeutic potential.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52.
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–33.
Pagliuca FW, Millman JR, Gürtler M, Segel M, van Dervort A, Ryu JH, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–39.
Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–53.
Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–7.
Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507.
Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC, et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci U S A. 2010;107:13426–31.
Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106:15768–73.
Millman JR, Xie C, van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463.
Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35:765–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Antonio Citro declares no conflict of interest. Harald C. Ott is founder and stockholder of IVIVA Medical Inc., a bioengineering company. This relationship had no effect on the content of this article. Also, Dr. Ott has a patent pending on bioartificial pancreas.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Immunology, Transplantation, and Regenerative Medicine
Rights and permissions
About this article
Cite this article
Citro, A., Ott, H.C. Can We Re-Engineer the Endocrine Pancreas?. Curr Diab Rep 18, 122 (2018). https://doi.org/10.1007/s11892-018-1072-7
Published:
DOI: https://doi.org/10.1007/s11892-018-1072-7