Abstract
Purpose of Review
Hyperglycemia in the emergency department (ED) is being recognized as a public health problem and presents a clinical challenge. This review critically summarizes available evidence on the burden, etiology, diagnosis, and practical management strategies for hyperglycemia in the ED.
Recent Findings
Diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) are hyperglycemic emergencies that commonly present to the ED. However, the most common form of hyperglycemia in ED is associated with non-hyperglycemic medical emergencies. The presence of hyperglycemia increases the mortality and morbidity associated with the primary condition. The related hospital admission rates and costs are also elevated. The frequency of DKA or HHS related mortality and morbidity has remained high over the last decade. However, attempts have been made to improve management of all hyperglycemia in the ED. Evidence suggests that better management of hyperglycemia in the ED with proper follow-up improves clinical outcomes and prevents readmission.
Summary
Optimization of the hyperglycemia management in the ED may improve clinical outcomes. However, more clinical trial data on the outcomes and cost-effectiveness of various management strategies or protocols are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prevalence of diabetes is increasing in the USA and globally, and is associated with a substantial burden of mortality and morbidity [1]. Population-based estimates indicate that approximately 14% of adult Americans have diabetes, mainly type 2 diabetes mellitus (T2DM). Approximately 25% of people with diabetes remained undiagnosed in 2012 [1].Of this latter group, a significant proportion is first diagnosed when they present to the emergency department (ED) with an acute illness or hyperglycemia. Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS) represent two extremes in the spectrum of dysglycemia, which pose enormous clinical and management challenges especially to the emergency physician. Presence of DKA often suggests that the patient has type 1 diabetes mellitus (T1DM), but DKA can also occur in T2DM under conditions of extreme stress. Similarly, whereas HHS occurs most commonly in T2DM, it can be seen in T1DM in conjunction with DKA [2]. However, the largest majority of hyperglycemic patients presenting to the ED have a non-hyperglycemic emergency. Because hyperglycemia may affect the outcomes of other acute illnesses, it is important to pay proper attention to this condition. Evidence shows that even non-diabetic hyperglycemia is associated with increased mortality and length of hospital stay in patients admitted through ED [3]. However, treatment of hyperglycemia in the ED remains inadequate [4].
Scope of the Problem
More than 20% of all ED visits include diabetes as a diagnosis, with an increase in these proportions over the last decade [5]. Accordingly, the costs of ED visits among people with diabetes have also increased, especially during the 2002–2011 period [6]. Most of these visits are due to non-hyperglycemia emergencies because data covering the last two decades indicate no change in the overall rates of ED visits related to hyperglycemic crisis [7•]. However, over the period 2006–2011 notable changes in subgroups of patients presenting to ED with hyperglycemic crises were seen, including significant increases of 29 and 17% in ED visit rates among women and adults aged 65–74 years, respectively [7•]. Also, the age- and sex-adjusted ED visits related to hyperglycemia have remained higher (up to fourfold) among Black patients compared with their White counterparts [8]. However, of the patients seen in the ED with hyperglycemia, a large proportion will have neither DKA nor HHS. In this pool of patients, an important proportion is unaware of their diabetes.
Undiagnosed Prediabetes or Diabetes
Population-based national US estimates indicate that a sizeable number of Americans with diabetes are unaware of their condition. In 2011–2012, 36.4% of Americans with diabetes were undiagnosed [1]. Additionally, up to 38% of American had undiagnosed prediabetes (impaired fasting glucose and impaired glucose tolerance) [1]. Accordingly, a number of patients presenting to the ED will be found to have previously undiagnosed hyperglycemia. Indeed the presence of known risk factors and therefore, estimated risk of undiagnosed diabetes among ED visitors’ is quite high [9, 10]. In a study that followed patients with relatively high blood glucose levels without known diagnosis of diabetes in the ED, found that 11% of these patients had diabetes and 55% had prediabetes on retesting 6 weeks later [11]. In those with unequivocal hyperglycemia (e.g., those with random blood glucose >200 mg/dL) including signs such as polyuria, polydipsia, and nocturia, further testing is not needed. For the rest of patients, the diagnosis of diabetes will require testing performed on two separate occasions, as per the American Diabetes Association (ADA) recommendations [12]. Typically, emergency department providers will not offer confirmatory testing for patients who are suspected to have prediabetes or diabetes, thus this can be done in the hospitals for those admitted or on an outpatient basis. Hemoglobin A1C may give a clue as to which patients will end up having diabetes, as it represents the average blood glucose levels over the last 3 months [10, 13]. Additional factors may also help in predicting those who will ultimately have diabetes. For example, in a cohort study in an urban ED, a number of risk factors (age > 45 years, polyuria, and polydipsia) and random blood glucose >155 mg/dL were predictive of later diagnosis of prediabetes or diabetes [14]. For patients with suspected diabetes, there is need for communication both with the patient and the next clinical care team, to help overcome clinical inertia and improve glycemic control. For patients who are to be hospitalized, a clear communication with the hospital team regarding the need for further evaluation and potential initiation of treatment in the hospital is essential. The communication is critical to avoid clinical inertia, especially as a previous study in the ED indicated that only 10% of patients with diabetes found in the ED were informed of their hyperglycemia, as assessed by their inpatient or ED discharge instructions [15]. The latter phenomenon could foster readmission for hyperglycemia.
Pathophysiological Considerations for Hyperglycemia in ED
For both DKA and HHS, the key pathogenic components are hyperglycemia and dehydration, with additional ketogenesis in the case of DKA. Both these conditions are characterized by absolute or relative insulin deficiency in association with increased circulating levels of counter-regulatory hormones including glucagon, catecholamines (norepinephrine, epinephrine), cortisol, and growth hormone [2]. Similar pathophysiological mechanisms can account for hyperglycemia in non-hyperglycemic acute illnesses. Increased levels of counter-regulatory hormones with concurrent insulinopenia stimulate gluconeogenic enzymes, especially phosphoenol pyruvate carboxykinase (PEPCK) [16, 17]. Proteins and fats are broken down to supply the precursors for gluconeogenesis, which is a crucial step in the development of severe hyperglycemia. Moreover, glucose utilization is decreased due to insulin deficiency and further exaggerated by increased availability of FFA [18]. Overall, the mechanism of hyperglycemia in acute illness is multifactorial, with the main abnormalities including increased gluconeogenesis and, glycogenolysis, and impaired glucose utilization by cells (primarily muscle and fat cells) due to insulin deficiency. Severe hyperglycemia leads to osmotic diuresis and dehydration. The latter results in a decrease in glomerular filtration rate and decreased clearance of glucose with further aggravation of hyperglycemia [2]. Delay in treatment will eventually lead to a hyperglycemic crisis.
Newly diagnosed individuals with T1DM account for 15% or more of cases of DKA [19]; with higher figures among children than among adults. Approximately 7–17% of cases of HHS cases are the initial manifestation of diabetes [20, 21].The two main precipitating factors described for inducing hyperglycemic crises have been inadequate insulin therapy and infection [22]. The inadequate insulin therapy may be related to noncompliance or in some cases failure of insulin pump [23]. The main infectious processes that have been implicated are pneumonia or urinary tract infection and less frequently other type of infections such as dental abscess or skin infection [22, 24]. Other precipitating factors include the situations associated with extreme stress, namely, surgery, trauma, pregnancy, cardiovascular events (e.g., myocardial ischemia, cerebrovascular events, or limb ischemia), medications (corticosteroids, sympathomimetics [terbutaline], pentamidine, atypical antipsychotic medications, and thiazide diuretics), acute abdominal processes (e.g., appendicitis or pancreatitis), substance abuse (alcohol or cocaine, which have been associated with frequent omissions of insulin administration, with the cocaine exerting a significant effect on counter-regulatory hormones as well) [2, 22, 24, 25]. In the specific case of HHS, as it develops slowly over time, decreased water intake can be an aggravating factor especially in elderly patients (with impaired thirst mechanism and/or inability to access water because of physical or neurologic limitations) [22]. In rare cases, there have been reports of DKA as the primary manifestation of acromegaly [26]. In a number of cases, no precipitating factor is identified despite a thorough workup.
Management of Hyperglycemia in ED
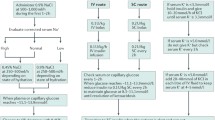
It is important to detect unknown diabetes, assess the status of diabetes control and diagnose the presence of DKA or HHS in any patient presenting to ED. A suggested algorithm for the diagnosis and management of diabetes in the ED is shown in Fig. 1. The current accepted tenets of therapy for any severe hyperglycemia include hydration, insulin therapy, and correction of electrolyte abnormalities.
Initial Assessment
As both DKA and HHS have potentially high mortality rates, this dictates the need for a careful initial evaluation of patients presenting to ED with hyperglycemia. This includes a non-specific set of measures that include assessing the need for airway protection to ensure adequate ventilation/and oxygenation, appropriate intravenous (IV) access, continuous cardiac monitoring, and strict monitoring of intake and output. History is extremely important to identify the cause of severe hyperglycemia in patient with known diabetes. Non-adherence with insulin or antidiabetic medications is common, as are psychiatric illnesses and substance abuse. The recommended biochemical assessment to evaluate the cause and severity of hyperglycemia includes serum glucose levels, hemoglobin A1C, basic metabolic panel, serum phosphate and magnesium, arterial blood gas, and serum ketones. A complete blood count (CBC) with differential, hepatic enzymes, urinalysis, cardiac enzymes, and coagulation profile are also recommended as part of the initial evaluation. Further assessment should aim at investigating potential underlying triggers such an infection (urine and blood cultures, chest radiograph, and possibly other tests in selected cases), pancreatitis (serum lipase and amylase), or cardiac events (cardiac enzymes, electrocardiogram) for the most part. In specific cases, there may be a need for further imaging (chest/abdomen/brain or other organs) based on the clinical picture.
Key biochemical calculations based on the initial laboratory results that are necessary to guide therapy include corrected serum sodium, serum osmolality, anion gap, and free water deficit [2]. Thereafter, the frequency of reassessment of serum glucose and electrolytes is a function of the response to therapy. This is detailed in the ADA guidelines treatment algorithm [2].
Therapies
Treatment of Hyperglycemic Crisis
The specific treatment of a hyperglycemic crisis has several components, as detailed below.
Intravenous fluids
Intravenous hydration is the mainstay of management of hyperglycemic crises and the first needed therapy in both DKA and HHS [2]. It decreases serum glucose and ketones (thus improving metabolic acidosis) through urinary clearance, which is improved by the restoration of intravascular volume and renal perfusion. Intravenous hydration also decreases counter-regulatory hormone concentrations. The initial fluid replacement is most often isotonic saline to restore perfusion and expand intravascular volume. The average fluid deficit in DKA ranges from 3 to 6 L, whereas it may be 8 to 10 L in patients with HHS. More precisely, the water deficit is estimated at 100 mL/kg of body weight in DKA and 100–200 mL/kg in HHS [2]. The water deficit is derived using the following formula: water deficit = (0.6) (body weight in kg) × (1–[corrected sodium/140]) [20]. To correct water deficit, isotonic saline is initially infused at 500–1000 mL/h for 2–4 h, followed by the infusion of 0.9 or 0.45% saline at 250–500 mL/h, with the choice between the two determined by the levels of serum sodium, the state of hydration and urine output. In general, isotonic saline (0.9% NaCl) is continued in patients with low serum sodium, whereas patients with normal or elevated sodium or hyperosmolality often receive 0.45% NaCl [20]. Intravenous dextrose (5 or 10%) is added once the plasma glucose level is between 200 and 250 mg/dL, to allow insulin administration until ketonemia is controlled while avoiding hypoglycemia [2].
Insulin Therapy
Insulin therapy is the key component of therapy, which should be commenced immediately. Hitherto, intravenous regular insulin has been the treatment of choice for the majority of patients with hyperglycemic emergency [27, 28]. However, for mild to moderate DKA subcutaneous insulin can be considered. A few studies have shown equivalent time to resolution of DKA when comparing subcutaneous insulin versus intravenous insulin. Subcutaneous insulin may be a better option in the ED setting where one to one staffing is often a problem. Insulin reduces hepatic gluconeogenesis and suppresses lipolysis and ketogenesis [29]. However, insulin should not be started unless the serum potassium is at least >3.3 mEq/L as this may cause a rapid shift of extracellular potassium into the intracellular space. Most physicians treating DKA start insulin infusions with a bolus of 0.1 unit/kg followed by a continuous infusion at a rate of 0.1 U/kg/h (5–10 U/h) [2]. However, a study showed no difference in outcomes or hypoglycemia risk between patients receiving an initial insulin bolus and those that did not [30]. In case the bolus is omitted, the initial infusion rate could be started at 0.14 U/kg/h. The infusion rate should be adjusted to ensure that serum glucose falls by at least 50 mg/dL/h, with the glucose level acting as a reliable surrogate marker for insulin action, including its action in the adipose tissue to suppress lipolysis. Evidence suggests that insulin therapy combined with rehydration leads to a predictable decrease in plasma glucose concentration [31, 32]. The insulin rate may be decreased (minimum rate 0.5 U/h) and dextrose added to the intravenous fluids when the plasma glucose concentration reaches <200 mg/dL. The insulin infusion rate can be decreased at a higher glucose level (<300 mg/dL) in patients with HHS [2]. The insulin infusion rate should be adjusted to maintain a plasma glucose level of 150–200 mg/dL until ketoacidosis is resolved, as evidenced by normalization of pH and anion gap among those with DKA, and until mental obtundation and the hyperosmolar state are corrected among those with HHS. The resolution of DKA and often HHS is heralded by a rapid increase in insulin sensitivity, which requires hourly adjustment of the insulin infusion rate while dextrose-containing fluids are infused to avoid hypoglycemia.
Potassium Therapy
Total body potassium depletion is a common feature in all patients with DKA and HHS, with a deficit of 3–5 mmol/kg [33]. The initial serum potassium level is often within the normal range or elevated, which is due to a shift of potassium from the intracellular to the extracellular compartment related to a combination of hypertonicity, insulin deficiency and acidosis [33, 34]. Fluid and insulin therapy promote a rapid intracellular shift of potassium, which may result in hypokalemia with the risk of cardiac arrest. Hence, early IV potassium therapy should be initiated when the serum potassium level is below 5.0 mEq/L with the goal level of 4–5 mEq/L during therapy [2]. An exception to this rule is the case of low urine output or severely decreased renal function whereby potassium should be given only if low and monitored carefully.
Bicarbonate Therapy
The use of bicarbonate in DKA has been controversial. Currently, it is recommended for the severe metabolic acidosis (pH < 6.9) by most experts including the ADA guidelines [2]. However, no study has shown benefit from the use of this therapy. In a systematic review of randomized clinical studies on the efficacy of bicarbonate therapy for severe acidemia in DKA, no benefit of this therapy in improving outcomes or the rate of recovery of hyperglycemia and ketoacidosis was found [35]. Bicarbonate therapy is not without risk. Potentially deleterious effects include cerebral edema, hypokalemia, rebound acidosis, hypoxia, and hypernatremia, and in view of evidence of lack of a therapeutic effect, it should generally be avoided [2, 36].
Phosphate Therapy
Phosphate repletion is not always needed in the management of DKA as mild degrees of hypophosphatemia usually self-correct once the patient has resumed eating, unless the phosphate levels fall below <1 mg/dL, especially in a patient with evidence of respiratory or cardiac distress. There is no evidence of the beneficial effect of phosphate replacement on clinical outcomes in DKA [37, 38]. Furthermore, aggressive intravenous phosphate therapy can cause hypocalcemia [39] and it should be avoided.
Hyperosmolality
Although hyperosmolality is associated with adverse outcomes, specific correction of this derangement is generally not indicated in adults. A gradual rate of change as a marker of effective and safe HHS therapy appears to be less important in adults than in children. Interestingly, data from pediatric studies no longer support the construct of cerebral edema caused by changes in osmolarity, but rather suggest it linked more to low overall brain perfusion and insufficient volume resuscitation [40].
Monitoring of Therapy and Resolution of Hyperglycemia Crisis
While in the ED, all patients should get frequent clinical and laboratory reassessment to ensure an adequate urine output, electrolyte correction, and avoid fluid overload. The main assessments include finger-stick glucose (every hour to prevent hypoglycemia) and basic metabolic panel (every 1 to 2 h mainly to monitor potassium levels and the anion gap, as these provide a good estimate of the serum ketoacid (anion) levels) [2]. In the ED, the average period over which fluid, metabolic, and electrolyte deficits will be corrected is 18 to 24 h. Criteria for resolution of DKA include a serum glucose ≤250 mg/dL and at least two of the following criteria: normalization of the anion gap, a venous or arterial pH ≥7.3, and a serum bicarbonate level ≥ 18 mEq/L [2]. Ketonemia and ketonuria may persist for 24 to 36 h due to slower elimination. Patients who recover from ketoacidosis may develop a secondary hyperchloremic non-anion gap metabolic acidosis resulting from aggressive saline administration. The serum bicarbonate may not normalize immediately for this reason as it is temporarily “replaced” by chloride. In HHS, the resolution occurs with a plasma glucose level ≤ 250 mg/dL and normal effective serum osmolality <310 mmol/kg in the setting of a restored baseline mental status.
Transitioning from Insulin Infusion to Subcutaneous Insulin
In patients treated with IV insulin, transition to a subcutaneous insulin regimen should be considered once the acute metabolic derangement has resolved and the patient is alert and can start oral nutrition. In instances of patient remaining under extreme stress such as those with critical illness (e.g., shock requiring pressor agents, mechanical ventilation) or underlying causes requiring additional interventions (e.g., need for surgery), continuation of insulin infusion therapy using an appropriate variable-rate continuous insulin infusion protocol designed to maintain glucose within the appropriate evidence-based range until clinically stable should be considered.
In transitioning from IV insulin to subcutaneous insulin, abrupt interruption of the insulin infusion is not recommended as the half-life of intravenous regular human insulin is less than 10 min. An overlap of the insulin infusion and subcutaneous insulin (continuation of IV insulin for 2 to 4 h after administration of subcutaneous insulin) is absolutely necessary to avoid rebound hyperglycemia and possible reopening of the anion gap from ketoacidosis. Patients with previously known diabetes treated with subcutaneous insulin prior to admission can be restarted on their previous insulin regimen, especially in those who were adequately controlled. Among insulin naive patients or newly diagnosed patients, insulin can be started at a total daily dose of 0.5–0.8 U/kg/dL. Subcutaneous insulin regimens with long acting basal insulin (glargine or detemir) and rapid acting insulin analogs (lispro, aspart, or glulisine) mimic normal insulin physiology and are preferred for transition over use of intermediate-acting insulin (neutral protamine Hagedorn, NPH) and regular human insulin. A randomized trial comparing the use of insulin analogs (glargine or glulisine) to regular human insulin and NPH for transitioning from intravenous to subcutaneous insulin among DKA patients found a lower incidence of hypoglycemia with insulin analogs [32]. Evidence suggests that administration of insulin glargine at a dose of 0.25 U/kg within 12 h of initiation of intravenous insulin infusions may help prevent rebound hyperglycemia following acute management of DKA. Indeed, the incidence of rebound hyperglycemia was lower without increased risk of hypoglycemia in the group receiving insulin glargine than in the control group, in a recent study [41]. It is important to keep in mind that insulin sensitivity changes quickly in critically ill and glucotoxic patients and lower doses of insulin may be required after recovery from acute illness. Lower insulin doses are also necessary in those with renal or pancreatic insufficiency.
Disposition of those with DKA or HHS
Patient with severe DKA or HHS often require ICU admission for adequate treatment, observation, and resolution of the underlying cause. However, the hyperglycemic crisis itself can often be resolved in an emergency department and some of these patients may be stable enough for general floor admission to continue subcutaneous insulin, pending improved volume status after resuscitation, closed anion gap, and ability to tolerate fluids by mouth. A number of patients warrant a higher level of care due to underlying sepsis, hypoxia, altered mental status, hypotension, persistent tachycardia despite fluid resuscitation, and significant laboratory derangements, such as acidosis or severe electrolyte abnormalities.
Acute comorbidities such myocardial infarction or cerebrovascular accidents may also dictate disposition to an ICU. Because of the scarcity of ICU beds, there may be longer ED stays requiring close attention. If appropriately managed, hyperglycemic crises itself can be treated in ED or another properly staffed observation unit and the need of ICU admission can be obviated. With proper treatment, the average time to the resolution of anion gap acidosis is 3 h [42].
Treatment of Hyperglycemia in Patients with Non-Hyperglycemic Emergency
There are no hard and fast rules as to how to approach therapy among those without DKA or HHS. Among those with glycemia levels not warranting admission, patients with newly diagnosed diabetes can be discharged on oral therapy, and those with previously known diabetes can have their therapy adjusted including oral therapy and/or insulin therapy. For the newly diagnosed patients, given that lifestyle interventions are generally unsuccessful, patients with a clear diagnosis of diabetes may be considered candidates for initiating an antidiabetic medication in the emergency department upon discharge. These patients include those with obesity, family history of diabetes, and polyuria who also have a blood glucose level > 200 mg/dL with no other obvious cause such as trauma or infection. In starting antidiabetic therapy in the ED, consideration should be given to comorbidities, renal and hepatic functions, with the understanding that a follow-up with a primary care physician is needed for medication adjustment. Following the recommendations of the ADA and the European Association for the Study of Diabetes for initiating therapy in patients with newly diagnosed diabetes, metformin is often the initial therapy to consider in a patient with T2DM [43]. Indeed, in a before-after pilot study conducted in an urban ED [44], starting metformin in the emergency department was found safe and effective among patients with hyperglycemia but no prior diagnosis of diabetes, with the majority of patients experiencing rapid and safe decreases in their blood glucose and HbA1C levels, as well as a decrease in the number of ED visits by 78%.
For hyperglycemia warranting immediate treatment and admission, the therapy should ideally begin before admission and should not be delayed for confirmatory testing. There is evidence to suggest that initiation of insulin in the ED may improve hospitalization outcomes, mainly the length of hospital stay [45, 46]. There is no consensus among experts regarding glycemic management goals, though there is ample data to suggest that appropriate blood glucose control improves patient outcomes and hospital stays [47]. The currently recommended targets are those of the inpatient standards for glycemic control, with goals based on random blood glucose levels [47]. In general, the goals are to avoid hypoglycemia, avoid severe hyperglycemia, volume depletion and electrolyte abnormalities, ensure adequate nutrition, assess patient educational needs and address deficiencies, ensure appropriate glucose control upon discharge until patient can be seen by the clinician managing his or her diabetes as an outpatient. The details of the recommendations would vary for different groups of patients.
Critically Ill Patients
Appropriate targets for patients with preexisting diabetes mellitus who are critically ill have not been firmly established. While some studies suggest that achieving normoglycemia (80 to 110 mg/dL) in cardiac surgery patients or those requiring admission to surgical ICU may reduce mortality, trials in mixed medical/surgical populations of critically ill patients did not show a benefit of targeting blood glucose of 80 to 110 mg/dL [48]. The ADA recommends a blood glucose target of 140 to 180 mg/dL in the ICU. Achieving this goal will usually require an insulin infusion, which should be initiated for persistent hyperglycemia greater than 180 mg/dL.
Non-Critically Ill Patients (Medical/Surgical Patients)
An appropriate glycemic goal would be one that would provide security to avoid hypoglycemia as the patient’s illness improves. In general, all glucose levels should be kept below 180 mg/dL to avoid further escalations, which may be associated with dehydration, glycosuria, and caloric loss, and to reduce the risk of infection and, although rare, of developing ketoacidosis. The goal for blood glucose are generally <140 mg/dL for fasting (pre-meal) and <180 mg/dL for random blood glucose levels, with an insulin regimen including basal, meal time (prandial) and correctional insulin being the preferred treatment regimen. Of note, sliding scale insulin alone is not the preferred method, as this is associated with high rate of suboptimal control of hyperglycemia [49]. For now, the use of non-insulin therapies or oral antidiabetic agents is generally not recommended in hospitalized patients due to the limited data available on their safety and efficacy, frequent contraindications, risk of hypoglycemia, and slow onset of action that may preclude achieving rapid glycemic control and daily dose adjustments. However, there is recent evidence suggesting that dipeptidyl peptidase-4 inhibitors may be effective and safe and a convenient alternative to the labor-intensive basal-bolus insulin regimen for the management of hyperglycemia in patients with T2DM in the non-intensive-care setting [50].
Preventing Revisits to ED and Improving Long-Term Diabetes Control
Experience has shown that ED visits with hyperglycemia are repetitive and associated with poor outpatient management of diabetes. Recently, there has been much emphasis on implementing programs with goal of preventing ED revisits after the index visit. Moreover, ED visit may be an opportunity to identify high risk patients in need of long-term diabetes control. Focused treatment of these patients is likely to be more cost-effective than any other approach. To improve access to outpatient diabetes care, a program that allowed ED staff with direct booking into the diabetes clinic was implemented at an academic medical center and showed a significant reduction in hospitalization, ED visits, HbA1c, and healthcare expenditures in the subsequent year [51••]. In another study, diabetes self-management education (DSME) for ED patients with random blood glucose levels ≥200 mg/dL showed improvement in glycemic control and medication adherence after 4 weeks [52•]. Close follow-up by a pharmacist or diabetes nurse educator after discharge from ED may be another effective strategy to prevent readmission and improve long-term glycemic control [53, 54]. However, data are mostly observational and highly inadequate to implement these strategies on a larger scale.
Conclusions
Hyperglycemic crises are the most common endocrine emergencies but hyperglycemia associated with non-hyperglycemic emergencies is also common. It is unlikely that the prevalence of hyperglycemia in the ED would decrease given the continued high prevalence of diabetes. The role of the emergency department in curbing the toll of these emergencies is key. The role of the ED is paramount in the appropriate diagnosis of hyperglycemic emergencies, aggressive elucidation of the underlying cause, prevention of complications, and thus ultimate improvement of clinical outcomes of these life threatening conditions. Particular attention should be directed at preventing their future recurrences. Providers should use the opportunity provided by an admission for marked hyperglycemia to address the underlying complexities inherent in diabetes management and enhance outpatient self-management skills. Moreover, management of hyperglycemia in association with non-hyperglycemic emergencies should not be ignored because this may affect the eventual outcomes of primary disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–9.
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes care. 2009;32:1335–43.
Martin WG, Galligan J, Simpson S, Greenaway T, Burgess J. Admission blood glucose predicts mortality and length of stay in patients admitted through the emergency department. Intern Med J. 2015;45:916–24.
Zelihic E, Poneleit B, Siegmund T, Haller B, Sayk F, Dodt C. Hyperglycemia in emergency patients–prevalence and consequences: results of the GLUCEMERGE analysis. Eur J Emerg Med [Internet]. 2015;22:181–7.
Centers for Disease Control. Number of Emergency Department Visits with Diabetes as Any-Listed Diagnosis, All Ages, United States, 2006–2009 [Internet]. [cited 2017 Jan 5] Available from: https://www.cdc.gov/diabetes/statistics/emergency/fig1.htm
Ozieh MN, Bishu KG, Dismuke CE, Egede LE. Trends in health care expenditure in U.S. adults with diabetes: 2002-2011. Diabetes Care. 2015;38:1844–51.
• Wang J, Geiss LS, Williams DE, Gregg EW. Trends in emergency department visit rates for hypoglycemia and hyperglycemic crisis among adults with diabetes, United States, 2006-2011. PLoS one. 2015;10. This study estimated the number of ED visits for hypoglycemia and hyperglycemic crisis among adults with diabetes during the period of 2006 to 2011, while ED visit rates for hypoglycemia declined, ED visit rates for hyperglycemic crisis did not change
Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174:1116–24.
Ginde AA, Delaney KE, Lieberman RM, Vanderweil SG, Camargo CA. Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey. Acad Emerg Med. 2007;14:492–5.
Ginde AA, Cagliero E, Nathan DM, Camargo CA. Value of risk stratification to increase the predictive validity of HbA1c in screening for undiagnosed diabetes in the US population. J Gen Intern Med. 2008;23:1346–53.
Charfen MA, Ipp E, Kaji AH, Saleh T, Qazi MF, Lewis RJ. Detection of undiagnosed diabetes and prediabetic states in high-risk emergency department patients. Acad Emerg Med. 2009;16:394–402.
Association AD. Standards of Medical Care in Diabetes—2016: classification and diagnosis of diabetes. Diabetes Care. 2016;39:S13–22. l.
Silverman RA, Thakker U, Ellman T, Wong I, Smith K, Ito K, et al. Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes care. 2011;34:1908–12.
Charfen MA, Ipp E, Kaji AH, Saleh T, Qazi MF, Lewis RJ. Detection of undiagnosed diabetes and prediabetic states in high-risk emergency department patients. Acad Emerg Med. 2009;16:394–402.
Ginde AA, Savaser DJ, Camargo CA. Limited communication and management of emergency department hyperglycemia in hospitalized patients. J Hosp Med. 2009;4:45–9.
Alberti KG. Role of glucagon and other hormones in development of diabetic ketoacidosis. The Lancet (London, England). 1975;1:1307–11.
Schade DS, Eaton RP. The temporal relationship between endogenously secreted stress hormones and metabolic decompensation in diabetic man. J Clin Endocrinol Metab. 1980;50:131–6.
McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–38.
Lévy-Marchal C, Patterson CC, Green A. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. Diabetologia. 2001;44:B75–80.
Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes care. 2001;24:131–53.
Ennis ED, Stahl v EJB, Kreisberg RA. The hyperosmolar hyperglycemic syndrome. Diabetes Rev. 1994;2:115–26.
Wachtel TJ, Silliman RA, Lamberton P. Predisposing factors for the diabetic hyperosmolar state. Arch Intern Med [Internet]. 1987;147:499–501.
Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz-Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32(1):21–39.
Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092.
Warner EA, Greene GS, Buchsbaum MS, Cooper DS, Robinson BE. Diabetic ketoacidosis associated with cocaine use. Arch. Intern. Med. 1998;158:1799–802.
Yoshida N, Goto H, Suzuki H, Nagasawa K, Takeshita A, Okubo M, et al. Ketoacidosis as the initial clinical condition in nine patients with acromegaly: a review of 860 cases at a single institute. Eur J Endocrinol. 2013;169:127–32.
Umpierrez GE, Latif K, Stoever J, Cuervo R, Park L, Freire AX, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med. 2004;117:291–6.
Kitabchi AE, Umpierrez GE, Fisher JN, Murphy MB, Stentz FB. Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93:1541–52.
Luzi L, Barrett EJ, Groop LC, Ferrannini E, DeFronzo RA. Metabolic effects of low-dose insulin therapy on glucose metabolism in diabetic ketoacidosis. Diabetes. 1988;37:1470–7.
Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38:422–7.
Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med. 1997;157:669–75.
Umpierrez GE, Jones S, Smiley D, Mulligan P, Keyler T, Temponi A, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: a randomized controlled trial. Diabetes Care. 2009;32:1164–9.
Adrogué HJ, Lederer ED, Suki WN, Eknoyan G. Determinants of plasma potassium levels in diabetic ketoacidosis. Medicine (Baltimore). 1986;65:163–72.
Beigelman PM. Potassium in severe diabetic ketoacidosis. Am J Med. 1973;54:419–20.
Chua HR, Schneider A, Bellomo R. Bicarbonate in diabetic ketoacidosis - a systematic review. Ann. Intensive Care. 2011;1:23.
Bureau MA, Bégin R, Berthiaume Y, Shapcott D, Khoury K, Gagnon N. Cerebral hypoxia from bicarbonate infusion in diabetic acidosis. J Pediatr. 1980;96:968–73.
Wilson HK, Keuer SP, Lea AS, Boyd AE, Eknoyan G. Phosphate therapy in diabetic ketoacidosis. Arch. Intern Med. 1982;142:517–20.
Fisher JN, Kitabchi AE. A randomized study of phosphate therapy in the treatment of diabetic ketoacidosis. J Clin Endocrinol Metab. 1983;57:177–80.
Winter RJ, Harris CJ, Phillips LS, Green OC. Diabetic ketoacidosis. Induction of hypocalcemia and hypomagnesemia by phosphate therapy. Am. J Med. 1979;67:897–900.
Watts W, Edge JA. How can cerebral edema during treatment of diabetic ketoacidosis be avoided? Pediatr Diabetes. 2014;15:271–6.
Hsia E, Seggelke S, Gibbs J, Hawkins RM, Cohlmia E, Rasouli N, et al. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab. 2012;97:3132–7.
Charfen MA, Fernández-Frackelton M. Diabetic ketoacidosis. Emerg Med Clin North Am. 2005;23:609–28. vii
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2009;32:193–203.
Dubin J, Nassar C, Sharretts J, Youssef G, Curran J, Magee M. Step-DC: stop emergency department visits for hyperglycemia project -DC. Ann Emerg Med. 2009;54:S18.
Munoz C, Villanueva G, Fogg L, Johnson T, Hannold K, Agruss J, et al. Impact of a subcutaneous insulin protocol in the emergency department: Rush Emergency Department Hyperglycemia Intervention (REDHI). J. Emerg. Med. 2011;40:493–8. 46
Bernard JB, Munoz C, Harper J, Muriello M, Rico E, Baldwin D. Treatment of inpatient hyperglycemia beginning in the emergency department: a randomized trial using insulins aspart and detemir compared with usual care. J Hosp Med. 2011;6:279–84.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes care. 2009;32:1119–31.
Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151–9.
Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120:563–7.
Pasquel FJ, Gianchandani R, Rubin DJ, Dungan KM, Anzola I, Gomez PC, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. The Lancet Diabetes Endocrinol. 2017;5(2):125–33.
•• Palermo NE, Modzelewski KL, Farwell AP, Fosbroke J, Shankar KN, Alexanian SM, et al. Open access to diabetes center from the emergency department reduces hospitalizations in the susequent year. Endocr. Pract. 2016;22:1161–9. Providing an appointment for diabetes clinic in the ED and thus removing barriers to ambulatory care was associated with significant reductions in hospitalization rate, ED recidivism rate, HbA1c, and healthcare expenditures in the subsequent year
• Magee MF, Nassar CM, Mete M, White K, Youssef GA, Dubin JS. The synergy to enable glycemic control following emergency department discharge program for adults with type 2 diabetes: step-diabetes. Endocr. Pract. 2015;21:1227–39. Diabetes self-management education was provided to ED patients with blood glucose (BG) levels ≥200 mg/dL. This focused approach led to improvement in short-term glycemic outcomes and medication adherence.
Chung N, Rascati K, Lopez D, Jokerst J, Garza A. Impact of a clinical pharmacy program on changes in hemoglobin A1c, diabetes-related hospitalizations, and diabetes-related emergency department visits for patients with diabetes in an underserved population. J Manag care Spec Pharm. 2014;20:914–9.
Magee MF, Nassar CM, Copeland J, Fokar A, Sharretts JM, Dubin JS, et al. Synergy to reduce emergency department visits for uncontrolled hyperglycemia. Diabetes Educ. 2013;39:354–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Justin B. Echouffo-Tcheugui and Rajesh Garg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hospital Management of Diabetes
Rights and permissions
About this article
Cite this article
Echouffo-Tcheugui, J.B., Garg, R. Management of Hyperglycemia and Diabetes in the Emergency Department. Curr Diab Rep 17, 56 (2017). https://doi.org/10.1007/s11892-017-0883-2
Published:
DOI: https://doi.org/10.1007/s11892-017-0883-2