Abstract
During menopause, women’s body composition, sex hormone profile, and metabolic profile may change dramatically. In this review, we summarize studies examining whether the menopausal transition and physiologic factors characterizing the transition are associated with increased risk of diabetes. We review the evidence for estrogen therapy and diabetes risk and studies examining the relationship between diabetes and menarche, which represents an extension of the reproductive life span at the opposite end of the age spectrum. Although studied less extensively, the presence of type 1 or type 2 diabetes may increase the risk of ovarian failure, and we review this literature. In conclusion, we note that the evidence linking menopausal sex hormone changes with increased diabetes risk is weak, although rapid changes as observed with oophorectomy may increase risk. Further studies should investigate the contradictory effects of estrogen therapy upon hepatic and glucose metabolism in mid-life women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes, like other disorders of metabolism, commonly manifests during the mid-life [1] and thus coincides with the timing of the menopausal transition in women. The menopausal transition is a period of rapid change in physiologic characteristics including endogenous sex steroid hormones, body composition and body fat distribution, and lipid and metabolic profiles [2•]. Thus, it has been hypothesized that these underlying changes represent a mechanistic link between diabetes and menopause [3]. This review will provide an overview of the menopausal transition and its association with diabetes risk. Further, the literature relating diabetes to variations in the timing of the menopausal transition will be examined. Finally, the burgeoning literature supporting a beneficial role for hormone therapy (HT) in reducing diabetes risk will be reviewed.
Pathophysiology and Characterization of the Menopausal Transition
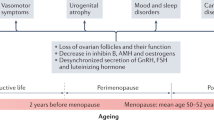
While menopause (i.e., the postmenopause) is clinically defined by the cessation of menses for at least 12 months, the menopausal transition itself actually begins 5–10 years before and is characterized by menstrual cycle and hormone variability [2•]. Utilization of longitudinal hormone data in combination with bleeding characteristics is accepted as the optimal methodology for staging menopause [2•]. As this data is not usually readily available, this is often impractical in the clinical setting. The recent 2011 International Stages of Reproductive Aging Workshop (STRAW+10) has defined the menopausal transition as an early and late stage preceding the final menstrual period (FMP) and postmenopause as an early and late stage following the FMP [2•]. Most clinical and research studies have implemented this paradigm with some simplification (see Fig. 1). The FMP can only be defined retrospectively after 12 months of amenorrhea, and anything after the FMP is designated as postmenopause. Perimenopause is the period before the FMP with menstrual cycle irregularity but before 12 months of amenorrhea. Premenopause occurs before perimenopause and is characterized by regular menses. Identifying a “natural” FMP can be complicated if women are using exogenous hormone therapy or if they have had a hysterectomy, bilateral oophorectomy, or both. Women who have stopped having menses due to surgical reasons are referred to as being in “surgical menopause.”
Hormone variability during the menopausal transition can be attributed to the more rapid depletion of the ovarian follicle pool and loss of negative feedback loops for hormonal regulation due to aging of the hypothalamic-pituitary axis [4, 5]. Several epidemiologic studies have indexed changes in sex steroid hormone levels in relation to the timing of the FMP. As shown in Fig. 2, estradiol (E2), the hormone traditionally implicated in sex differences in disease risk, has fairly stable levels up until 2 years prior to the FMP, followed by a 4-year sharp decline until about 2 years after the FMP [5, 6]. Rising follicle-stimulating hormone (FSH) levels can be detected as early as 10 years prior to the FMP [4], and levels rise more rapidly approximately 2 years prior to the FMP and then stabilize 2 years after the FMP [6]. Because FSH increases precede changes in E2, it is a more widely used biomarker of endocrine change during the menopausal transition.
Anti-mullerian hormone (AMH) is a dimeric glycoprotein produced by the preantral or functional ovarian follicles [7]. AMH levels decline with age and are the most accurate marker of ovarian reserve [8, 9], particularly in late reproductive life before the onset of marked cycle irregularity [10]. AMH has primarily been used for risk stratification of women undergoing assisted reproductive technology interventions [11]. However, over the past decade, it has been increasingly applied to the diagnosis and management of endocrinopathies including polycystic ovarian syndrome [12] and to the prediction of the age at the FMP [7].
Menopausal Status and Diabetes Risk
Whether the increased prevalence of diabetes during the mid-life is due to the menopausal transition independent of chronological aging is of great interest. In cross-sectional studies, evidence linking menopausal status with prevalent diabetes is mixed. Using a survey of more than 10,000 Japanese women, Heianza et al. reported that those with natural menopause had 40 % higher odds (odds ratio (OR) = 1.40, 95 % confidence interval (CI) 1.03–1.89) and women with surgical menopause had 59 % higher odds of diabetes (OR = 1.59, 95 % CI 1.07–2.37) as compared to premenopausal women [13]. Di Donato et al. reported a similar increase in the odds of diabetes among postmenopausal women as compared to premenopausal women (OR = 1.38, 95 % CI 1.03–1.84), a finding that persisted after adjustment for age [14]. Other cross-sectional studies have not found an association between menopausal status and several metabolic parameters including fasting plasma glucose, insulin, and insulin secretion rate or beta cell glucose sensitivity [15] or diabetes [16, 17]. Several longitudinal studies including the 6-year Pizarra Study [18], the 8-year Australian Longitudinal Study on Women’s Health [19], and the 3-year Diabetes Prevention Program [20] found no association between natural postmenopausal status and diabetes risk.
Whether age at menopause is associated with diabetes risk has also been investigated with conflicting results. In both cross-sectional [21] and longitudinal [22, 23•, 24] studies, earlier age at menopause was associated with type 2 diabetes. In the Study of Women’s Health Across the Nation, diabetes was more prevalent among women with premature ovarian failure (cessation of menses before age 40), but this finding was not significant after adjustment for confounders [24]. However, in the InterACT Study, a subcohort of more than 8000 postmenopausal women nested within the larger European Prospective Investigation into Cancer and Nutrition (EPIC) study, women with early age at menopause (less than 40 years) had 32 % greater risk (95 % CI 1.04–1.69) of type 2 diabetes as compared to women with menopause at age 50–54 years [23•]. However, an analysis of all women in the EPIC study, the largest prospective study examining diabetes and age at natural menopause which includes women from France, Italy, Spain, the UK, the Netherlands, Greece, Germany, Sweden, Denmark, and Norway, found no association between age at natural menopause and diabetes risk [25••]. A lack of an association between menopause and diabetes risk was also reported in cohorts from Latin America [16], Italy [14], China [26, 27], Mexico [28], and Japan [17]. These studies suggest that the association between menopausal status or age at menopause and diabetes risk is not particularly strong, and at least part of the association is confounded by other factors (see Table 1 for hypothesized direct effects and confounders).
Unlike natural menopause whereby declines in E2 occur over several years, the event of a bilateral oophorectomy (known as surgical menopause) is a more abrupt end to a woman’s reproductive capacity as the ovarian production of E2 is suddenly absent. Thus, studies of surgical menopause should be considered separately from those of natural menopause. Such studies have been more consistent regarding the impact of rapid and abrupt loss of E2 due to surgical menopause upon glycemic control. Two recently published longitudinal studies have reported an increased risk of diabetes among women with surgical menopause [22, 29]. Women in the NHANES I Epidemiologic Follow-up Study with both hysterectomy and bilateral oophorectomy had a 57 % increased risk (95 % CI 1.03–2.41) of developing diabetes as compared to women with natural menopause [22]. A deleterious effect of oophorectomy on insulin resistance and glucose tolerance has also been demonstrated in animal studies [30, 31].
Studies relating age at menarche to diabetes may also be informative given that an earlier age at menarche, like a later age at menopause, would extend a woman’s reproductive life span. Further, like menopause, menarche is a period of change in the hormonal milieu. Despite different definitions of early puberty or early age at menarche, several studies have identified an increased risk of diabetes or metabolic dysfunction associated with early commencement of reproductive age [32–36]. In a study of Korean women, Baek et al. defined early age at menarche as less than 13 years and average age at menarche as 13–16 years; early menarche was significantly associated with prediabetes, diabetes, and hyperglycemia [32]. In a study of women in the USA, Chen et al. defined early menarche as ≤12 years and average age at menarche as 12–14 years of age and observed greater levels of fasting insulin, the homeostasis model assessment of insulin resistance (HOMA-IR), and the homeostasis model assessment of beta cell function (HOMA-β) among women with early menarche as compared to those with an average age at menarche [33]. The association between decreased menopausal age with diabetes and decreased menarcheal age with diabetes suggests that factors aside from the reproductive-aged hormonal milieu may influence glucose metabolism as an early age at menarche and an early age at menopause have different impacts of the length of the reproductive lifespan.
Mechanisms Linking Menopause and Diabetes Risk
Potential mechanisms linking menopause and diabetes include changes in body composition as well as sex steroids. While the mid-life is a vulnerable period for the development of obesity, longitudinal studies have shown that this increase in weight is due to chronological aging rather than reproductive aging [37–39] whereas changes in body composition and body fat distribution are related to both chronological and reproductive aging [39–41]. In the Study of Women’s Health Across the Nation, FSH was positively correlated with fat mass and waist circumference change during the menopausal transition [41]. Further, obesity status is associated with differences in sex steroid trajectories during the menopausal transition; compared to women who are not obese, women with obesity have lower premenopausal E2 levels but higher postmenopausal E2 levels [6]. This may be due to the inhibitory effect of obesity on E2 production from the ovary prior to the menopausal transition [42, 43] whereas after menopause, adipose tissue is the main source of postmenopausal estrogen [44, 45]. The changes in body fat distribution and elevated inflammatory cytokines [46, 47] that occur during the menopausal transition have been associated with decreased tissue insulin sensitivity and glucose tolerance [48–51]. However, the potentially beneficial effect of less rapid changes in menopausal E2 levels among obese women may be counteracted by the impact of excess adipose tissue on carbohydrate metabolism [52].
Unique to the menopausal transition, however, is a state of increased androgenicity during the postmenopause due to declines in ovarian production of estrogen [6], increased levels of testosterone [53], and decreases in sex hormone-binding globulin (SHBG) [53]. The combination of increased testosterone and decreased SHBG results in an increase in free circulating testosterone or greater overall androgenicity. Testosterone and SHBG levels have been associated with insulin resistance and diabetes. In the Rancho Bernardo Study, a prospective cohort study of older men and women, free testosterone was positively associated with fasting glucose, insulin, and insulin resistance among postmenopausal women [54] and SHBG was inversely associated with impaired glucose tolerance and diabetes incidence [55]. These findings have been replicated in other studies as well including the Multi-Ethnic Study of Atherosclerosis [56, 57], the Women’s Health Study [58, 59], and the Study of Women’s Health Across the Nation [60].
While estrogens are known to have insulin-sensitizing properties and to be protective for pancreatic beta cells [61], associations between estrogen levels and diabetes risk have been mixed. Among postmenopausal women, the Women’s Health Study [58] and the Multi-Ethnic Study of Atherosclerosis [57] reported a significant association between higher levels of E2 and risk of diabetes, but this was not confirmed in the Rancho Bernardo Study [54] or among pre- or postmenopausal women in the Diabetes Prevention Program [62].
The autoimmunity characterizing type 1 diabetes may extend to other organ systems and co-existing thyroid autoimmunity is particularly common [63]. As premature ovarian failure may also be triggered by increased autoimmunity, investigators have examined whether women with type 1 diabetes are at risk for earlier menopause. Several studies have identified an earlier age at menopause among women with type 1 diabetes compared to women without diabetes [64–66], but whether these findings are generalizable to older cohorts is not clear. For example, using data from the Familial Autoimmune and Diabetes study of nearly 500 women, Dorman et al. [64] and Strotmeyer et al. [65] were the first to report a younger age at menopause (41.6 years) among women with type 1 diabetes as compared to their sisters without diabetes (49.9 years) or unrelated controls (48.0 years). Overall, the authors determined that this resulted in a 6-year relative reduction in reproductive lifespan among women with type 1 diabetes. However, given the length of follow-up of this study, only a small proportion of subjects reached natural menopause (10 %), thereby impacting the external validity of the study. Similarly, onset of diabetes before 20 years of age was associated with an earlier menopause in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort [25••]; although type 1 diabetes and type 2 diabetes could not be distinguished, the young age of onset is most consistent with type 1 diabetes [67]. However, other studies among women with validated diagnoses of type 1 diabetes have not confirmed an earlier age at menopause [68–70]. In a recently published analysis among more than 5000 women in the Ovarian Ageing in type 1 Diabetes mellitus (OVADIA) study, the age at natural menopause was identical among women with type 1 diabetes as compared to women without diabetes (49.8 years) [70].
Relative to type 1 diabetes, type 2 diabetes is a more heterogeneous disorder and usually not associated with autoimmunity. Interestingly, two studies have reported that women with type 2 diabetes have an earlier age at menopause than women without diabetes, consistent with the hypothesis that diabetes has an accelerating effect upon age at menopause [25••]. Data from a large multi-national Latin American study found that among 40–44-year-olds, the presence of diabetes was associated with a threefold risk of menopause before the age of 45 years [16]. In the longitudinal Study of Women’s Health Across the Nation, women with diabetes had their FMP 3 years earlier than women without diabetes (49 vs. 52 years, respectively) [71]. In contrast, Brand et al. noted that women with later onset of diabetes (after age 50) tended to have later age at menopause. However, this finding may be due to misclassification of the timing sequence for diabetes onset and the FMP, as the two events likely occurred in close proximity to one another.
Exogenous Hormone Therapy and Diabetes Risk
For women who may be at an increased risk of diabetes due to surgical menopause, treatment with exogenous hormone therapy may be a reasonable treatment option to attenuate their risk profile as the results of a protective effect are encouraging. Data from both the Heart and Estrogen/progestin Replacement Study (HERS) and Women’s Health Initiative (WHI) Hormone Trial, both large randomized controlled trials, showed a reduction in type 2 diabetes incidence associated with hormone therapy [72–74]. In the HERS study, women treated with 0.625 mg of conjugated estrogen plus 2.5 mg of medroxyprogesterone acetate daily had a 35 % reduction in diabetes incidence versus women treated with placebo after 4 years of follow-up [72]. Similarly, the WHI trial which utilized the same hormone therapy treatment as HERS found a 21 % reduction of incident diabetes after 5.6 years of follow-up [73]. Other RCTs [75] and observational longitudinal studies [76–78] further support a protective effect of HT on diabetes risk. A meta-analysis including data from 18 randomized controlled trials or crossover trials reported that postmenopausal hormone therapy was associated with a 30 % reduction in incident diabetes risk and reduced HOMA-IR, fasting glucose, and fasting insulin in diabetic and non-diabetic women [79]. These findings were repeated in a recent meta-analysis of articles published from 1997 to 2011; the pooled estimate suggested that postmenopausal use of combined estrogen replacement therapy reduced diabetes incidence by nearly 40 % and that women using estrogen replacement therapy had lower fasting plasma glucose and hemoglobin A1c [80••].
However, estrogen supplementation elevates postprandial glucose even as it suppresses fasting glucose. In the Postmenopausal Estrogen/Progestin Intervention Study, women randomized to estrogen had decreases in their fasting glucose and insulin levels while having increases in their postchallenge glucose and insulin levels [81]. The clinical implications of these postchallenge glucose elevations were unclear and suggest a differential effect on hepatic and peripheral insulin sensitivity. However, the majority of women in studies reporting a decreased risk of diabetes were diagnosed with the use of the fasting glucose. Thus, it is unclear whether the criteria used for diabetes diagnosis adequately captured postprandial glucose elevations. This is particularly an issue in aging women, who more commonly present with postchallenge glucose elevations than with fasting glucose elevations. More consideration of these potential differences is needed as the results from ongoing studies are being analyzed.
Due to concerns of increased risk for other chronic diseases including breast malignancy and thromboembolism, estrogen use is not currently recommended for diabetes prevention in postmenopausal women [82]. While estrogen is known to have insulin-sensitizing properties [83] and may improve insulin signaling and glucose transport, there is much to learn about the optimal treatment protocol, particularly the dosing and route of estrogen that would be most efficacious. Finally, it is well recognized that diabetes is a heterogeneous disorder, and affected individuals vary as to whether they have elevations in fasting glucose or postchallenge glucose. It is unclear whether the use of estrogen therapy would have different effects on these different populations of persons with diabetes. Confirmation of these potential differences should be a priority in future analyses of ongoing studies.
The so-called timing hypothesis regarding hormone therapy, whereby maximum benefit may be experienced with postmenopausal women who are treated within a certain time frame, has been suggested for cardiovascular health outcomes [84]. Now, a recently published small clinical study of the effectiveness of short-term treatment with transdermal E2 administration found that insulin-mediated glucose disposal rate was improved only among women in early menopause (≤6 years). In fact, glucose disposal rate worsened with E2 treatment among women in late menopause (≥10 years) [85••]. These findings highlight the importance of more careful consideration of HT as a treatment strategy for diabetes in mid-life women.
Conclusions
For women, the mid-life period, which coincides with the timing of the menopausal transition, is characterized by increasing levels of glucose and incidence of diabetes. The key characteristics of menopause, particularly decreases in estrogen and the presence of absence of bleeding, do not appear to have strong correlations with diabetes risk. However, the subtler changes in body composition that also occur during the transition may negatively influence glucose metabolism, as does the relative increase in androgenicity. Treatment with exogenous estrogen may decrease fasting glucose levels, and the application of this therapy to particular subpopulations with elevating fasting glucose levels should be explored. Finally, the interaction between diabetes and menopause on other comorbidities common in aging women, particularly cognitive function, cardiovascular conditions, and malignancy, has been infrequently examined. As the majority of women with diabetes are elderly and diagnosed during and after the menopausal transition, attention to the joint impact of these two extremely common conditions on other comorbidities is a critical public health priority.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90.
Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–68. This paper provides the contemporary methodology for defining the stages of reproductive aging and makes recommendations on the future directions for staging the menopausal transition.
Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Womens Health. 2012;8(2):155–67.
Sowers MR, Zheng H, McConnell D, et al. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93(10):3958–64.
Sowers MR, Zheng H, McConnell D, et al. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93(10):3847–52.
Randolph Jr JF, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–54.
Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–83.
Rosen MP, Johnstone E, McCulloch CE, et al. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril. 2012;97(1):238–43.
van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–87.
de Vet A, Laven JS, de Jong FH, et al. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–62.
Ledger WL. Clinical utility of measurement of anti-mullerian hormone in reproductive endocrinology. J Clin Endocrinol Metab. 2010;95(12):5144–54.
Lauritsen M, Bentzen J, Pinborg A, et al. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-mullerian hormone. Hum Reprod. 2014;29(4):791–801.
Heianza Y, Arase Y, Kodama S, et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care. 2013;36(12):4007–14.
Di Donato P, Giulini NA, Bacchi Modena A, et al. Risk factors for type 2 diabetes in women attending menopause clinics in Italy: a cross-sectional study. Climacteric. 2005;8(3):287–93.
Muscelli E, Kozakova M, Flyvbjerg A, et al. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. Am J Hypertens. 2009;4:364–70.
Monterrosa-Castro A, Blumel JE, Portela-Buelvas K, et al. Type II diabetes mellitus and menopause: a multinational study. Climacteric. 2013;16:663–72.
Lee JS, Hayashi K, Mishra G, et al. Independent association between age at natural menopause and hypercholesterolemia, hypertension, and diabetes mellitus: Japan nurses’ health study. J Atheroscler Thromb. 2013;20(2):161–9.
Soriguer F, Morcillo S, Hernando V, et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause. 2009;16(4):817–21.
Mishra GD, Carrigan G, Brown WJ, et al. Short-term weight change and the incidence of diabetes in midlife: results from the Australian Longitudinal Study on Women’s Health. Diabetes Care. 2007;30(6):1418–24.
Kim C, Edelstein SL, Crandall JP, et al. Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause. 2011;18(8):857–68.
Malacara JM, Huerta R, Rivera B, et al. Menopause in normal and uncomplicated NIDDM women: physical and emotional symptoms and hormone profile. Maturitas. 1997;28(1):35–45.
Appiah D, Winters SJ, Hornung CA. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care. 2014;37(3):725–33.
Brand JS, van der Schouw YT, Onland-Moret NC, et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care. 2013;36(4):1012–9. This is a subcohort analysis of the larger EPIC study, reference 25.
Luborsky JL, Meyer P, Sowers MF, et al. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18(1):199–206.
Brand JS, Onland-Moret NC, Eijkemans MJ, et al. Diabetes and onset of natural menopause: results from the European Prospective Investigation into Cancer and Nutrition. Hum Reprod. 2015;30(6):1491–8. This is the largest prospective study examining the association between diabetes and age at natural menopause.
Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab. 2013;98(4):1612–21.
He L, Tang X, Li N, et al. Menopause with cardiovascular disease and its risk factors among rural Chinese women in Beijing: a population-based study. Maturitas. 2012;72(2):132–8.
Lopez-Lopez R, Huerta R, Malacara JM. Age at menopause in women with type 2 diabetes mellitus. Menopause. 1999;6(2):174–8.
Lejskova M, Pit’ha J, Adamkova S, et al. Bilateral oophorectomy may have an unfavorable effect on glucose metabolism compared with natural menopause. Physiol Res. 2014;63 Suppl 3:S395–402.
Riant E, Waget A, Cogo H, et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–17.
Zhu L, Martinez MN, Emfinger CH, et al. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab. 2014;306(10):E1188–97.
Baek TH, Lim NK, Kim MJ, et al. Age at menarche and its association with dysglycemia in Korean middle-aged women. Menopause. 2015;22(5):542–8.
Chen L, Zhang C, Yeung E, et al. Age at menarche and metabolic markers for type 2 diabetes in premenopausal women: the Biocycle Study. J Clin Endocrinol Metab. 2011;96(6):E1007–12.
Day FR, Elks CE, Murray A, et al. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;18(5):11208.
He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171(3):334–44.
Lakshman R, Forouhi N, Luben R, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia. 2008;51(5):781–6.
Sternfeld B, Wang H, Quesenberry Jr CP, et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;160(9):912–22.
Davies KM, Heaney RP, Recker RR, et al. Hormones, weight change and menopause. Int J Obes Relat Metab Disord. 2001;25(6):874–9.
Davis SR, Castelo-Branco C, Chedraui P, et al. Understanding weight gain at menopause. Climacteric. 2012;15(5):419–29.
Toth MJ, Tchernof A, Sites CK, et al. Menopause-related changes in body fat distribution. Ann N Y Acad Sci. 2000;904:502–6.
Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901.
De Pergola G, Maldera S, Tartagni M, et al. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity. 2006;14(11):1954–60.
Freeman EW, Sammel MD, Lin H, et al. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17:718–26.
Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3 Suppl):S116–124.
Szymczak J, Milewicz A, Thijssen JH, et al. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63(5-6):319–21.
Pfeilschifter J, Koditz R, Pfohl M, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119.
Malutan AM, Dan M, Nicolae C, et al. Proinflammatory and anti-inflammatory cytokine changes related to menopause. Prz Menopauzalny. 2014;13(3):162–8.
Vryonidou A, Paschou SA, Muscogiuri G, et al. Mechanisms in endocrinology: metabolic syndrome through the female life cycle. Eur J Endocrinol. 2015;173(5):R153–163.
Sites CK, Calles-Escandon J, Brochu M, et al. Relation of regional fat distribution to insulin sensitivity in postmenopausal women. Fertil Steril. 2000;73(1):61–5.
Barrett-Connor E, Schrott HG, Greendale G, et al. Factors associated with glucose and insulin levels in healthy postmenopausal women. Diabetes Care. 1996;19:333–40.
Campbell AJ, Busby WJ, Horwath CC, et al. Relation of age, exercise, anthropometric measurements, and diet with glucose and insulin levels in a population aged 70 years and over. Am J Epidemiol. 1993;138:688–96.
Kim C, Halter JB. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr Cardiol Rep. 2014;16(4):467.
Sowers MF, Zheng H, McConnell D, et al. Testosterone, sex hormone-binding globulin and free androgen index among adult women: chronological and ovarian aging. Hum Reprod. 2009;24(9):2276–85.
Oh JY, Barrett-Connor E, Wedick NM, et al. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25(1):55–60.
Goodman-Gruen D, Barrett-Connor E. Sex hormone-binding globulin and glucose tolerance in postmenopausal women. The Rancho Bernardo study. Diabetes Care. 1997;20(4):645–9.
Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92(4):1289–95.
Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127–35.
Ding EL, Song Y, Manson JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076–84.
Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–63.
Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168(14):1568–75.
Cignarella A, Kratz M, Bolego C. Emerging role of estrogen in control of cardiometabolic disease. Trends Pharmacol Sci. 2010;31(4):183–9.
Mather KM, Kim C, Christophi CA, et al. Steroid sex hormones, sex hormone binding globulin and diabetes incidence in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2015;100(10):3778–86.
Buschur E, Sarma A, Pietropaolo M, et al. Self-reported autoimmune disease by sex in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care. 2014;37(2):E28–29.
Dorman JS, Steenkiste AR, Foley TP, et al. Menopause in type 1 diabetic women: is it premature? Diabetes. 2001;50(8):1857–62.
Strotmeyer ES, Steenkiste AR, Foley Jr TP, et al. Menstrual cycle differences between women with type 1 diabetes and women without diabetes. Diabetes Care. 2003;26(4):1016–21.
Snell-Bergeon JK, Dabelea D, Ogden LG, et al. Reproductive history and hormonal birth control use are associated with coronary calcium progression in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2008;93(6):2142–8.
SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among U.S. youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–8.
Sjoberg L, Pitkaniemi J, Harjutsalo V, et al. Menopause in women with type 1 diabetes. Menopause. 2011;18(2):158–63.
Kim C, Cleary PA, Cowie CC, et al. Effect of glycemic treatment and microvascular complications on menopause in women with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort. Diabetes Care. 2014;37(3):701–8.
Yarde F, van der Schouw YT, de Valk HW, et al. Age at menopause in women with type 1 diabetes mellitus: the OVADIA study. Hum Reprod. 2015;30(2):441–6.
Khalil N, Sutton-Tyrrell K, Strotmeyer ES, et al. Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos Int. 2011;22(5):1367–76.
Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9.
Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–87.
Bonds DE, Lasser N, Qui L, et al. The effect of conjugated equine estrogen on diabetes incidence: the Women’s Health Initiative randomized trial. Diabetologia. 2006;49(3):459–68.
Rossi R, Origliani G, Modena MG. Transdermal 17-beta-estradiol and risk of developing type 2 diabetes in a population of healthy, nonobese postmenopausal women. Diabetes Care. 2004;27(3):645–9.
Manson JE, Rimm EB, Colditz GA, et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2(5):665–73.
Pentti K, Tuppurainen MT, Honkanen R, et al. Hormone therapy protects from diabetes: the Kuopio Osteoporosis Risk Factor and Prevention Study. Eur J Endocrinol. 2009;160(6):979–83.
de Lauzon-Guillain B, Fournier A, Fabre A, et al. Menopausal hormone therapy and new-onset diabetes in the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l’Education Nationale (E3N) cohort. Diabetologia. 2009;52(10):2092–100.
Salpeter SR, Walsh JM, Ormiston TM, et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–54.
Xu Y, Lin J, Wang S, et al. Combined estrogen replacement therapy on metabolic control in postmenopausal women with diabetes mellitus. Kaohsiung J Med Soc. 2014;30(7):350–61. This is a meta-analysis of studies published between 2001 and 2011 which evaluated the association of hormone therapy and diabetes risk.
Espeland MA, Hogan PE, Fineberg SE, et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998;21(10):1589–95.
Moyer VA, U.S. Preventative Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: U.S. Preventative Services Task Force recommendation statement. Ann Intern Med. 2013;158(1):47–54.
Cignarella A, Bolego C. Mechanisms of estrogen protection in diabetes and metabolic disease. Horm Mol Biol Clin Investig. 2010;4(2):575–80.
Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol. 2014;142:68–75.
Pereira RI, Casey BA, Swibas TA, et al. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015. [Epub ahead of print]. This study evaluated the hypothesized ‘timing hypothesis’ with regard to estrogen therapy and diabetes. Findings from this study may explain some of the discordant findings regarding menopause and diabetes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carrie A. Karvonen-Gutierrez, Sung Kyun Park, and Catherine Kim declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Other Forms of Diabetes
Rights and permissions
About this article
Cite this article
Karvonen-Gutierrez, C.A., Park, S.K. & Kim, C. Diabetes and Menopause. Curr Diab Rep 16, 20 (2016). https://doi.org/10.1007/s11892-016-0714-x
Published:
DOI: https://doi.org/10.1007/s11892-016-0714-x