Abstract
Purpose of Review
Treatment options for patients with metastatic colorectal cancer continue to advance as the therapeutic implications of the molecular subtypes of this disease are becoming better understood. DNA sequencing and mismatch repair assessment are now standard of care analyses for patients with metastatic colorectal cancer This review describes important aspects of the biology of the clinically relevant molecular subtypes of colorectal cancer based on the current standard of care testing. In addition, the clinical treatment strategies available now and potentially in the future for these colorectal cancer subtypes are discussed.
Recent Findings
Currently, for metastatic colorectal cancer, standard of care molecular testing is done for mutations in exons 2, 3, and 4 of KRAS and NRAS, and BRAF V600E. Testing for mismatch repair (MMR) deficiency/microsatellite instability (MSI) status is also done. These aberrations are well known to change the clinical prognosis and guide patients’ treatment strategies. Additionally, three new subtypes have emerged: PIK3CAmut, HER2 amplified, and NTRK fusions. With the addition of these emerging subtypes, tumor heterogeneity further validates the need to examine mCRC as a heterogeneous disease. Here, we present recent exciting data from translational research and clinical trials exhibiting possible distinct treatment strategies for these different subtypes.
Summary
Altogether, these data show promising treatment strategies for many of these well-known and emerging subtypes of mCRC. In addition, these also give better clinical prognostic and predictive information. We believe that as molecular testing expands, PIK3CA mutation, HER2 amplification, and NTRK fusion molecular testing will be included in standard of care analyses. This incorporation of testing in clinical practice will generate further information regarding prognostic and therapeutic options for these and other CRC subtypes in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third-leading cause of cancer-related deaths in the USA with ~ 50,000 deaths each year [1]. Standard cytotoxic chemotherapy remains the mainstay of treatment for metastatic CRC; however, a growing appreciation for the molecular subtypes of CRC has led to recent advancements in the manner in which this disease is treated [2]. FOLFOX (leucovorin, 5-FU, and oxaliplatin) or FOLFIRI (leucovorin, 5-FU, and irinotecan) chemotherapy regimens are largely utilized in the first-line setting, commonly in conjunction with bevacizumab, an anti-vascular endothelial growth factor antibody, regardless of molecular subtype [3, 4]. Bevacizumab is a humanized monoclonal antibody that targets the vascular endothelial growth factor. Initial investigations of bevacizumab showed evidence of clinical benefit when added to bolus irinotecan, 5-FU, and leucovorin (IFL) including improvements in median overall survival (OS) and progression-free survival (PFS) [5]. This was extrapolated to current use in combination with FOLFOX chemotherapy [6]. Pooled analysis of phase II and III trials has shown improvement for the addition of bevacizumab to chemotherapy with improvement in OS 18.7 versus (vs) 16.1 months (p = 0.0003), PFS 8.8 vs 6.4 months (p < 0.0001), and response rate (RR) 39% vs 33% [7].
The current standard of care molecular testing for metastatic colorectal cancer is to examine the mutational status of multiple genes. This includes exons 2, 3, and 4 of KRAS and NRAS. In addition, sequencing for the BRAF V600E mutation is needed. Beyond RAS and RAF testing, it is also important to test for mismatch repair (MMR) deficiency/microsatellite instability (MSI). This is now most commonly done using immunohistochemistry for MLH1, MSH2, PMS2, and MSH6. Next-generation sequencing approaches are also commonly being utilized, and the previously widely utilized PCR-based analyses are now done less frequently. RAS/RAF testing and MMR/MSI testing can be done as stand-alone tests, though these are now being more commonly performed as part of larger next-generation sequencing panels, allowing for the examination of other alterations of potential interest.
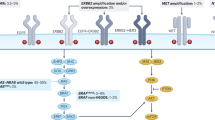
The standardized practice of analyzing CRCs for these alterations has identified subtypes of CRC with distinct treatment strategies (Table 1). These subtypes include RAS/RAF wild-type (wt) microsatellite stable (MSS), KRAS/NRAS mutant (mt) MSS, microsatellite instability-high (MSI-H)/MMR deficient, and BRAF V600E mt. Beyond standard of care testing, there are three emerging populations of interest in metastatic CRC including HER2-amplified, PIK3CA mt, and NTRK fusions.
Colorectal Cancer Molecular Subtypes
RAS/RAF Wild-Type, Microsatellite Stable
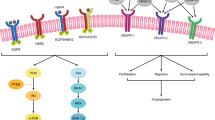
The RAS/RAF wt MSS subtype represents about 30–40% of all CRC [8]. Cytotoxic chemotherapy remains the primary treatment backbone though this subtype is targetable with agents against the epidermal growth factor receptor (EGFR). EGFR is a transmembrane glycoprotein with extracellular ligand-binding domain and intracellular tyrosine kinase domain that mediates downstream signaling [9]. Upon ligand binding, EGFR functions as a homodimer with downstream activation of the RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways resulting in cell growth, proliferation, and regulation of other critical cellular functions [10]. Pre-clinical models have demonstrated the therapeutic benefit of these agents, including the dependence of models with loss of APC requiring signaling through EGFR [11]. Two monoclonal antibodies targeting EGFR are approved for use in metastatic CRC including chimeric human-murine cetuximab and fully humanized panitumumab [12, 13].
EGFR inhibitors, such as cetuximab and panitumumab, have undergone extensive clinical investigation and demonstrated benefit in the first-line in combination with chemotherapy and later-lines across multiple investigations [14,15,16,17]. The phase III multicenter prospective CALGB/SWOG 80405 clinical trial examined first-line chemotherapy in combination with either bevacizumab or cetuximab [14]. These regimens resulted in a median OS of 31.2 months in those patients with extended spectrum RAS/BRAF wt cancers. This study failed to show a significant difference between OS when comparing bevacizumab to cetuximab in this setting [18]. Despite selection for extended spectrum RAS, EGFR inhibitor resistance remains common at up to 60% of patients for which an improve understanding of mechanistic resistance and novel therapeutic strategies are needed [19].
An additional analysis of CALGB/SWOG80405 stratified clinical outcomes by primary disease sidedness. Right-sided disease was defined as proximal to the splenic flexure and left-sided distal to the spleen flexure [20]. In the extended-spectrum RAS/RAF wt population, right-sided primary tumors were found to have inferior clinical outcomes with the use of anti-EGFR therapy in the front-line setting. This was consistent with the population-based cohort from Canada showing inferior median OS for the use of EGFR inhibitors in the right-sided primary cohort at 30.5 vs 39.3 months [21]. Additionally, retrospective analysis of the FIRE-3 and CRYSTAL studies revealed improvement for left-sided tumors treated with FOLFIRI in combination with EGFR inhibition and improved OS for right-sided tumors when starting in combination with bevacizumab [22]. The mechanistic basis for these differential clinical outcomes remains of great clinical interest at this time for which an improved mechanistic understanding is needed and may be accounted for due to differences in molecular profiling [23].
Despite the clinical utility of EGFR inhibition in the first-line setting, the molecular profiling might not be available at the time of treatment initiation. The first-line use of EGFR inhibitors also prolongs exposure and toxicities for patients including acneiform rash. The timing of incorporating EGFR inhibition into the treatment paradigm remains controversial among oncologists [18, 24,25,26,27,28].
We recently reviewed the impact of the metastatic disease bulk in predicting the late-line utility of EGFRi [29]. Using a retrospective cohort, disease bulk was found to be an independent marker of therapeutic resistance to EGFR inhibition [29]. This study demonstrated consistent results with prior studies that also revealed delayed presentations for right-sided tumors with resultant increased rate of both bulky disease and multi-site metastases at presentation [30,31,32]. In addition to bulk harboring complex intratumor heterogeneity, alternative considerations for bulk resulting in therapeutic resistance include reduced antibody drug delivery [33], increased hydrostatic pressure [34], and the complexities of local blood supply [35]. This work requires validation in prospective trial design to tune the durability of EGFR inhibition in the non-bulky cohort.
KRAS/NRAS and BRAF mutations lead to primary resistance to EGFR inhibition, and multiple other alterations are also implicated in this resistance, including amplifications in KRAS (< 2%), ERBB2 (5%), or MET (2%) [36,37,38,39]. The effect of alterations in the PI3K/AKT/PTEN pathway in predicting response to EGFR inhibition remains unclear due to limited prospective data and the high rate of concomitant mutations in both KRAS and BRAF [40]. Mutations in EGFR including S492R at the extracellular domain inhibit the binding of cetuximab as an acquired or secondary mechanism of resistance [41, 42]. Mechanisms of resistance are generally established; however, the rarity of individual mutations provides a formidable challenge for prospective trial design to overcome these mechanisms. Limitations of traditional profiling with core biopsy may not capture the molecular profile of subclonal populations, which may be overcome by enhanced sensitivity in sequencing techniques [43, 44]. While intratumor heterogeneity leads to acquired resistance, further innovation is needed to improve the reliable characterization of these subclones.
Tracking the clonal dynamics over the course of therapy with circulating tumor DNA (ctDNA) may provide additional insights into the acquisition of different mechanisms of resistance for individual patients [45]. Interestingly, the depth of the initial response and the time since progression from prior EGFR inhibition predicts future success with EGFR inhibition retreatment [46]. ctDNA data indicates selection for RAS mutations in response to EGFR inhibition through therapeutic pressure driving this mechanism of secondary resistance [31]. The mutant alleles responsible for secondary resistance undergo exponential decay following discontinuation of EGFR inhibition [47]. These techniques, using ctDNA, help to explain the clonal basis for both secondary resistance and successful of retreatment strategies. These should be prospectively investigated as a powerful clinical tool to optimize EGFR inhibition retreatment strategies in the RAS/RAF wt MSS subtype.
KRAS/NRAS Mutant, Microsatellite Stable
KRAS and NRAS are members of the family of Ras oncogenes, small GTPases important in RAS/MAPK signaling. Activation of this pathway leads to cell growth, cell cycle progression, migration, and cell survival. Mutations in KRAS are found in approximately 40% of all CRC cases and are most often found at exons 2 (codon 12, 13), and less commonly at 2–5% in exons 3 (codon 61), and 4 (codon 146). Mutations in NRAS are found in only 2–5% of all CRC cases and are mutually exclusive with mutations in KRAS. Mutations in either KRAS or NRAS result in constitutive activation of the RAS/MAPK pathway signaling and lead to resistance upstream EGFR targeting [40, 48,48,49,50,52]. Some clinical investigations have even demonstrated worse outcomes when EGFR inhibitors are used in the setting of activating RAS alterations [19, 50, 53].
Targeted therapies aimed at the RAS oncoproteins or the downstream signaling cascades have been ineffective and strategies specifically benefitting this subtype of CRC remain elusive. Recently, there was excitement in this area with early investigations of the combination of cobimetinib, a MEK inhibitor, and atezolizumab, an anti-PD-L1 agent, showing promise in treatment refractory CRC [54]. Excitingly, in the phase I trial, increased activity was seen specifically in those cancers with enhanced RAS/RAF/MEK/ERK signaling [36]. The confirmatory phase III clinical trial, IMblaze370, examined the combination of cobimetinib and atezolizumab in chemotherapy-refractory metastatic CRC compared to atezolizumab or regorafenib, a multi-targeted receptor tyrosine kinase inhibitor, alone. Over half of the patients in this study were KRAS mt. Unfortunately, this study did not meet its primary endpoint of an improvement in OS and the RR to this regimen was only 3% [55•]. Studies using other similar regimens are on-going with the benefit of this approach specifically for the KRAS mt MSS yet to be determined. Additional therapies of interest for targeting this subtype of CRC include agents that target CEA expression, often found in KRAS mt cancers. This includes agents such as a cibisatuzumab which is a CEA CD3 bispecific antibody that has demonstrated interesting activity in an early phase clinical trial [56].
Microsatellite Instability-High/Mismatch Repair Deficient
Microsatellites are short, tandemly-repeated sequences of DNA throughout the genome and are commonly shortened in the setting of deficient mismatch repair (dMMR) protein activity [57]. Metastatic MSI-high/dMMR patients represent 3–4% of all metastatic CRC [58]. The most commonly altered DNA MMR genes are MLH1, MSH2, MSH6, and PMS2 with greater than 90% of those dMMR cancers having alterations in MLH1 and MSH2. The genes can undergo mutation or epigenetic regulation resulting in loss of the functional protein. MMR deficiency can be detected either on a protein, mutation status, or analyses of microsatellite status [59].
MMR-deficient cancers have been an area of active interest for the development of immunotherapeutic strategies. Secondary to the dMMR status, these tumors develop possessing 100 to 1000 s of mutations. These mutations result in an enhanced neoantigen load leading to the potential for enhanced immune recognition [60]. These cancers also tend to have lower rates of alterations in WNT signaling compared to MSS CRCs, which has been implicated in immunotherapy resistance [61, 62]. Recent investigations have examined the use of anti-PD1 therapies for patients with MSI-H/dMMR metastatic CRCs in the treatment refractory setting [63]. Pembrolizumab demonstrated a RR of 40% and the progression free survival (PFS) at 20 weeks was 78% [64]. Similarly, nivolumab demonstrated a RR of 31% and 69% had a PFS of 12 weeks or greater [65]. Of note, durable responses are seen with these patients, especially in those who develop a clinical response. This has led to the FDA approval of both pembrolizumab and nivolumab for patients with treatment refractory MSI-H/dMMR metastatic CRCs.
Dual immune checkpoint has also recently been reported examining the combination of nivolumab with the CTLA4 antibody ipilimumab as part of the CheckMate 142 clinical trial. This combination resulted in a RR of 31% and a 12-month PFS of 71% [66••]. Additionally, this regimen was also recently investigated in the first-line metastatic setting in CRC and demonstrated a RR of 60% and a 12-month PFS of 77% [67, 68]. Given this success, other immuno-oncology treatment strategies targeting anti-PD1 therapies in combination with agents against other immune targets, such as LAG3 and TIM3, are being actively pursued.
BRAF Mutant
BRAF is a serine/threonine kinase found downstream of RAS in the RAS/RAF/MEK/ERK pathway. Mutations in BRAF are found in about 10% of CRC patients with the majority of them being V600E (80% of all BRAF mutations). This mutation leads to constitutive activation of BRAF and the downstream signaling pathway. BRAFV600 mt CRCs have a worse prognosis and relative chemotherapy resistance, and upon progression, these patients clinically deteriorate quickly resulting in many patients being unable to receive second-line therapy [2, 69]. In addition, secondary to the effects of mutant BRAF downstream from EGFR signaling, anti-EGFR therapies have not shown consistent benefit for patients. Given this lack of benefit, patients with BRAF mt CRC are not treated with anti-EGFR therapies as single-agents [70,70,71,73].
Since the BRAFV600 mutation activates the BRAF kinase, agents targeting BRAF have known clinical utility across multiple cancers including melanoma. However, BRAFV600 mt CRC is not nearly as sensitive to single agent BRAF inhibitors as compared to BRAF mt melanoma. Combination strategies have emerged to overcome resistance mechanisms, including signaling through EGFR. The recent SWOG1406 phase III clinical trial examined BRAF mt CRC patients treated with the combination of irinotecan (topoisomerase I inhibitor), cetuximab (anti-EGFR), and vemurafenib (BRAF inhibitor) compared to irinotecan and cetuximab. This study demonstrated an increase in median PFS (4.4 vs 2 months, p < 0.001) and increased RR (16% vs 4%, p = 0.09) for the triplet combination compared to the standard of care arm, respectively [74]. Though the additional benefit of this regimen is modest, for this particular subtype of CRC, these results are important and have led to this becoming a standard treatment option for these patients.
Additionally, the combination of encorafenib (Raf kinase inhibitor), cetuximab (anti-EGFR), and binimetinib (MEK inhibitor) was examined in BRAFV600E mt CRC patients in a phase I/II and in an on-going phase III clinical trial. In the phase II, 30 patients were enrolled. A RR of 40% and a median PFS of over 8 months were observed. Given the results of the phase II study, the outcomes of the phase III clinical trial are expected to be practice changing and a first-line study is expected to start enrolling soon [75•].
It is important to note that BRAFnon-V600 mutations largely possess a different biology than BRAFV600 mutations. BRAFnon-V600 mutations occur in 2% of all CRC cases, and many of the common BRAFnon-V600 mutations actually inactivate the BRAF kinase [53]. BRAFnon-V600 mt CRCs have a better prognosis than BRAFV600 mt. BRAFnon-V600 mt CRC patients have a longer OS compared to those BRAFV600 (60.7 vs. 11.4 months, respectively). Additionally, BRAFnon-V600 CRCs are more likely to co-occur with RAS mutations, but interestingly have a similar clinical prognosis to RAS WT patients [76]. These results demonstrate the importance of identifying the particular mutation and its change in the protein function when determining treatment strategies.
Emerging Colorectal Cancer Subtypes
PIK3CA Mutant
PIK3CA is the gene that encodes for the p110ɑ catalytic subunit of PI3K, a phosphoinositide kinase important in the PI3K/mTOR signaling pathway. Activation of this pathway leads to enhanced protein synthesis, cell cycle progression, cell growth, and survival. Mutations in PIK3CA are found in approximately 18% of CRC cases with 48% of those mutations occurring in the kinase domain (most commonly H1047R) and 43% occurring in the helical domain (most commonly E542K and E545K) [77]. While genetic profiling of this gene is not routinely recommended for metastatic CRC patients, many medical centers nationwide have begun including PIK3CA sequencing to guide clinicians in choosing appropriate targeted therapies as part of genetic sequencing panels.
Currently, there are several inhibitors of the PI3K/mTOR pathway which directly target PI3K alone, mTOR alone, and or the combination (dual PI3K/mTOR inhibitors). To date, no clinical trial has looked specifically at these inhibitors in the context of patients with PIK3CA mutant CRC. Our group has demonstrated the preclinical benefit of targeting MTORC1/2 for PIK3CA mutant CRCs. Using a novel transgenic mouse model possessing the hotspot Pik3caH1047R mt and loss of APC, mTORC1/2 inhibition resulted in treatment response. Using tumor-derived spheroid cultures, we demonstrated that these cancers were resistant to MTORC1 inhibition with everolimus and the PI3K alpha isomer specific inhibitor BYL-719. These treatment strategies need to be prospectively examined in PIK3CA mt CRCS without concomitant mutations in RAS or RAF [78].
HER2-Amplified
Human epidermal growth factor receptor 2 (HER2) is a member of the epidermal growth factor receptor family in which activation leads to upregulation of the RAS/MAPK and PI3K/mTOR pathways. HER2 amplification is found in about 3–5% of all CRC cases and can lead to relative resistance to anti-EGFR therapies [79]. It is not currently standard of care to examine for HER2 amplification at most centers, though this subtype of CRC is targetable using agents that target HER2. This is especially true for those cancers with a copy number greater than 10 [80].
One of the initial studies to target this subset of CRC was the HERACLES phase II trial which examined combination of trastuzumab, a HER2 directed antibody, and lapatinib, an oral dual HER2/EGFR kinase inhibitor. Of those enrolled in the study, 30% of patients (8/27 patients) had an objective response [81]. The MyPathway trial enrolled patients with HER2-amplified CRC to receive the combination of pertuzumab (anti-HER2) and trastuzumab. In the initial report of this study, 38% of the cohort have received an objective response rate [82•]. Additionally, there is an ongoing phase II clinical trial SWOG1613 examining the combination of trastuzumab and pertuzumab vs cetuximab and irinotecan in metastatic HER2-amplified CRC. Given the promising results of those studies, it is anticipated that it will become a national standard to test all patients with metastatic CRC for HER2 amplification.
NTRK Fusions
The neurotrophic tropomyocin receptor kinase (NTRK) genes encode for the TrkA, TrkB, and TrkC receptor tyrosine kinases that are important in the function of the nervous system in human neuronal tissue. These proteins are activated by the nerve growth factors neurotrophins (NTs) which lead to activation of the RAS/MAPK pathway, PI3K pathway, or the phospholipase C-γ (PLC-γ) pathway resulting in neuronal differentiation and survival. However, gene fusions of the NTRK gene can occur in which the 3′ region is joined with the 5′ sequence of a fusion partner gene leading to constitutive activation or overexpression of the Trk kinase [83,83,84,86]. These fusion proteins have been reported to occur in ~ 1.5% of all CRCs. Although these fusion proteins are rare in CRC, the FDA has recently approved larotrectinib, a Trk inhibitor, for all solid tumors with NTRK1, NTRK2, and NTRK3 fusion genes. This is the second time the FDA has approved a drug independent of cancer type but the first time a targeted therapy has been approved in this setting. Three clinical trials were used to assess the efficacy of this drug: a phase I trial in adults, a phase 1–2 study in children, and a phase 2 study in adolescents and adults. The combined analysis endpoint had an overall response rate of 75%, including achieving complete response [87••]. Given these results and FDA approval, it is anticipated that examining NTRK fusions will become a part of the standard molecular screening of CRCs.
Summary and Conclusions
Colorectal cancer is a diverse disease with multiple histological and molecular subtypes. Standard of care molecular testing for metastatic CRC includes the assessment of mismatch repair deficiency and analysis for mutations in KRAS, NRAS, and BRAF. This is now often being performed clinically using IHC to determine the MMR status and a sequencing panel for the mutation analyses. This information directly informs clinical practice with both prognostic and predictive information. Each of the identified molecular subtypes of CRC outlined above has distinct treatment strategies that continue to evolve. The sequencing analyses being performed now should be done as part of large clinical sequencing platforms. Large panel testing allows for the standard of care testing to be done, and additionally, those alterations that we believe will soon be part of standard testing including PIK3CA mutations, HER2 amplification, and NTRK fusions. As further testing is being incorporated into clinical practice, further information will be generated regarding the prognostic and therapeutic options for these and other CRC subtypes in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93.
Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7.
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol. 2007;25(30):4779–86.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30.
Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan Z-Z, Mitchell L, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18(9):1004–12.
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17(2):79–92.
Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108(2):564–80.
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–74.
Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1(11):1311–8.
Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95(4):1897–905.
Yang X-D, Jia X-C, Corvalan JR, Wang P, Davis CG. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38(1):17–23.
Jonker DJ, O'callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au H-J, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–8.
Van Cutsem E, Kohne C-H, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346–55.
Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15(6):569–79.
Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–401.
Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091–6.
Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O’Neil BH et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). American Society of Clinical Oncology; 2016.
Segelov E, Earle C, Venook A, Saskin R, Mofid L, Singh S. 587PSurvival by sidedness of metastatic colorectal cancer (mCRC) treated with epidermal growth factor receptor antibodies (EGFR-Ab) in the refractory setting: A population-based study of 1509 patients. Ann Oncol. 2017;28(suppl_5)): v158–v208. https://doi.org/10.1093/annonc/mdx393.
Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33(1):125–36 e3.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45.
Wasan H, Meade AM, Adams R, Wilson R, Pugh C, Fisher D, et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15(6):631–9.
Amado R, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman D, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–64.
Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–72. https://doi.org/10.1056/NEJMoa0808268.
Kratz JD, Uboha NV, Lubner SJ, Mulkerin DL, Clipson L, Yi Y, et al. Metastatic bulk independently predicts outcomes for EGFR precision targeting in colorectal cancer. J Natl Compr Cancer Netw. 2018;16(12):1442–50.
Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64. https://doi.org/10.1007/DCR.0b013e3181c703a4.
Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–22 discussion 22-4.
Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121(6):830–5. https://doi.org/10.1002/cncr.29129.
Lee CM, Tannock IF. The distribution of the therapeutic monoclonal antibodies cetuximab and trastuzumab within solid tumors. BMC Cancer. 2010;10:255. https://doi.org/10.1186/1471-2407-10-255.
Hofmann M, McCormack E, Mujic M, Rossberg M, Bernd A, Bereiter-Hahn J, et al. Increased plasma colloid osmotic pressure facilitates the uptake of therapeutic macromolecules in a xenograft tumor model. Neoplasia. 2009;11(8):812–22.
Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50(3 Suppl):814s–9s.
Mekenkamp LJ, Tol J, Dijkstra JR, de Krijger I, Vink-Börger ME, van Vliet S, et al. Beyond KRAS mutation status: influence of KRAS copy number status and microRNAs on clinical outcome to cetuximab in metastatic colorectal cancer patients. BMC Cancer. 2012;12(1):292.
Smith G, Bounds R, Wolf H, Steele R, Carey F, Wolf C. Activating K-Ras mutations outwith ‘hotspot’codons in sporadic colorectal tumours–implications for personalised cancer medicine. Br J Cancer. 2010;102(4):693–703.
Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C et al. A molecularly annotated platform of patient-derived xenografts (‘xenopatients’) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–23.
Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658–73.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–62.
Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18(2):221–3.
Voigt M, Braig F, Göthel M, Schulte A, Lamszus K, Bokemeyer C, et al. Functional dissection of the epidermal growth factor receptor epitopes targeted by panitumumab and cetuximab. Neoplasia. 2012;14(11):IN2–3.
Richman SD, Chambers P, Seymour MT, Daly C, Grant S, Hemmings G, et al. Intra-tumoral heterogeneity of KRAS and BRAF mutation status in patients with advanced colorectal cancer (aCRC) and cost-effectiveness of multiple sample testing. Anal Cell Pathol. 2011;34(1–2):61–6.
Kosmidou V, Oikonomou E, Vlassi M, Avlonitis S, Katseli A, Tsipras I, et al. Tumor heterogeneity revealed by KRAS, BRAF, and PIK 3 CA pyrosequencing: KRAS and PIK 3 CA intratumor mutation profile differences and their therapeutic implications. Hum Mutat. 2014;35(3):329–40.
Khan KH, Cunningham D, Werner B, Vlachogiannis G, Spiteri I, Heide T, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov. 2018;8(10):1270–85.
Liu X, George G, Tsimberidou A, Naing A, Wheler J, Kopetz S, et al. Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response. BMC Cancer. 2015;15(1):713.
Parseghian CM, Loree JM, Morris VK, Liu X, Clifton K, Napolitano S, et al. Anti-EGFR resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann Oncol. 2018; (in press).
Sievers CK, Kratz JD, Zurbriggen LD, LoConte NK, Lubner SJ, Uboha N, et al. The multidisciplinary management of colorectal cancer: present and future paradigms. Clin Colon Rectal Surg. 2016;29(03):232–8.
Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Lievre A, Bachet J-B, Le Corre D, Boige V, Landi B, Emile J-F, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–5.
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti–epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643–8.
Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais M, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96(8):1166–9.
Lievre A, Bachet J-B, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374–9.
Tapia Rico G, Price TJ. Atezolizumab for the treatment of colorectal cancer: the latest evidence and clinical potential. Expert Opin Biol Ther. 2018;18(4):449–57.
• Bendell J, Ciardiello F, Tabernero J, Tebbutt N, Eng C, Di Bartolomeo M, et al. LBA-004Efficacy and safety results from IMblaze370, a randomised Phase III study comparing atezolizumab+cobimetinib and atezolizumab monotherapy vs regorafenib in chemotherapy-refractory metastatic colorectal cancer. Ann Oncol. 2018;29(suppl_5):mdy208.003-mdy208.003. https://doi.org/10.1093/annonc/mdy208.003 Important clinical trial highlighting the need for more work to better understand the clinical significance of RAS mutations in mCRC.
Oberst MD, Fuhrmann S, Mulgrew K, Amann M, Cheng L, Lutterbuese P et al. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs; Taylor & Francis; 2014.
Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options in Oncol. 2015;16(7):30.
Koopman M, Kortman G, Mekenkamp L, Ligtenberg M, Hoogerbrugge N, Antonini N, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100(2):266–73.
Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–87 e3.
Germano G, Lamba S, Rospo G, Barault L, Magrì A, Maione F, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552(7683):116–20.
Panarelli NC, Vaughn CP, Samowitz WS, Yantiss RK. Sporadic microsatellite instability-high colon cancers rarely display immunohistochemical evidence of Wnt signaling activation. Am J Surg Pathol. 2015;39(3):313–7.
Wang B, Tian T, Kalland K-H, Ke X, Qu YJ. Targeting Wnt/β-Catenin Signaling for Cancer Immunotherapy. Trends Pharmacol Sci. 2018;39(7):648–58.
Gupta R, Sinha S, Paul RN. The impact of microsatellite stability status in colorectal cancer. Curr Probl Cancer. 2018;42:548–59.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91.
•• Overman MJ, Lonardi S, Wong KYM, Lenz H-J, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–9 The largest single group assignment immunotherapy combination study done in dMMR/MSI-H mCRC with encouranging overall response rates.
Overman MJ, Kopetz S, McDermott RS, Leach J, Lonardi S, Lenz H-J, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. 2016;34(15_suppl):3501. https://doi.org/10.1200/JCO.2016.34.15_suppl.3501.
Overman MJ, Bergamo F, McDermott RS, Aglietta M, Chen F, Gelsomino F, et al. Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): Long-term survival according to prior line of treatment from CheckMate-142. 2018;36(4_suppl):554. https://doi.org/10.1200/JCO.2018.36.4_suppl.554.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54.
Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–94.
Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466–75.
Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Rowland A, Dias M, Wiese M, Kichenadasse G, McKinnon RA, Karapetis C, et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112(12):1888–94.
Kopetz S, McDonough SL, Morris VK, Lenz H-J, Magliocco AM, Atreya CE, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). 2017;35(4_suppl):520. https://doi.org/10.1200/JCO.2017.35.4_suppl.520.
• Cutsem EV, Cuyle P-J, Huijberts S, Yaeger R, Schellens JHM, Elez E, et al. BEACON CRC study safety lead-in (SLI) in patients with BRAFV600E metastatic colorectal cancer (mCRC): Efficacy and tumor markers. 2018;36(4_suppl):627. https://doi.org/10.1200/JCO.2018.36.4_suppl.627 An exciting ongoing clinical trial for patients with a BRAF V600E mutation which promises to be a new clinical practice for this subset of patients.
Jones JC, Renfro LA, Al-Shamsi HO, Schrock AB, Rankin A, Zhang BY, et al. Non-V600 BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol. 2017;35(23):2624–30.
Akinleye A, Avvaru P, Furqan M, Song Y, Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6(1):88.
Fricke SL, Payne SN, Favreau PF, Kratz JD, Pasch CA, Foley TM et al. MTORC1/2 inhibition as a therapeutic strategy for PIK3CA mutant cancers. Mol Cancer Ther. 2018:18(2):346–55.
Raghav KPS, Overman MJ, Yu R, Meric-Bernstam F, Menter D, Kee BK, et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. Proc Am Soc Clin Oncol. 2016;34:3517.
Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz H, McCall S, Penault-Llorca F, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29(5):1108–19.
Marsoni S, Bertotti A, Sartore-Bianchi A, Leone F, Lonardi S, Ciardiello F, et al. Dual anti-HER2 treatment of patients with HER2-positive metastatic colorectal cancer: the HERACLES trial (HER2 amplification for Colo-rectaL cancer enhanced stratification). J Clin Oncol. 2013;31, no. 15_suppl.
• Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6):536–42 An important basket trial which found significant objective response rates among HER2 receptor amplified mCRC patients.
Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1(2):e000023.
Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32(1):147–53.
Pietrantonio F, Di Nicolantonio F, Schrock AB, Lee J, Tejpar S, Sartore-Bianchi A, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J Natl Cancer Inst. 2017;109(12).
Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846.
•• Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9 The first tissue-agnostic targeted therapy approved by the FDA.
Khan KH, Cunningham D, Werner B, Vlachogiannis G, Spiteri I, Heide T, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov. 2018;8(10):1270–85.
Financial Support
This project was supported by P30 CA014520 (Core Grant, University of Wisconsin Carbone Cancer Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rebecca A. DeStefanis declares that she has no conflict of interest.
Jeremy D. Kratz declares that he has no conflict of interest.
Philip B. Emmerich declares that he has no conflict of interest.
Dustin A. Deming has received clinical trial funding from Merck, and has received compensation from Bristol-Myers Squibb, Genentech, Bayer, and Array Pharmaceuticals for service on advisory boards.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Systemic Therapies in Colorectal Cancer
Rights and permissions
About this article
Cite this article
DeStefanis, R.A., Kratz, J.D., Emmerich, P.B. et al. Targeted Therapy in Metastatic Colorectal Cancer: Current Standards and Novel Agents in Review. Curr Colorectal Cancer Rep 15, 61–69 (2019). https://doi.org/10.1007/s11888-019-00430-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-019-00430-6