Abstract
Purpose of Review
Prediction of clinical complete response is pivotal in the management of patients with rectal cancer. The ability to determine tumor response to neoadjuvant therapy in rectal cancer might guide subsequent treatment modalities. We review the current literature on predictors of complete response after neoadjuvant for rectal cancer with an emphasis of clinical complete response rather than pathological complete response.
Recent Findings
Clinical and radiological findings have been used to predict response, as well as a myriad of biomarkers. There is limited evidence validating most of these strategies. The role of imaging in defining tumor response has been assessed retrospectively. The TRIGGER trial is a randomized trial that will evaluate stratified management of rectal cancer based on their tumor regression grade.
Summary
The management of locally advanced rectal cancer is evolving. The ability to predict clinical complete response in patients that have undergone neoadjuvant chemoradiation will allow us to select potential patients that can benefit from a “watch and wait” strategy. Identifying patients that will have a complete response will result in decreased surgical overtreatment, favoring organ-sparing strategies. Treatment individualization will require further research. Emphasis should be made in validating prediction markers; these should be cost-effective and of minimally invasive retrieval. Surveillance protocols to assess for tumor regrowth are yet to be determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current management of locally advanced rectal cancer includes neoadjuvant chemoradiation (nCRT) followed by total mesorectal excision. Preoperative CRT is associated with improved clinical outcomes and toxicity profile when compared with postoperative CRT [1]. However, tumor response to neoadjuvant therapy may be variable ranging from complete obliteration of the lesion to no tumor response [2]. Of the patients who undergo neoadjuvant therapy, 25–45% will have little to no response with minimal regression of the tumor [3]. The most commonly used neoadjuvant protocol includes a 5-fluorouracil-based chemotherapy regimen in combination with radiation therapy.
Pathologic complete response (pCR) is defined as the absence of tumor on pathological examination of the surgical specimen after nCRT and has been found in 15 to 27% of patients [4]. However, clinical complete response (cCR) lacks a precise definition and it can vary across studies [5, 6]. Literature reports that up to 40% of the patients after neoadjuvant CRT exhibit cCR [6]. This response is assessed with a combination of digital rectal examination, endoscopic examination with biopsy, and imaging techniques. Different rates of pCR and cCR may arise from the presence of microscopic tumor residual that were not detected on clinical assessment, but rather identified during pathological analysis after surgery [7].
The importance of being able to predict patients who may have a cCR cannot be overstated. Those who have a cCR after neoadjuvant therapy could potentially avoid surgery and its associated morbidity. Rates of permanent colostomy after surgical excision are as high as 45% [8]. Several studies have evaluated the use of a nonoperative approach in patients with a potential cCR to neoadjuvant therapy [9•, 10, 11]. In 2004, Habr-Gama et al. showed similar results between operative and nonoperative strategy for patients that achieved stage 0 after neoadjuvant CRT. Surgical resection did not result in improved outcomes and was associated with the inherent morbidity of the surgical procedure [12].
It remains a challenge to prospectively determine which patients will benefit from an operative versus nonoperative strategy. CRT might be the definite treatment in a subset of patients. In a “wait and watch” strategy, salvage surgery is an option for those who develop local recurrence [7]. Some series have shown promising outcomes [9•, 10, 13,14,15,16,17,18,19]. Moreover, those with a predicted poor response might benefit from a different neoadjuvant strategy, with either a more aggressive CRT regimen or different chemotherapy drugs combinations. Valentini et al. analyzed 163 patients treated with different CRT regimens and found variable tumor responses [3]. Futility can also be evaluated within this stratification based on the predicted tumor response after neoadjuvant therapy [20]. Most of the currently used predictors focus on tissue analysis from resected specimens to evaluate on genetic, epigenetic, or molecular factors, while some use biopsy samples before neoadjuvant therapy [21]. Frequently, these markers are not routinely applied in the clinical setting due to conflicting study results and the lack of prospective validation [22]. A clinically useful test should be sensitive, specific, and cost-effective, providing prognostic information and guiding therapy [23•].
The Habr-Gama’s group first presented their data on a watch-and-wait strategy to the American Society of Colon and Rectal Surgeons in San Diego in 1998 [24]. They proposed a definition of complete response after CRT and trialed an organ conservative strategy. Glynne-Jones et al. reviewed rates of cCR after nCRT and found heterogeneity in the definition of cCR among the different studies [25].
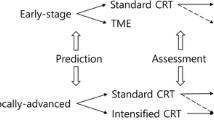
Thus, determining if we can reliably predict a clinical complete response is a fundamental issue to proceed with further treatment modalities and to determine if they need to be altered. The current review paper addresses the current strategies to assess a cCR to determine if we have made progress in this area. A definition of cCR is a pertinent good start.
Definition
Clinical response after neoadjuvant therapy is assessed by digital rectal examination to determine any residual palpable tumor, endoscopic examination with biopsy, and repeat imaging [5]. Several clinical and endoscopic features are used to describe response of distal rectal cancer after neoadjuvant therapy.
Glynne et al. found significant heterogeneity in the cCR definition used by several reports. Most studies relied on clinical evaluation with digital rectal examination (DRE) or CT scanning while the minority used ultrasound or magnetic resonance imaging (MRI) to assess response to nCRT. In general, cCR was defined as absence of detectable residual lesion on clinical examination, or clinical examination and endoscopy. The use of microscopic evaluation with biopsies was not widespread. This review also reported a 30% association of cCR with pCR [25].
Similarly, Kong et al. reported the variability to define this term (Table 1) [26••]. The description proposed by Habr-Gama et al. appears to be the most widespread used definition for assessment of cCR [5]. Complete clinical responders can exhibit whitening of the mucosa, presence of telangiectasias, and decreased pliability of the rectal wall that contains the residual scar. Addition, the tumor cannot be palpated or visualized. The presence of the following represents incomplete clinical response: ulceration regardless of the presence of necrosis, palpable nodule, and stenosis precluding proctoscopic evaluation [5]. In another analysis using prospectively collected data, exclusively used endoscopic findings to determine complete response. In this study, an endoscopic complete response was defined by (1) flat, pale or reddish scar or flat healing ulcer with regular borders surrounded by healthy mucosa, (2) disappearance of the neoplastic pit without visual amplification, and (3) disappearance of the neoplastic nodule or stenosis. The endoscopic findings associated with cCR reflected pCR with an accuracy of 88.7% [27].
While some authors have supported the definition of cCR through post-biopsy analysis [14, 15, 18], Perez et al. stated that biopsies after neoadjuvant therapy have a very low negative predictive value, so its use as a sole defining factor of cCR complete response is discouraged [28]. Habr-Gama et al. favor clinical assessment as a minimal requirement for clinical response determination [29••].
The available data regarding the criteria to define cCR is heterogeneous. Clinical and endoscopic findings can be subjective and rely on the expertise of the clinician. Various imaging modalities, specifically MRI may be used as a way to improve the accuracy of determining cCR. In a series by Bhoday et al., MRI after neoadjuvant therapy was able to predict pCR more accurately than preoperative clinical assessment alone [30].
Imaging
Magnetic Resonance Imaging
MRI has been used as predictor of neoadjuvant therapy response in rectal cancer [31]. Moreover, restaging MRI after CRT is highly accurate in predicting pathological response in those patients who were deemed clinical responders (κ coefficient = 0.72) [32]. Unfortunately, there is not enough evidence to individualize treatment in rectal cancer based exclusively on imaging results [33]. Conventional MRI has limited ability to distinguish fibrosis from residual tumor, hence restaging after preoperative CRT is challenging. A systematic review evaluated the ability of MRI to restage locally advanced rectal cancer after neoadjuvant CT; the analysis reported a mean sensitivity of 50.4% for conventional MRI, while the use of functional MRI showed an improved sensitivity of 83.6% [31]. Diffusion-weighted images can evaluate the vascularity of a tumor and its variation in response to treatment, which represents an upgrade from conventional MRI [34].
Shen et al. showed that the extramural depth (EMD) to mesorectum ratio was a predictor for tumor response to neoadjuvant CRT in T3 rectal cancer. They defined extramural depth as the distance from the outer edge of the muscularis propria to the most exterior edge of the tumor. The mesorectum was measured at the same plane. An EMD to mesorectum ratio of 0.5 was determined to be a reasonable cutoff for patients with T3 rectal cancer as those patients had higher rates of cCR. Moreover, a ratio of 0.5 or less was independently associated with better disease-free survival in a multivariate analysis [35].
The vascular characteristics within a neoplastic focus have significant influence on its sensitivity to CRT. Perfusion-based MRI criteria can be used in the evaluation of a tumor microcirculation, neoangiogenesis, and response to treatment [36]. Recently, perfusion parameters have been reported to change with neoadjuvant therapy, but specific cutoff values have not been determined because of high variability between subjects. In a prospective cohort, peak tumor blood volume measurements in a high-density enhancement area assessed 1–2 weeks after treatment initiation were strong independent predictors of cCR to neoadjuvant therapy [37]. Krishan et al. proposed using a ratio with the tumor-free rectal wall, normalizing these values. Responders are expected to have tumor perfusion parameters close to normal tissue, in consequence the normalized values approach 1 [36].
Pham et al. have designed a protocol for a prospective clinical study to use different MRI modalities to improve MRI prediction accuracy of treatment response. They suggest that joining diffusion-weighted imaging and dynamic contrast-enhanced MRI would achieve a better three-dimensional volumetric analysis of the tumor to assess its heterogeneity and can help develop standardized multiparametric protocols to quantitatively assess treatment response [20]. Multiple objective parameters have been evaluated in diffusion-weighted MRI (DWI), which supports this idea. The results have been encouraging. Zhu et al. designed a study to assess the performance of DWI models in predicting tumor response to CRT. Their models showed reasonable reliability and diagnostic performance [38].
MRI represents a frequently used tool to determine cCR. So far, the studies evaluating its use have shown promising results, yet a prospective trial still needs to be published assessing its role in individualized management of the rectal cancer.
Positron Emission Tomography (PET/CT)
PET/CT is an imaging modality with limited availability to some patients due to cost and one that involves the risk of radiation exposure. Nevertheless, it has been used to evaluate cCR to nCRT. Perez et al. evaluated its performance in 99 patients. PET/CT was able to detect residual cancer with an 85% accuracy. The addition of PET/CT to clinical assessment achieved an overall accuracy of 96% [39]. Presence of FDG uptake restricted to the rectal wall at baseline PET/CT and a decrease from baseline in the maximum standardized uptake value (SUVmax) are findings that favor complete response [40]. The total lesion glycolysis (TLG) parameter is a value that depends on metabolic activity and tumor volume. A decrease greater than 92% in the TLG was used as cutoff to stratify patients between complete versus incomplete response. This was associated with a negative predictive value of > 90%. The authors of this study concluded that volumetric PET/CT value can be used to predict response to neoadjuvant CRT and to isolate potential candidates for alternative tactics after clinical complete response [41].
Tumor Grading Using Imaging
The magnetic resonance tumor regression grade (mrTGR) classifies response to neoadjuvant therapy based on post-CRT MRI findings. It is a 5-point scale where lower score represents greater regression (Table 2). Poor response is defined as mrTGR 4–5. This imaging-related grading scale has a directly proportional association with clinical outcomes, including local recurrence, distant recurrence, disease-free survival, and overall survival [42]. The scale depends on the assessment of the proportion of tumor replaced by fibrosis, which is more reliable than attempting to delineate T stage after treatment [43].
The Magnetic Resonance Tumor Regression Grade as Biomarker for Stratified Management of Rectal Cancer Patients (TRIGGER) trial is a multicentric, randomized control study that aims to evaluate the use magnetic resonance tumor regression grade (mrTRG) as a tool to stratify management of rectal cancer patients based on their response to neoadjuvant CRT [44]. This is a needed step to make imaging a standard tool in the individualized management of rectal cancer.
Biological Markers
DNA Mutations
Recent research has focused on the use of biomarkers before neoadjuvant therapy to predict response to neoadjuvant therapy. DNA mutations, including KRAS mutations, have been suggested to confer radio-resistance to the tumor [45]. While mixed results have been reported, association with p53 mutations has been widely linked to tumor resistance to radiotherapy [7, 46, 47]. The upregulation of XRCC3, a gene that codes for a protein involved in DNA repair, confers chemo-resistance to 5-fluorouracil in patients with rectal adenocarcinoma [48]. Other DNA mutations studied that predict response to CRT include DNA repair gene SMC1, apoptotic genes LUM and THBS2, and DNA repair gene XRCC3 [49]. The glycoprotein YKL-40 and the oncogene c-Met receptor tyrosine kinase are markers of chemo- and radio-resistance [50]. The methylation of the TIMP3 gene has also been correlated with resistance to CRT [51] while the expression of lincRNA-p21 favors improved outcomes [52]. These last two represent examples of non-DNA targets of research as predictors of cCR.
Liquid Biopsy
Circulating tumor DNA (ctDNA) are tumoral DNA fragments present in the patient’s bloodstream. A quantitative analysis of the ctDNA correlates with tumor staging and prognosis [53]. The detection of ctDNA in the plasma of cancer patients is known as liquid biopsy. With the use of bioinformatic technology, it is possible to identify genome-wide patient-specific mutations, which can be used as individualized biomarkers for the monitoring of ctDNA. Carpinetti et al. used this technology to evaluate the value of early changes of ctDNA to assess cCR after neoadjuvant CRT. They concluded its use would complement clinical and radiological evaluation. Moreover, ctDNA levels could be used to monitor early response to nCRT [54•].
Micro-RNA
Micro-RNA is a type of noncoding RNA that participates in difference cellular processes, including cell proliferation, differentiation and apoptosis. Their role in cancer has been studied and how it can affect radiotherapy response. Its dysregulation is a factor leading to carcinogenesis and altered gene expression. As a biomarker, its structural characteristics allow it to be highly stable in vitro and in vivo. Extracellular miRNAs have been identified in circulating blood of healthy and diseased patients, which makes them an interesting novel biomarker [55, 56]. The studies evaluating the use of micro-RNA have been heterogeneous and have yet to make an impact in the clinical setting. Lopes-Ramos et al. studied miRNA expression profiles of patients with complete or incomplete response aiming to determine predictive biomarkers. They identified miR-21-5p overexpression as a strong predictor of cCR to neoadjuvant therapy [57]. While these studies are compelling and had yielded promising results, this technology has not made it to standard clinical practice.
RNA Sequencing
RNA sequencing (RNA-Seq) can be used to detect the presence of RNA in a sample and quantify it. It has been a promising technology to recognize groups of genes with specific patterns of gene expression, called gene expression signatures. RNA-Seq has shown potential to replace microarrays for transcriptome sequencing. Many authors have tried to determine a gene expression signature able to predict cCR to CRT. Unfortunately, the results of these reports have been difficult to replicate in subsequent studies, regardless of the use of microarray or RNA-Seq for gene expression analysis. Lopez-Ramos et al. concluded in their work that the use of gene signatures is dependent on the sample used, and the accuracy is not superior to current clinical and radiological criteria to assess response [46, 58,59,60,61,62,63]. While Agostini et al. suggested that the use of integrative computational biology and gene expression patterns can be useful to predict cCR in patients undergoing neoadjuvant therapy. Their promising work suggests that these biomarkers can predict tumor responsiveness to CRT and delineate new strategies based on specific identified molecular pathways [48].
Other Serum Biomarkers
Serum markers are cheaper alternatives to response prediction. Carcinoembryonic antigen (CEA) is the most used tumor marker in colorectal cancer. Its most important role is surveillance after complete resection of the tumor. Levels of <5 ng/mL after neoadjuvant is associated with higher rates of cCR [64, 65], but there is no consensus among clinicians in its role for preoperative rectal cancer [21]. Abnormal CEA levels after neoadjuvant therapy should prompt PET/CT evaluation because it can assess tumor response but may also aid in the determination of unsuspected metastasis [29].
Higher hemoglobin levels before neoadjuvant therapy are associated with cCR, but it represents a poor association (area under ROC curve 0.673). Other markers, for example neutrophil to lymphocyte ratio, have shown similar results [21]. On the basis that hypoalbuminemia and leukocytosis have been found to correlate with poor outcomes in pancreatic cancer, a neutrophil to albumin ratio (NAR) was evaluated in rectal cancer patients. Higher neoadjuvant CRT ratios independently correlated with a complete response [23•].
Bitterman et al. determined that a CEA level > 5 ng/mL at diagnosis, a tumor size > 3 cm, distance greater than 3 cm between the tumor and the anal verge, clinically node-positive disease, and more than 8-week interval from CRT to surgery were independent predictors of poor response [66].
Although many biomarkers have been evaluated, their use is not standard to predict response to nCRT. Most studies lack a direct comparison between different sets of biomarkers, limiting the ability to assess its relative performance. Dayde et al. emphasizes the molecular heterogeneity of colorectal cancer. Hence, a single biomarker is improbable to achieve a reasonable sensitivity and specificity to predict cCR [67]. CEA levels remain an important tool. It has a proven role in follow-up after resection and normal levels after nCRT are suggestive of cCR.
Nomograms
Sun et al. have prepared a novel approach to predict cCR using a nomogram. Multiple factors that were independently associated with pCR were evaluated and subsequently integrated in a prediction nomogram. The proposed model included CEA levels before and after CRT, and residual tumor characteristics after CRT such as distance of the tumor from the anal verge, tumor size, and circumferential extent of the tumor. This tool was internally and externally validated with good concordance index [68].
Future Strategies
As the use of selective surgery for patients with clinical complete response to neoadjuvant therapy becomes more frequent, it will be necessary to determine which patients are at risk of tumor regrowth. Approximately 20% of the patients managed nonoperatively will develop local recurrence within the first 12 months [16, 69]. The results of a small, retrospective study show that more superficial tumors at baseline are less likely to develop early local recurrence, i.e., cT2 tumor versus a cT3/4 [70].
The following are different roads to achieve individualized management of rectal cancer: detection of circulating tumor DNA to determine incomplete response to neoadjuvant therapy [54•, 71]; developing and validated micro-RNA profiles that correlate with clinical outcomes and that can predict response to therapy; emphasis should be made on specimen of low invasiveness collection, as serum and urine RNA [7]; and use of deregulation scores to study and categorize disease-specific pathway to help stratify these tumors [72].
Conclusion
Developing a reliable prediction strategy will allow us to stratify patients based on their predicted response to neoadjuvant therapy and individualize treatment. Most authors agree on the most common clinical and endoscopic findings that represent cCR; however, there is no universal definition. The TRIGGER trial promises to appraise the role of imaging in this setting. Many biomarkers have been studied, yet there is no consensus on their ability to predict response to neoadjuvant therapy. Currently, a consistent method to predict cCR remains elusive, but as we continue to emphasize on this subject, prediction of tumor response after neoadjuvant therapy will become standard of care. Prospective studies are needed to clarify the optimal approach for those predicted with cCR, of preference with close follow-up in centers of excellence.
Abbreviations
- cCR:
-
Clinical complete response
- pCR:
-
Pathologic clinical response
- TME:
-
Total mesorectal excision
- mrTGR:
-
Magnetic resonance tumor-grade regression
- CRT:
-
Chemoradiation
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Fokas, E., et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J Natl Cancer Inst 2017. 109(12).
Valentini V, et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys. 2001;51(2):371–83.
Maas M, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. The Lancet Oncology. 2010;11(9):835–44.
Habr-Gama A, et al. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–8.
Glynne-Jones R, Hughes R. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg. 2012;99(7):897–909.
Pettit C, et al. Molecular profiling of locally-advanced rectal adenocarcinoma using microRNA expression (review). Int J Oncol. 2017;51(2):393–404.
McCarthy K, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:Cd008368.
• Renehan AG, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174–83. Large propensity-score matched cohort analysis assessing for outcomes after conservative treatment for rectal cancer. Suggests a standardized definition for clinical complete response.
Maas M, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633–40.
Habr-Gama A, et al. Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol. 2011;21(3):234–9.
Habr-Gama A, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7. discussion 717-8
Lai CL, et al. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait”. Int J Color Dis. 2016;31(2):413–9.
Appelt AL, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–27.
Nakagawa WT, et al. Chemoradiation instead of surgery to treat mid and low rectal tumors: is it safe? Ann Surg Oncol. 2002;9(6):568–73.
Habr-Gama A, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(4):822–8.
Smith JD, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6):965–72.
Dalton RS, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Color Dis. 2012;14(5):567–71.
Smith RK, et al. Surveillance after neoadjuvant therapy in advanced rectal cancer with complete clinical response can have comparable outcomes to total mesorectal excision. Int J Color Dis. 2015;30(6):769–74.
Pham TT, et al. Study protocol: multi-parametric magnetic resonance imaging for therapeutic response prediction in rectal cancer. BMC Cancer. 2017;17(1):465.
Clarke TL, et al. Predicting response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer with serum biomarkers. Ann R Coll Surg Engl. 2017;99(5):373–7.
Grade M, et al. The molecular basis of chemoradiosensitivity in rectal cancer: implications for personalized therapies. Langenbeck's Arch Surg. 2012;397(4):543–55.
• Tawfik B, et al. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anti-Cancer Drugs. 2016;27(9):879–83. Survival analysis of serum biomarkers as predictors of complete response.
Habr-Gama A, et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41(9):1087–96.
Glynne-Jones R, et al. Complete clinical response after preoperative chemoradiation in rectal cancer: is a “wait and see” policy justified? Dis Colon Rectum. 2008;51(1):10–9. discussion 19-20
•• Kong JC, et al. Outcome and salvage surgery following “watch and wait” for rectal cancer after neoadjuvant therapy: a systematic review. Dis Colon Rectum. 2017;60(3):335–45. Systematic review evaluating use of salvage surgery for patients that underwent non-operative strategy. Analyzed 5 restrospective and 4 prospective studies. Described variability in the definition of clinical complete response among studies.
Lim SG, Kim YB, Oh SY. Clinical significance of the endoscopic finding in predicting complete tumor response to preoperative chemoradiation therapy in rectal cancer. World J Surg. 2016;40(12):3029–34.
Perez RO, et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Color Dis : Off J Assoc Coloproctol Great Britain and Ireland. 2012;14(6):714–20.
•• Habr-Gama A, São Julião GP, Perez RO. Nonoperative management of rectal cancer: identifying the ideal patients. Hematol Oncol Clin North Am. 2015;29(1):135–51. Report on complete response assessment, clinical significance and management using a 'Watch and wait' protocol.
Bhoday J, et al. Magnetic resonance tumor regression grade and residual mucosal abnormality as predictors for pathological complete response in rectal cancer postneoadjuvant chemoradiotherapy. Dis Colon Rectum. 2016;59(10):925–33.
van der Paardt MP, et al. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101–12.
Lee JH, et al. Prediction of pathologic staging with magnetic resonance imaging after preoperative chemoradiotherapy in rectal cancer: pooled analysis of KROG 10-01 and 11-02. Radiother Oncol. 2014;113(1):18–23.
Moszkowicz D, et al. Can we predict complete or major response after chemoradiotherapy for rectal cancer by noninvasive methods? Results of a prospective study on 61 patients. Am Surg. 2014;80(11):1136–45.
Yu Y, Yue J, Yu J. Value of functional magnetic resonance imaging in predicting outcomes of neoadjuvant chemoradiotherapy in rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20(5):491–4.
Shen, L., et al. T3 sub-classification using the EMD/mesorectum ratio predicts neoadjuvant chemoradiation outcome in T3 rectal cancer patients. Br J Radiol 2017: p. 20170617.
Krishan, S., et al. Rectal perfusion parameters normalised to tumour-free rectal wall can predict response to neoadjuvant chemoradiotherapy. Clin Radiol 2017.
Kino A, et al. Perfusion CT measurements predict tumor response in rectal carcinoma. Abdominal radiology (New York). 2017;42(4):1132–40.
Zhu HB, et al. Assessment of pathological complete response to preoperative chemoradiotherapy by means of multiple mathematical models of diffusion-weighted MRI in locally advanced rectal cancer: a prospective single-center study. J Magn Reson Imaging. 2017;46(1):175–83.
Perez RO, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683). Cancer. 2012;118(14):3501–11.
Perez RO, et al. Predicting complete response to neoadjuvant CRT for distal rectal cancer using sequential PET/CT imaging. Techn Coloproctol. 2014;18(8):699–708.
Dos Anjos DA, et al. Semiquantitative volumetry by sequential PET/CT may improve prediction of complete response to neoadjuvant chemoradiation in patients with distal rectal cancer. Dis Colon Rectum. 2016;59(9):805–12.
Siddiqui MRS, et al. Defining response to radiotherapy in rectal cancer using magnetic resonance imaging and histopathological scales. World J Gastroenterol. 2016;22(37):8414–34.
Patel UB, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol : Off J Am Soc Clin Oncol. 2011;29(28):3753–60.
Battersby NJ, et al. A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials. 2017;18:394.
Bernhard EJ, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60(23):6597–600.
Ghadimi BM, et al. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol : Off J Am Soc Clin Oncol. 2005;23(9):1826–38.
Huerta S, et al. Role of p53, Bax, p21, and DNA-PKcs in radiation sensitivity of HCT-116 cells and xenografts. Surgery. 2013;154(2):143–51.
Agostini M, et al. A functional biological network centered on XRCC3: a new possible marker of chemoradiotherapy resistance in rectal cancer patients. Cancer Biol Ther. 2015;16(8):1160–71.
Conde-Muino R, et al. Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed Res Int. 2015;2015:921435.
Senetta R, et al. YKL-40/c-Met expression in rectal cancer biopsies predicts tumor regression following neoadjuvant chemoradiotherapy: a multi-institutional study. PLoS One. 2015;10(4):e0123759.
Molinari C, et al. Gene methylation in rectal cancer: predictive marker of response to chemoradiotherapy? J Cell Physiol. 2013;228(12):2343–9.
Wang G, et al. LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/beta-catenin signaling pathway. Oncol Rep. 2014;31(4):1839–45.
Bettegowda C, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24.
• Carpinetti P, et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget. 2015;6(35):38360–71. Evaluation of liquid biopsies to detect recurrence and monitor treatment response to neoadjuvant CRT. Proposes further avenues of research.
Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101(10):2087–92.
D'Angelo E, et al. MicroRNAs as tools and effectors for patient treatment in gastrointestinal carcinogenesis. Curr Drug Targets. 2015;16(4):383–92.
Lopes-Ramos CM, et al. Overexpression of miR-21-5p as a predictive marker for complete tumor regression to neoadjuvant chemoradiotherapy in rectal cancer patients. BMC Med Genet. 2014;7:68.
Lopes-Ramos C, et al. Comprehensive evaluation of the effectiveness of gene expression signatures to predict complete response to neoadjuvant chemoradiotherapy and guide surgical intervention in rectal cancer. Cancer Gen. 2015;208(6):319–26.
Watanabe T, et al. Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res. 2006;66(7):3370–4.
Kim I-J, et al. Microarray gene expression profiling for predicting complete response to preoperative chemoradiotherapy in patients with advanced rectal cancer. Dis Colon Rectum. 2007;50(9):1342–53.
Rimkus C, et al. Microarray-based prediction of tumor response to neoadjuvant radiochemotherapy of patients with locally advanced rectal cancer. Clin Gastroenterol Hepatol : Off Clin Prac J Am Gastroenterol Assoc. 2008;6(1):53–61.
Brettingham-Moore KH, et al. Pretreatment transcriptional profiling for predicting response to neoadjuvant chemoradiotherapy in rectal adenocarcinoma. Clin Cancer Res : Off J Am Assoc Cancer Res. 2011;17(9):3039–47.
Watanabe T, et al. Prediction of response to preoperative chemoradiotherapy in rectal cancer by using reverse transcriptase polymerase chain reaction analysis of four genes. Dis Colon Rectum. 2014;57(1):23–31.
Perez RO, et al. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum. 2009;52(6):1137–43.
Restivo A, et al. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann Surg Oncol. 2013;20(3):864–71.
Bitterman DS, et al. Predictors of complete response and disease recurrence following chemoradiation for rectal cancer. Front Oncol. 2015;5:286.
Dayde, D., et al. Predictive and prognostic molecular biomarkers for response to neoadjuvant chemoradiation in rectal cancer. Int J Mol Sci 2017. 18(3).
Sun, Y., et al. A nomogram predicting pathological complete response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer: implications for organ preservation strategies. Oncotarget 2017.
Habr-Gama A, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–17.
Habr-Gama A, et al. Baseline T classification predicts early tumor regrowth after nonoperative management in distal rectal cancer after extended neoadjuvant chemoradiation and initial complete clinical response. Dis Colon Rectum. 2017;60(6):586–94.
Crowley E, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472–84.
Domany E. Using high-throughput transcriptomic data for prognosis: a critical overview and perspectives. Cancer Res. 2014;74(17):4612–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Surgery and Surgical Innovations in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Taveras, L.R., Cunningham, H.B. & Imran, J.B. Can We Reliably Predict a Clinical Complete Response in Rectal Cancer? Current Trends and Future Strategies. Curr Colorectal Cancer Rep 14, 56–63 (2018). https://doi.org/10.1007/s11888-018-0401-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-018-0401-1