Abstract
New strategies for the treatment of cancer in the rectum should be directed towards the improvement of micrometastatic disease and the reduction of long-term sequelae, without prejudice to good local control. To achieve this, in the last decade, new strategies have been postulated. Treatment with preoperative chemotherapy (CT) alone or induction CT followed by chemoradiation CRT/short course radiation (CRT/SCPRT) or CRT/SCPRT and consolidative CT is being planned. We currently have data from phase II studies with results of stimulating efficacy and/or compliance. New single-arm and randomized trial is underway and will allow us to know the impact on survival outcomes and long-term sequelae of these strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prior to the current surgical operation of total mesorectal excision (TME), surgeons employed blunt dissection techniques with the fingers. High rates of local pelvic recurrence were reported, which caused devastating symptoms and were difficult to palliate. Also, 30–50% required a permanent stoma. Hence, avoidance of LR and the possibility of sphincter sparing have long dominated decision-making.
Postoperative trials during the 1980s showed a significant benefit for adjuvant concurrent chemoradiation (CRT) in terms of local recurrence (LR), disease-free survival (DFS) and overall survival (OS) [1, 2], which has now been extrapolated to the preoperative setting. Preoperative CRT was then compared to postoperative CRT in the German Trial CAO/ARO/AIO—94 [3]. Acute and late toxicity was significantly less with the preoperative approach. Loco-regional failure was significantly lower at 7% in the preoperative arm vs. 10% in the postoperative arm. There was, however, no difference in the distant metastases rate or OS [4]. Further trials investigating preoperative CRT all showed a reduction in LR [5, 6], but did not impact on OS. For more advanced unresectable cases, the addition of fluoropyrimidines to radiation has favourable effects on response and relapse-free survival compared to RT alone, with a trend to improved OS [7]
In Europe, randomized trials [8,9,10,11] examined short course preoperative radiotherapy (SCPRT), accelerated and hypo-fractionated radiotherapy, administering 25 Gy in five fractions over 5 days. SCPRT also reduced LR rates and established SCPRT as a standard component of treatment for rectal cancer.
Thus, there are now three distinct current options which can deliver radiotherapy locally to tumour in the pelvis to reduce LR. SCPRT and long-course preoperative CRT are both considered standard neoadjuvant strategies. The integration of chemotherapy (CT) is attractive as a radio-sensitizing agent within the radiation field in the pelvis and may have systemic effects.
In addition, high dose rate endoluminal brachytherapy (HDR-BRT) offers a third preoperative alternative. A pCR rate of 27% and an actuarial LR rate of only 4.8% were reported after HDR-BRT [12].
A matched analysis compared SCPRT and HDR-BRT in terms of LR and OS between Dutch and Canadian centres [13] and demonstrated no significant differences. SCPRT and immediate surgery have also been compared directly to long-course CRT in randomized studies. CRT and SCPRT appear equivalent in terms of LR, DFS and overall survival (OS) [14,15,16]. More recently, the Polish Rectal study showed more adverse events associated with CRT compared with SCPRT followed by consolidative FOLFOX for 3 cycles, but no statistically significant differences in postoperative complications [17••].
CRT leads to downstaging/downsizing, and 10–20% of patients achieve a pathological complete response (pCR). Hence, many believe this excellent response facilitates sphincter-preserving surgery (SPS). The results of the German trial indirectly support this view [3], but specifically designed trials [18•] and meta-analyses have failed to confirm CRT increases the chance of SPS. However, efforts are continuing to intensify multimodality treatments both to increase SPS and for organ preservation with avoidance of the need for surgery. CRT has been considered the treatment of choice to maximize downstaging in locally advanced, fixed or unresectable tumours, although SCPRT followed by systemic CT with oxaliplatin may provide similar results with less acute toxicity [18•].
Currently, with the standard of good quality TME, LR has become a rare event in patients with stage I, II and III rectal cancer. However, outcomes with the standard treatment strategies are still far from optimal. There are significant long-term sequelae related to surgery and radiotherapy, There is considerable overtreatment relating to inaccurate staging techniques. A substantial risk of distant relapse also highlights the ineffectiveness of the subsystemic fluoropyrimidine component of chemoradiotherapy against micrometastatic disease. All of which dictate a pressing need for improvement.
Patients treated with SCPRT prior to TME have less potential for LR compared to patients undergoing TME alone, but this benefit is mainly limited to subgroups (i.e. stage III), underscoring the need for accurate preoperative staging [19].
In the case of patients treated with preoperative CRT or SCPRT, adjuvant CT has shown no benefit compared to observation in any of the four phase III studies performed to date [20,21,22,23]. Poor adherence to systemic therapy in some studies [20,21,22], inclusion of low-risk patients due to the use of old methods of staging, lack of statistical power by early closure of the study [21, 23] as well as the use of suboptimal systemic treatments [20, 22, 23] are among the causes of negative outcomes. More recently, modern studies with adjuvant CT regimens with FOLFOX have shown benefit compared with FU/LV in patients previously treated with CRT and surgery [24, 25].
Based on these observations, a new generation of clinical trials in stages II and III rectal cancer that mandate MRI as a staging technique include more effective CT regimens and utilize novel strategies that allow an optimal sequence of treatment modalities are being carried out in recent years.
In this article, we review the arguments that justify these new treatment strategies, current data, as well as ongoing efforts to further evaluate the impact of this approach
Preoperative CT Without Radiation
SCPRT and CRT have improved local control, but both treatment strategies are associated with an increased incidence of serious side effects, with approximately 5–10% of patients experiencing grade 3–4 late morbidity [10, 15, 16, 26]. The concept of locally advanced rectal cancer (LARC) encompasses the group of middle and distal third rectal tumours (i.e. with the lower border of the tumour ≤12 cm from the anal verge) with a heterogeneous prognosis. Patients with rectal cancer in the middle third and T3 tumours with no predicted involvement of the mesorectal fascia (MRF) represent an intermediate risk group in which the risk of distant relapse predominates over the risk of LR. In a recent MERCURY study, 122 patients with MRI-predicted safe circumferential resection margins (CRM), regardless of MRI N stage, were included. Patients were treated with good quality TME surgery alone. The population included 57 cStage II tumours and 66 patients with good prognosis cStage III tumours (i.e. mrT2, T3a and T3b and mrN+). In this later group, 4 patients (6%) had a pathological involved CRM. Overall, the LR and distant relapses rates were 3 and 20%, respectively [27]. In addition, retrospective series of metastatic colorectal cancer patients with intact primary tumours have shown significant primary tumour regression with pCR following modern systemic CT [28]
These observations suggest that induction with CT in this group of patients (i.e. T3 with clear MRF) is an attractive strategy, producing the local benefits of preoperative treatment and allowing early treatment of micrometastases at therapeutic doses without the long-term side effects of radiation.
Selected Published Series
Results of two single-arm trials in T3, middle third rectal tumours with clear MRF have been reported. Both trials used a similar treatment strategy with 12 weeks of induction with bevacizumab and systematic CT treatment, while CRT was only given for poor response. The MSKCC pilot trial included 32 patients with locally advanced rectal cancer, who were candidates low anterior resection and cT2-3 N+ and cT3 any N (with ERUS and MRI). Induction CT consisted of 4 cycles neoadjuvant of FOLFOX + Bevacizumab and 2 subsequent cycles FOLFOX alone. Patients with stable/progressive disease went on to receive CRT; however, responders proceeding straight to TME. Postoperative CRT was considered for close/positive margins. Postoperative FOLFOX × 6 was recommended, but adjuvant regiments were left to the clinician discretion. pCR and R0 resection rates were 25 and 100%, respectively. With a median follow-up of 54 months, local and distant relapse were 0 and 12.5%, respectively, and 4-year DSF was 92%. Only two participants, both intolerant of FOLFOX/bevacizumab, received preoperative CRT [29••].
The Grupo Español Multidisciplinar Cancer Digestivo (GEMCAD) 0801 multicentric, phase II trial recruited 46 patients with T3, middle third and MRF clear rectal cancer selected by MRI. Patients receive 4 cycles of CAPOX-bevacizumab (last cycle without Bev), and preoperative CRT was recommended only in cases of evidence of progression. The overall response rate was 78%, R0 100%, pCR 20% and downstaging 48% [30•]. An unexpectedly high rate (15%) of anastomotic leakage (AL) and two deaths were probably related to the use of bevacizumab during the treatment period. No patient received preoperative CRT. In a second report from the same trial with a median follow-up of 41 months, LR only and distant relapse were 6 and 21%, respectively, and DFS was 61%. Baseline MRI assessment of extramural venous invasion (EMVI) status has been identified as the most important clinical risk factor related with DFS and recurrence. Three-year DFS for mrEMVI-positive patients was 44 vs. 96% for mrEMVI-negative patients (p = 0.0001), and 3-year cumulative incidence of recurrence for mrEMVI-positive patients was 44 vs. 4% for mrEMVI-negative patients (p = 0.0019). The authors conclude these patients should benefit from intensive preoperative CT [31]
In addition, two Japanese studies used a scheme identical to that of GEMCAD 0801, but in a high-risk population of middle or distal third cancers. Patients underwent surgery and CRT was not offered. Uehara et al. recruited 32 patients, of which 60% were cT4. The pCR was 13%, and the anastomotic leakage rate was 28% with 3% mortality [32]. Hasegawa et al. recruited 25 high-risk patients (T4 in 75%) and achieved a pCR rate of 4% [33]. A third trial from Japan with 8 weeks induction CT (2 cycles of Irinotecan, Fluorouracil, Leucovorin (IFL)) in T3, T4 middle or low rectal cancer also reported a 4% pCR [34].
Primary results from a three-arm randomized phase III trial has been recently reported [35•] comparing neoadjuvant fluorouracil/leucovorin (5FU/LV) combined with RT followed by adjuvant 5FU/LV (arm A) with neoadjuvant mFOLFOX6 combined with RT and adjuvant mFOLFOX6 (arm B) with the same regimen without RT (arm C). A total of 165 patients were randomized to each group. Patients with rectal cStage II or III were included. No stratification was made, and T4 patients were 34.6% in arm A, 34% in arm B and 30.3% in group C. The rates of pCR were 14, 27.5 and 6.6% in each group. Patients in arm C without RT achieved less pCR rates but a lower rate of postoperative surgical complications. Three-year DFS (primary endpoint) will be available during 2017.
In summary, systemic treatment in T3 tumours results in promising activity, high R0 resection and low LR rates, which justify new studies in this population with this strategy. However, the considerable surgical morbidity with the addition of bevacizumab to fluoropyrimidine/oxaliplatin CT does not support further investigation of this specific regimen. Pathologic CR seem to be related to tumour size, as evidenced by the lower rate observed in the other studies analyzed that included T4 tumours.
Ongoing Trials
The Preoperative Radiation or Selective Preoperative Radiation and Evaluation Before Chemotherapy and TME study (NCT01515787) is a phase II/III randomized trial. Patients who are candidates for sphincter preservation at presentation and with cT2N1, cT3N0 or T3N1 are randomized to standard CRT followed by TME vs. neoadjuvant FOLFOX followed by objective response determination. Responding patients proceed directly to surgery. The primary endpoint is complete resection rates (phase II portion) and disease-free survival/time to LR (phase III portion). This trial could establish whether selective omission of radiotherapy to patients deriving clinical benefit from systemic neoadjuvant CT provides adequate oncologic outcomes with less toxicity.
A British trial investigated the addition of bevacizumab to combination chemotherapy in rectal cancer until surgery trial (NCT01650428). In this multi-centre randomized phase II study, patients with T3, MRF clear tumours in the middle third were randomized to 12 weeks of neoadjuvant systemic therapy with either FOLFOX with bevacizumab or FOLFOXIRI (5FU, leucovorin, oxaliplatin and irinotecan) with bevacizumab. This trial stopped early because of lack of recruitment, and results are not yet reported.
In this intermediate risk population, the GEMCAD is now performing a phase II trial exploring the value of incorporating anti-epidermal growth factor receptor therapies to the appropriately extended BRAF, PIK3CA, RAS wild-type population neoadjuvant setting (NCT03000374).
As other new therapies become clinically relevant in the metastatic disease setting, the opportunity to bring them into the neoadjuvant rectal cancer treatment paradigm represents a new era in rectal cancer personalized patient management and clinical trial design.
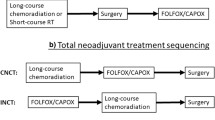
Upfront Chemotherapy Followed by CRT (SCPRT) and TME
A more effective systemic treatment against micrometastatic disease should be a priority in the new generation of clinical trials in LARC. Current figures for distant disease in stages II and III overall are 30% and are still significantly higher in some subgroups of patients with poor prognostic features (i.e. T4, N2 or EMVI-positive tumours) [30•, 36, 37]
Upfront CT offers some theoretical advantages, such as the early treatment of micrometastases, with optimal exposure to systemic CT, rapid relief of local symptoms and a shorter time to cope with a stoma. This strategy, however, may also be associated with its own caveats, such as selection of radioresistant clones, induction of accelerated repopulation [38] and overtreatment if we are not able to detect high-risk patients accurately. However, although currently a reliable assessment of the pretherapeutic mesorectal regional lymph node status is not possible by the present imaging modalities, thin-slice high-resolution MRI can identify accurately the extent of extramural tumour spread, the EMVI and the status of MRF [36, 39]
Evidence from Selected Series
The British Expert trial showed that intensification of systemic therapy with neoadjuvant combination oxaliplatin-based CT before CRT and surgery was feasible in high-risk potentially operable rectal cancer, with acceptable safety and promising outcomes [40•].
Based on these encouraging results, the Spanish GCR-3 completed a randomized phase II trial in a high-risk population selected by MRI, comparing this approach with conventional preoperative CRT followed by surgery and postoperative adjuvant CT. Primary results were reported in 2010 [41••]. Compared with postoperative adjuvant capecitabine-oxaliplatin (CAPOX), induction CAPOX before CRT had similar pCR and complete resection rates while at the same time achieving a significant more favourable compliance (51 vs. 92% in the adjuvant vs. induction group (p = 0.0001) and toxicity profiles (g3/4 toxicities 51% during adjuvant vs. 19% during induction (p = 0.0004) [41••]. Updated results of this trial showed that delay from diagnosis to surgery due to the induction CT had no negative impact in 5-year LR (2.1 and 2% in the R0 + R1 population in the adjuvant vs. induction arm) 5-y DFS Five-year DFS in both arms (64% in adjuvant vs. 62% induction arm) [42].
Two randomized phase II trials [43, 44] and several single-arm studies [45,46,47,48] have been published since 2010. The randomized phase II EXPERT-C study evaluated, in high-risk patients selected by MRI, the addition of cetuximab to neoadjuvant CT before CRT [43]. As compared with induction CAPOX, the addition of cetuximab to neoadjuvant CT did not improve either pCR or 5-year PFS and OS, although a significant increase in radiological response rate was observed. In a biomarker subanalysis, TP53 wild-type patients (n = 69) who received cetuximab had a statistically significant better PFS (89.3 vs. 65.0%; p = 0.02) and 5-year OS (92.7 vs. 67.5%; p = 0.02) [49].
Marechal et al. compared preoperative CRT vs. induction FOLFOX followed by CRT. Adjuvant CT was at the discretion of the treating physician. pCR rate was similar, but higher overall toxicity was associated to the induction group [44].
Single-arm series included patients with middle and distal third cancers, clinical stage T3-T4/N+, except series from Perez et al. that also included some patients with clinical stage T1-T2/N+. Three used CAPOX, 2 or 4 cycles before CRT, and two used FOLFOX as the induction regimen. Compliance was high for both induction CT (85–92%) and CRT (83–100%). pCR rates varied between 20 and 36%. Notably, the higher pCR was seen in the trial that combined bevacizumab with induction CT and with CRT, but in this trial, 24% of patients required reoperation. High 4% mortality rate occurred in the series using CAPOX as induction CT.
The UK COPERNICUS multicentre phase II study evaluated the strategy of induction CT prior to SCPRT (instead of CRT) with 8 weeks of FOLFOX-like induction CT before SCPRT, then immediate surgery. Of 60 enrolled patients (88% cStage III), 57 underwent surgical resection with 100% complete resections (R0 surgical resections) and a 12% pCR [50]. The main aspects of these phase II trials are summarized in Table 1.
Ongoing Trials with Induction CT Followed by CRT
Efforts to augment the standard backbone of cytotoxic CT (fluoropyrimidine and oxaliplatin) with novel therapeutics are well under way.
The Spanish GEMCAD group is testing the inclusion of anti-vascular endothelial growth factor (VEGF) therapy with neoadjuvant FOLFOX (5FU, leucovorin and oxaliplatin). The induction FOLFOX with or without aflibercept followed by chemoRT in high risk locally advanced rectal cancer (RIA Trial) (NCT02340949; GEMCAD-1402) is a randomized phase II multi-centre study. The primary hypothesis is that the administration of aflibercept and CT prior to chemoRT and surgery can improve efficacy (via normalization of the tumour vasculature) without compromising safety (by moving surgery further away from VEGF administration). Primary endpoint is pCR.
Two French randomized trials are ongoing: Prodige23 is a phase III comparing preoperative CRT vs. induction CT with 6 cycles of FOLFIRINOX followed by CRT and surgery for patients with resectable high-risk LAR cancer with 3-year DFS as the primary endpoint (NCT01804790). The GRECCAR 12 compares 4 cycles of FOLFIRINOX followed by CRT vs. standard CRT. All patients would then proceed to undergo either conventional surgery or local excision depending on response. Patients must have cT2 or cT3 tumours, and the primary study end point is rate of organ preservation and absence of stoma at 1 year (NCT02514278)
Upfront Chemoradiation/Radiation
Given the three radiotherapy options described above (CRT, SCPRT and HD-BRT), investigators have explored a number of different strategies to intensify neoadjuvant treatment to increase the rate of complete clinical response and pCR and improve long-term oncological outcomes. Histopathological evidence after CRT suggests about a third of patients have tumours that are resistant and not down-staged by CRT [51, 52].
These intensification strategies include escalation of the radiotherapy component or adding CT before, concurrently or after the radiotherapy/CRT. The intensity of SCPRT does not easily allow integration of concurrent preoperative CT, although it could be argued that standard fluoropyrimidine-based CRT does not utilize fully systemic doses of CT. Most intensification strategies have focused on a CRT platform, but SCPRT also allows some integration of induction [50], concurrent [53] and consolidation CT [54, 55] prior to, during and following SCPRT, respectively.
Intensification of Radiotherapy
NCCN guidelines recommend preoperative CRT (45–50 Gy in 25–28 fractions, with an optional boost of 5.4 Gy in 3 fractions), SCPRT and/or neoadjuvant CT in a total neoadjuvant approach. The overall duration inclusive of CT and radiation therapy should not exceed 6 months (NCCN guidelines 2016) [56]. This represents a relatively modest total dose (45–50.4 Gy).
A phase III randomized trial comparing a novel arm, where both oxaliplatin was added and radiotherapy escalated to 50 Gy against a control arm using capecitabine and 45 Gy, increased the pCR rate and concluded that 50 Gy is a new standard [57].
The efficacy could be enhanced by dose escalation of the radiotherapy with additional fractions of external beam radiotherapy by adding an HD-BRT boost or by shortening the overall treatment time. These strategies can be achieved by intensity-modulated radiotherapy (IMRT) using a simultaneous integrated boost [58] because of the increased precision of IMRT and image-guided radiotherapy [59].
Studies imply a significant dose-response relationship for radiotherapy within preoperative CRT regimens [60, 61]. The former derived a theoretical model suggesting that to achieve a pCR in 50% of cases requires a dose escalation to at least 90 Gy to primary tumour [60]. However, such doses cannot be delivered safely via external beam treatments and require an HD-BRT boost, but it is harder to escalate nodal doses in the pelvis safely. When a randomized trial explored the escalation of RT dose within CRT with a HD-BRT boost, there was no increase in the pCR rate [62]. The number of R0 resection rates increased, but long-term outcomes did not improve. A prospective study in small early cancers dose-escalated radiotherapy within CRT (60 Gy in 30 fractions to primary tumour, 50 Gy in 30 fractions to elective lymph node volumes) with a 5-Gy HD-BRT boost [63]. Preliminary results showed 40 patients achieved a clinical CR, and local recurrence at 1 year was only 15·5% with acceptable sphincter function.
Intensification of CRT
In rectal cancer, more sophisticated computer technology, imaging and radiation delivery have been matched by the development of an increasing array of biological agents. However, improvements in concurrent chemoradiotherapy have not kept pace, and fluoropyrimidines remain the mainstay of concurrent chemoradiation regimens. Other cytotoxic and biological agents have been investigated as partners of 5FU-based chemoradiation, but without consistent success.
Fluoropyrimidine-Based Chemoradiation
In the original randomized phase III trials, the addition of 5FU to preoperative radiation tripled the pCR rate from 4–7% to 12–17% and reduced local recurrence [5, 6, 64]. The short half-life of 5FU supports the use of prolonged venous infusions of 5FU to radiosensitize every radiotherapy fraction. This method of delivery administers near to maximum doses with minimal toxicity. Hence, prolonged venous infusions of 5-fluorouracil or oral capecitabine are recommended rather than bolus 5FU [65, 66]. Capecitabine at a dose of 800–900 mg/m2 twice daily for 5 days each week (Monday–Friday) of the 5-week radiation therapy is considered standard practice in many countries.
Irinotecan-Based Chemoradiation
Small initial phase II studies suggested irinotecan added to standard fluoropyrimidine-based CRT increased response rates [67, 68], but a randomized trial (RTOG0012) showed no benefit from a combination of weekly irinotecan to continuous infusional 5FU and concurrent radiation in 106 patients with T3–T4 distal tumours [69]. A current ongoing phase III randomized trial has randomized over 400 patients in the UK (ARISTOTLE) between capecitabine-based CRT, the same combination with concurrent weekly irinotecan in MRI-defined high-risk rectal cancer (www.controlled-trials.com/ISRCTN09351447).
Oxaliplatin-Based Chemoradiation
Early attempts in phase II trials to integrate oxaliplatin into fluoropyrimidine-based CRT showed promising results in terms of high rates of pCR. Consequently, five phase III trials (STAR-01, ACCORD 12/0405 PRODIGE 2, NSABP R-04, PETACC-6 and the CAO/ARO/AIO-04 study) [24,71,72,, 66, 70–73] and two additional trials performed in China [35•, 74] were designed to add oxaliplatin to concurrent 5FU or capecitabine-based chemoradiotherapy. Most added oxaliplatin as a radiosensitizer (with low weekly doses, i.e. 50–60 mg/m2 rather than systemically active doses of 85–130 mg/m2). The results have been generally disappointing. In four out of five of these randomized phase III trials, the chemoradiation arm adding oxaliplatin was associated with a significant increase in grade 3–4 acute gastrointestinal toxicity [66,71,72,, 70–73]
Two trials showed an increase in the pCR rate [24, 71], but in only one of these was the increase statistically significant [24]. Two trials suggest oxaliplatin may improve DFS [74, 75]. However, the design of the German trial is partly flawed by an inadequate control arm and the inclusion of oxaliplatin as postoperative adjuvant CT [75]. Hence, it is difficult to determine whether the preoperative chemoradiation component, the postoperative adjuvant component or both improve the DFS. Given the increase in toxicity without clear outcome benefit, concurrent oxaliplatin is not routinely recommended as an addition to fluoropyrimidine-based CRT outside clinical trials. Meta-analyses suggest oxaliplatin may increase pCR rates, but enhances acute toxicity [76].
Consolidation Chemotherapy after CRT/SCPRT
The introduction of additional chemotherapeutic agents such as oxaliplatin and irinotecan and the proven role of oxaliplatin in the adjuvant setting in colon cancer—plus a variety of targeted agents—have stimulated the use of neoadjuvant CT as an additional strategy to achieve systemic control. A long interval/delay after CRT has traditionally been recommended partly to allow the acute inflammatory and immunosuppressive effects of CT and RT to subside and lessen the risk of surgical complications. Additional systemic ‘consolidation’ CT before surgery could increase response rates and enable more patients to be managed nonoperatively. In series where radical surgery is performed after CRT, sequential additional courses of FOLFOX after chemoradiation increased the pCR rate from 18% with CRT alone and surgery performed at 8 weeks to 38% when six courses of FOLFOX were administered and surgery was performed at 19 weeks [77].
SCPRT followed by immediate surgery does not achieve tumour regression, but delayed surgery can result in substantial downstaging for patients with involvement of the MRF [78,79,80]. This longer interval has also been extrapolated to the use of SCPRT, which potentially allows the introduction of CT. An American study used SCPRT followed by 4 course of mFOLFOX6 in 44 evaluable patients (4 staged as cT4 and 40 as cT3) reported histopathological downstaging to ypT0-2 in 75% of patients and to ypT0 in 30% [54].
The Dutch Colorectal Group treated patients presenting with rectal cancer and synchronous resectable metastases with SCPRT followed by six cycles of capecitabine and oxaliplatin plus bevacizumab in the Dutch M1 phase II trial. Compliance to CT was very high and 90% received ≥4 cycles. In total, 36/50 (72%) of patients eventually underwent radical surgical treatment [81].
A randomised trial in patients with fixed cT3 or cT4 or locally recurrent rectal cancer showed this combination (SCPRT and FOLFOX) achieved a microscopically radical resection ie R0 (primary endpoint) resection rate of 77% and a 16% pCR rate with this schedule [17••].
This concept is tested in ‘The Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation’ (RAPIDO) trial [55]. The trial randomly assigned patients with more locally advanced rectal cancer to SCPRT followed by six cycles of oral capecitabine with intravenous oxaliplatin, and then TME vs. preoperative long-course CRT followed by TME and optional postoperative CT. Results are awaited.
Following neoadjuvant treatment (and prior to surgery), MRI can define different types of response (fibrous, desmoplastic and colloid), categorize histopathologic TN downstaging and accurately define a radiologic tumour regression grade (mriTRG). If sufficient downstaging/downsizing is confirmed, the surgeon may modify the initial treatment plan or even avoid surgery. In contrast, failure to respond can potentially be salvaged by additional CT at this point, prior to surgery, rather than waiting several months until surgery has taken place and the patient recovered sufficiently.
An alternative strategy is to alternate CT and RT. If RT when extended is susceptible to repopulation, integrating systemic CT between a split course of RT may minimize such repopulation [82].
Conclusions
Upfront therapy with CT alone or administered before or after CRT /SCPRT followed by surgery represents a rational way of treating LARC. Under- or overtreatment remains a potential problem, so optimal staging is necessary for accurate risk assessment. In middle third cT3 tumours with free MRF, CT alone has shown good efficacy in phase II studies. Its definitive role is being evaluated in phase III studies. In higher-risk patients, induction CT prior to CRT has not impacted adversely on local control and been associated with better compliance and lower toxicity. Given these advantages, this induction strategy is now considered a new treatment alternative for patients with high-risk rectal cancer in some European institutions and the USA.
Following SCPRT or CRT with systemic ‘consolidation’ chemotherapy is a further rational approach which obeys De Ruysscher’s Principle of SER (the interval between the start of treatment and the end of radiotherapy), which ideally should be as short as possible to avoid repopulation [83] In the RAPIDO trial, the SCPRT arm allows earlier administration of full systemic chemotherapy compared to CRT. Hence, results of this trial could change practice.
In addition, since metastatic disease is now the predominant cause of recurrence and death, many believe that systemic neoadjuvant CT might, in selected cases, substitute for CRT. However, any future results for neoadjuvant CT alone will need to be benchmarked against the outcomes with SCPRT and CRT in terms of R0 resection anastomotic leaks, failure to reverse a temporary colostomy and local recurrence.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gastrointestinal Tumour Study Group–GiTSG 7175. Prolongation of disease free interval in surgical treated rectal carcinoma. N Engl J Med. 1985;312:1464–72.
Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–15. 5.
Sauer R, Becker H, Hohenberger W, German Rectal Cancer Study Group, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-T4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–25.
Bosset JF, Collette L, Calais G, et al. Chemotherapy with pre-operative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23 13.
Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26(22):3687–94.
Stockholm Colorectal Cancer Study Group Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996;3:423–430.
Anon. Improved survival with preoperative radiotherapy in resectable rectal cancer: Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987.
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46.
Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–20.
Vuong T, Devic S. High-dose-rate pre-operative endorectal brachytherapy for patients with rectal cancer. J Contemp Brachytherapy. 2015;7(2):183–8.
Breugom AJ, Vermeer TA, van den Broek CB, Vuong T, Bastiaannet E, Azoulay L, et al. Effect of preoperative treatment strategies on the outcome of patients with clinical T3, non-metastasized rectal cancer: a comparison between Dutch and Canadian expert centers. Eur J Surg Oncol. 2015;41(8):1039–44.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Pudełko M, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72(1):15–24.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomised trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23.
Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol. 2012;30(31):3827–33.
•• Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Polish Colorectal Study Group. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42. Very important Randomised trial examining short course preoperative radiotherapy (SCPRT) followed by FOLFOX and long course chemoradiation (CRT). There were no differences were observed in local efficacy (pCR and R0 resection rate) between 5 × 5 Gy with consolidation FOLFOX chemotherapy versus long-course chemoradiation.But, an improved overall survival and lower acute toxicity favoured the 5 × 5 Gy schedule with FOLFOX consolidation chemotherapy.
• Ansari N, Solomon MJ, Fisher RJ, et al., Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann Surg. 2016. Randomised trial examining short course preoperative radiotherapy (SCPRT) and long course chemoradiation (CRT). There were significantly less AEs for SCPRT compared with CRT but no statistically significant differences in postoperative surgical complications.
Van Gijn W, Marijnen C, Nagtegaal I, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82.
Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90.
Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–62.
Sainato A, Cernusco Luna Lunzia V, Valentini V, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113:223–9.
Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomised phase III trial. Ann Oncol. 2015;26:696–701.
Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–87.
Hong Y, Nam B, Kim K, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53.
Marijnen C, Van de Velde C, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–58.
MERCURY. Study Group: Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779.
Chang GJ, Agarwal A, Maru DM, et al. Histopathologic responses in primary tumors of patients with colorectal carcinoma after neoadjuvant systemic chemotherapy alone. European Society of Medical Oncology 12th World Con- gress on Gastrointestinal Cancer, Barcelona, España, June 30-July 3, 2010 (abstr P-0032).
•• Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–8. First trial demonstrating encouraging local activity with high PCR rate with systemic chemotherapy only (no CRT) in intermediate-risk rectal cancer.
• Fernandez-Martos C, Brown G, Estevan R, et al. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19(10):1042–3. First phase II multicentric trial with MRI selected high/intermediate-risk patients, confirming local activity of neoadjuvant systemic chemotherapy treatment.
Patel U, Brown G, Machado I, et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for Primary Rectal Cancer: longterm results from the GEMCAD 0801 trial. Ann Oncol. In press.
Uehara K, Hiramatsu K, Maeda A, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: NSOG 03 phase II trial. Jpn J Clin Oncol. 2013;43(10):964–71.
Hasegawa J, Nishimura J, Mizushima T, et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73(5):1079–87.
Ishii Y, Hasegawa H, Endo T, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36(11):1061–5.
• Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34(27):3300–7. This randomised trial compares neoadjuvant chemotherapy alone versus two CRT regimes (with and without oxaliplatin) in cT3/T4 patients. Preliminary results showed better local efficacy parameters in the combined modality arms but with similar R0 resection rates and more toxicity.
Chand M, Bhangu A, Wotherspoon A, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25:858–63.
Bhangu A, Fitzgerald JE, Slesser A, et al. Prognostic significance of extramural vascular invasion in T4 rectal cancer. Color Dis. 2013;15(11).
Glynne-Jones R, Grainger J, Harrison J, et al. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: should we be more cautious? Br J Cancer. 2006;94:363–71.
Taylor F, Quirke P, Heald R, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY Study. J Clin Oncol. 2013;32:34–43.
• Chua Y, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–48. This phase II trial demonstrated that the strategy of induction with chemotherapy at therapeutic doses prior to CRT and surgery was feasible with good activity.
•• Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiation followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiation and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 Study. J Clin Oncol. 2010;28(5):859–65. Phase II randomized trial demonstrating better compliance and less toxicity of chemotherapy when given before than after CRT and surgery without compromise of local control.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722–28.
Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. 2012;30(14):1620–7.
Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30.
Nogue M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging–defined poor-prognosis locally advanced rectal cancer: the AVACROSS Study. Oncologist. 2011;16:614–20.
Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–33.
Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Cancer Netw. 2014;12:513–9.
Perez K, Safran H, Sikov W, et al. Complete neoadjuvant treatment for rectal cancer: the Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2014.
Sclafani F, Gonzalez D, Cunningham D, et al. TP53 mutational status and cetuximab benefit in rectal cancer: 5-year results of the EXPERT-C trial. J Natl Cancer Inst. 2014;106(7).
Gollins S, Sebag-Montefiore D, Adams R, et al. A phase II single arm feasibility trial of neoadjuvant chemotherapy (NAC) with oxaliplatin/fluorouracil (OxMdG) then short-course preoperative radiotherapy (SCPRT) then immediate surgery in operable rectal cancer (ORC): COPERNICUS (NCT01263171). J Clin Oncol. 2015;33(suppl; abstr 3609).
Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowska-Guttmejer A, Tokar P, et al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: an analysis of outcomes in a randomized trial. Int J Radiat Oncol Biol Phys. 2007;67(2):369–77.
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Yeo SG, Oh JH, Kim DY, Baek JY, Kim SY, Park JW, et al. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase 2 multicenter study (KROG 10-01). Int J Radiat Oncol Biol Phys. 2013;86(1):3.
Myerson RJ, Tan B, Hunt S, Olsen J, Birnbaum E, Fleshman J, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys. 2014;88(4):829–36.
Nilsson PJ, van Etten B, Hospers GA, Påhlman L, van de Velde CJ, Beets-Tan RG, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279. doi:10.1186/1471-2407-13-279.
National Clinical Practice Guidelines in Oncology (NCCN Guidelines): Rectal Cancer Version 2.2017 — December 22, 2016 www.ncrn.org. (last accessed 01/01/2017).
Gerard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30(36):4558–65.
Hernando-Requejo O, López M, Cubillo A, et al. Complete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiation. Strahlenther Onkol. 2014;190(6):515–20.
Engels B, Tournel K, Everaert H, et al. Phase II study of preoperative helical tomotherapy with a simultaneous integrated boost for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):142–8.
Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):74–80. 35.36.
Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113(1):1.
Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84(4):949–5.
Appelt AL, Vogelius IR, Pløen J, Rafaelsen SR, Lindebjerg J, Havelund BM, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–27.
Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol. 2005;23(24):5620–7.
Hoffheinz RD, Wenz F, Post S. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32(18):1927–34.
Navarro M, Dotor E, Rivera F, et al. A Phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66(1):201–5.
Gollins S, Sun Myint A, Haylock B, et al. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcome. J Clin Oncol. 2011;29(8):1042–9.
Mohiuddin M, Paulus R, Mitchell E, Hanna N, Yuen A, Nichols R, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2013;86(3):523–8.
Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773–80.
Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–44.
Schmoll H-J, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: first results of the PETACC-6 randomized trial (abstract). J Clin Oncol. 2013; 31(suppl; abstr 3531).
Allegra CJ, Yothers G, O’Conelll MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11).
Jiao D, Zhang R, Gong Z, Liu F, Chen Y, Yu Q, et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: a 3-year follow-up study. Chin J Cancer Res. 2015;27:588–96.
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–89.
Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D, et al. Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta-analysis. Oncotarget. 2016;7(29):45513–24.
Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Timing of rectal cancer response to chemoradiation consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66.
Radu C, Berglund Å, Påhlman L, Glimelius B. Short course preoperative radiotherapy with delayed surgery in rectal cancer—a retrospective study. Radiother Oncol. 2008;13:343–9.
Hatfield P, Hingorani M, Radhakrishna G, Cooper R, Melcher A, Crellin A, et al. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol. 2009;13:210–4.
Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg. 2012;13:577–83.
van Dijk TH, Tamas K, Beukema JC, Beets GL, Gelderblom AJ, de Jong KP, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24(7):1762–9.
Michael M, Chander S, McKendrick J, MacKay JR, Steel M, Hicks R. Phase II trial evaluating the feasibility of interdigitating folfox with chemoradiotherapy in locally advanced and metastatic rectal cancer. Br J Cancer. 2014;111(10):1924–31.
De Ruysscher D, Pijls-Johannes MM, Bentzen S, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24(7):1053–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carlos Fernandez-Martos and Alfonso Garcia-Fadrique declare that they have no conflict of interest. Rob Glynne-Jones has received research funding through grants from Roche and Merck Serono, has received speaker’s honoraria from Roche, Amgen, Servier, Sanofi and Merck Serono and has received compensation from Eli Lilly, Roche, Home Nutrition, Servier, Sanofi, Eisai and Amgen for service on advisory boards.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Radiation Therapy and Radiation Therapy Innovations in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Fernandez-Martos, C., Fadrique, A.G. & Glynne-Jones, R. Optimal Sequencing of Neoadjuvant Therapies (NAT) in Rectal Cancer: Upfront Chemotherapy vs. Upfront Chemoradiation. Curr Colorectal Cancer Rep 13, 154–164 (2017). https://doi.org/10.1007/s11888-017-0358-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-017-0358-5