Abstract
A fraction of patients with rectal cancer can achieve clinical complete response following long-course chemoradiotherapy (CRT), and there is accumulating clinical evidence that these patients can be managed non-surgically with acceptable oncological outcome. Consequently, strategies for increasing the proportion of complete responders are actively being explored. Some, although limited, experience with high-dose radiotherapy indicates that there might exist a dose-response relationship for local tumour control after radiotherapy alone. Thus, tumour dose escalation could be indicated for selected patients, particularly in cases with small tumours and limited local disease. This report discusses several radiotherapy techniques for tumour boosting, focusing on technical challenges and clinical experiences with each technique. Specifically, external beam radiotherapy, brachytherapy and contact X-ray treatment for dose escalation are considered. Ultimately, no technique provides definitive advantage over others, and the choice in clinical practice will have to depend on the patient population treated as well as the technical capabilities of the treating department.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organ-preserving and non-surgical strategies for treatment of rectal cancer have been the subject of considerable attention in the last decade [1•]. Tumours in the upper or middle part of the rectum can usually be managed with resection, i.e. without the need for a permanent colostomy. However, many patients with low cancers will—unless the tumours are very small (T1, early T2)—be treated with an abdominoperineal resection (APR) and a permanent stoma, a mutilating procedure with substantial impact on quality of life [2–4]. Patients often express a strong wish to avoid a colostomy if possible [5], and some elderly and heavily co-morbid patients may not be candidates for radical surgery at all [6, 7]. Consequently, alternative treatment strategies for local tumour control are currently the focus of significant research efforts.
Radiotherapy, used as (neo-)adjuvant treatment, has been shown in randomized trials to reduce the risk of local recurrence, even with optimal surgical techniques [8, 9]. The current standard in many parts of the world is to treat locally advanced disease with long-course chemoradiotherapy (CRT), followed by surgery 6–12 weeks after the end of treatment. The accepted treatment regimen consists of 45–50 Gy delivered in 1.8–2.0 Gy fractions to the tumour and regional lymph nodes, with concomitant fluorouracil-based chemotherapy. With this approach, the majority of patients will present with some degree of tumour regression at the time of surgery [10], and a subset of patients will even show pathological complete response (pCR) to the neoadjuvant treatment, i.e. no remaining tumour cells in the pathological specimen [11]. This has raised substantial interest as to whether selected patients can be treated with CRT alone, and some groups have allowed patients with clinical complete response (cCR) after radiotherapy to defer surgery and follow a close surveillance program instead. A small number of studies have reported encouraging outcomes with careful patient selection and close follow-up (the so-called watchful waiting or watch-and-wait approach) [12–16], including one prospective trial conducted by our own group [17••]. There is still a great deal of uncertainty, however, as to the optimal treatment strategy for maximising the probability of tumour control with CRT alone, especially with respect to the choice of radiotherapy treatment regimen and technique.
Rationale for Dose-Escalation
Predictive factors for pCR after CRT have previously been examined in individual studies and in meta-analyses (see, e.g. [18]). Radiation dose does seem to play a role, with higher doses resulting in higher pCR rates. Studies by our own group demonstrated the existence of a dose-response relationship for pathological tumour regression [19] and provided a quantitative estimate for the effect of dose [20•]. A meta-analysis of studies utilizing high-dose pre-operative radiotherapy also concluded that the use of radiation dose ≥60 Gy results in high rates of pCR. Collectively, these studies indicate that the number of patients with good response to CRT may be optimized by escalating the radiation dose to the tumour. It is worth noting, though, that pCR does not work well as a surrogate endpoint for evaluation of pre-operative treatment strategies [21–23], i.e. increased rates of pCR do not appear to translate into clinical benefit for patients who subsequently undergo surgery. Similarly, there is currently not enough data to evaluate whether treatment approaches that optimize cCR rates will also result in more patients with long-term tumour control without surgery— on the contrary it may be that the additional patients converted to responders by the higher doses ultimately end up with tumour recurrence anyway, i.e. no local control.
There are some suggestions, however, that the use of high-dose radiotherapy may allow for a higher rate of local control after non-surgical treatment than with standard radiation doses. The seminal work done by the Brazilian group lead by Habr-Gama, which established watch-and-wait for clinical complete responders as a feasible and safe treatment strategy, has been reported in a series of publications [12, 14, 24–27, 28•, 29••]. Of those, the one that reports the highest rate (50 %) of local control with CRT alone is a study combining higher tumour dose (54 Gy) with additional chemotherapy in the interval between end of radiotherapy and response assessment [28•]. Gérard and colleagues have been using combinations of external beam radiotherapy, contact X-ray treatment (Papillon treatment, see below) and interstitial brachytherapy for very high dose irradiation of (mainly inoperable) rectal cancers and have historically demonstrated very high rates of response and acceptable local control [16]. Finally, our own recently published trial of watchful waiting after high-dose external radiotherapy (60 Gy) plus endocavitary brachytherapy (5 Gy) reported over half of all treated patients with T2-3 cancers to have local control without surgery at 2 years [17••]. These reports can be compared to the small number of publications that describe watch-and-wait after standard (45–50 Gy) CRT; in general 10–20 % of treated patients are reported to have clinical complete response and/or long-term non-surgical control [13, 15, 30, 31]. Any attempt at understanding the dose-response relationship of clinical response is complicated, however, by the confounding effect of tumour stage. The degree of tumour regression, and especially the probability of complete or near-complete response seem—as would be expected—to relate to the pre-treatment tumour stage, such that smaller tumours and patients with less advanced disease are more likely to respond well to radiotherapy. This has been observed for pathological response grade [10, 11, 20•] and will likely be the case for clinical response as well. The Brazilian study discussed above [28•] included a substantial number of patients with T2N0 disease, and over half of the patients in the Danish watchful waiting trial had T2 tumours. This probably affected the rates of complete responders observed in the studies. A simple estimate of the radiation dose-response for local control after definitive CRT, based on selected studies, is presented in Fig. 1.
Local control at 2 years with chemoradiotherapy alone, as the proportion of all treated patient, in selected studies of non-surgical management of rectal cancer. Dose is given in grays [Gy] in equivalent dose in 2-Gy fractions (EQD2), using an α/β value of 10 Gy. Studies were selected based on availability of information on total number of patients treated, local control at 2 years amongst patients managed non-surgically (i.e. allowed to be followed with observation), and radiotherapy dose delivered. The data point for Habr-Gama et al. [29••] is based on the newest publication from the Brazilian group; radiotherapy was reported as “50.4–54 Gy”, and hence the average (52.2 Gy) has been used. Vuong et al. [47] is based on data for medically inoperable patients treated with combined external beam and brachytherapy, which are only published in abstract form. The radiotherapy regimen consisted of 40 Gy in 15 fractions delivered with external beam radiotherapy, as well as 30 Gy brachytherapy given weekly in three fractions. For the brachytherapy treatments, the prescription dose of 10 Gy per fraction has been used for calculation of EQD2 dose, without any attempt at estimating the actual tumour dose delivered. Appelt et al. [17••] is from our own prospective study of high-dose radiotherapy and brachytherapy for definitive treatment of low rectal cancer. The tumour dose is calculated from the external beam dose (60 Gy) combined with exact brachytherapy dose calculations for a subset of patients treated with MRI-guided brachytherapy (12.9 Gy). The curve shows the dose-response for major pathological tumour regression (Mandard regression grade 1–2) as estimated from the model in Appelt et al. [20•]—i.e. the curve is not fitted to the data points
In summary, traditional long-course CRT with standard radiation dose and fractionation has been optimized to limit local recurrence after surgery while keeping the risk of additional toxicity as low as possible. While this treatment regimen does induce tumour regression in many patients, it may not provide optimal therapeutic ratio for non-surgical strategies—both dose schedule and treatment technique may need to be optimized for use in a definitive rather than neoadjuvant setting. Specifically, there might be a rationale for radiation dose escalation in rectal cancer patients where the therapeutic aim is local tumour control without surgery. The standard dose of 45–50 Gy appears to be sufficient to control nodal disease in patients with limited, N0-1, clinical lymph node involvement, and therefore dose escalation is mainly relevant for the primary tumour. The question then remains as to which technique to use for dose escalation. The main options are external beam radiotherapy, endocavitary brachytherapy, and contact X-ray therapy; we will discuss these techniques in turn.
External Beam Radiotherapy
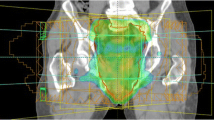
Increasing the radiation dose with external beam radiotherapy is technically relatively simple: The use of an external boost is familiar from other cancer sites, e.g. anal cancer, and has previously been integrated into the neoadjuvant treatment of locally advanced rectal cancer [32–34]. As such, many of the technical challenges of treatment delivery are well understood, including estimation of treatment margins and use of imaging for treatment verification. Additionally, employing an external boost has the advantage that integration into standard treatment regimens is straightforward, and this can be done using equipment available in all radiotherapy departments.
External beam boost treatment allows for delivery of a homogeneous dose distributions over the entire tumour; i.e. with no hot spots in tumour or surrounding normal tissue. However, the volume treated will be relatively large: External beam radiotherapy does not allow for very sharp dose gradients, and the treated volume has to take into account uncertainties in daily treatment delivery, such as organ movement. This can result in large parts of the mesorectum—and for very low tumours, sphincter musculature—to be irradiated to high doses alongside the tumour. Modern intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) techniques do alleviate some of this problem. They provide dose distributions that are closely shaped to the treatment planning volume (PTV)—i.e. the amount of excess normal tissue irradiated will be limited, compared to traditional 2D or 3D conformal techniques [35–37]. In our experience, using IMRT for integrated boost treatment (see below) to 60 Gy allows for a ratio of ~0.85 between the high dose PTV and the total volume treated to 95 % of the prescription dose (i.e. V 57Gy) for an average patient. Ultimately, though, the escalation dose level is limited by co-irradiation of normal tissue inside the PTV, and hence, most reports use boost doses of no more than 10–15 Gy in addition to an elective volume dose of 45–50 Gy.
Two different techniques can be used for delivery of an external boost: integrated (also called concomitant) boost or sequential boost. An integrated boost is delivered by increasing slightly the daily treatment dose to the tumour compared to the elective volume; e.g. a treatment plan with 60 Gy tumour dose and 50 Gy elective dose in 30 fractions requires a daily dose to the tumour of 2 Gy and a daily dose to the elective volume of 1.67 Gy. An integrated boost is robust to treatment delivery uncertainties, as the dose gradient from the boost volume to the elective volume is relatively shallow [17••, 38]. Additionally, the use of an integrated boost allows for the overall treatment time to be kept short, if so desired. A sequential boost involves limiting the treatment fields to the tumour only on additional treatment days, either prior to or following the primary treatment. This allows for adaptive treatment strategies, e.g. with re-planning and re-optimization for the boost treatment, to ensure the smallest possible boost volume, or for use of additional imaging and surveillance on boost treatment days [39]. The tumour will often be visible on cone beam computed tomography (CT) early in the treatment course, so treatment verification will be easier if the sequential boost is given prior to the primary treatment. On the other hand, delivering the boost after the primary treatment may allow for a smaller boost treatment volume, as most tumours will have shrunk after 4–5 weeks of treatment. A combined approach is also possible, using an adaptive strategy with re-planning halfway through the treatment, individual margins based on on-treatment imaging, and the boost delivered as an integrated part of the second half of the treatment course.
The published experience with external beam boost for definitive CRT for rectal cancer is limited. Habr-Gama et al. [28•] treated 70 patients using 3D conformal radiotherapy with concomitant 5-FU, with primary treatment consisting of 45 Gy in 25 fractions to the tumour and lymph nodes, followed by a 9 Gy sequential boost to the tumour, and additional 5-FU after end of radiotherapy. They found that around 45 % of the patients had long-term local control without major surgery or local excision. The Danish watchful waiting trial treated patients with an integrated boost technique (60 Gy to the tumour, 50 Gy to elective volumes, in 30 fractions) with concomitant peroral UFT, plus a 5 Gy endocavitary tumour boost [17••]. Fifty-eight percent of patients had local control with CRT alone at 2 years; it is not clear, however, how much of the tumour control could be attributed to the external beam dose and how much to the brachytherapy boost.
Endocavitary Brachytherapy
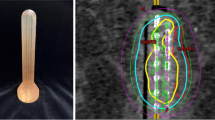
Endocavitary brachytherapy for rectal tumours is typically delivered as high dose rate (HDR) treatment with an iridium-192 source, using a remote afterloader with a cylindrical applicator. The applicator can be either rigid [40] or flexible [41], and a number of applicator designs exist: single, central channel designs or multichannel designs, with multiple peripheral channels (and optionally a central channel). Designs also differ in whether they integrate shielding capabilities. Shields are inserts of high-density material, usually lead or tungsten, that allow for protection of the uninvolved part of the rectal mucosa from the radioactive source. Some designs use a central, cylindrical shield [42, 43], others a semicircular shield covering part of the applicator periphery [40]. Newer, more innovative designs also exist, e.g. with channels arranged symmetrically in grooves on the surface of a solid applicator made of high-density material [44]. These novel designs allow for highly conformal delivery of dose to the tumour but have not yet been tested in clinical practice.
Optimal delivery of endorectal brachytherapy can be technically and logistically challenging. High-precision treatment requires imaging of the target volume with the applicator in place, and normal procedure is to perform treatment planning and treatment in the same session, directly after applicator placement and imaging. Early experience was based on simple, orthogonal X-rays for verification of applicator position, without individual treatment optimization [45]. For this approach, placement of radio-opaque clips at the proximal and distal edges of the tumour provides a minimum of optimization regarding tumour length. However, in order for full treatment planning to be feasible, 3D imaging is needed, and this must be acquired with the applicator in place, as the applicator distorts local anatomy. Computed tomography (CT) is useful with respect to dose calculation, as it easily permits for heterogeneity corrections [43], but while gross local anatomy can be visualised on CT, differentiation between tumour and normal rectum can be suboptimal. Magnetic resonance imaging (MRI) provides superior visualisation of the tumour [46], but dose calculation in most commercial dose planning systems is limited to dose-to-water with no heterogeneity corrections. Additionally, MRI compatibility requirements place limits on applicator construction, especially shield materials.
Brachytherapy treatment results in very inhomogeneous dose distributions and can thus provide very high doses to the tumour with limited irradiation of nearby normal tissue. As an example, consider an applicator with a 2-cm diameter and a single, central channel. If dose is prescribed at 1 cm from the applicator surface, then the dose 2 cm from the surface is ∼50 % of the prescription dose and ∼25 % further 1 cm out [46]. However, dose at applicator surface is excessive; in the previous example, the surface dose will exceed 300 % of the prescription dose—higher if prescription is more than 1 cm from the rectal wall, e.g. if the tumour thickness exceeds this depth. Thus, normal tissue close to the applicator, mainly the rectal mucosa, will be exposed to very high doses. Techniques have been developed to mitigate this: Shielding was discussed above, and some centres have been using an approach with water- or iodine-filled balloons, which displace the rectal mucosa away from the radiation source [42]. These balloons have two purposes: first, to supplement shielding, i.e. to move the uninvolved parts of the rectal wall further from the source positions, and second, to increase the distance from the source to the tumour itself. This makes the dose gradients across the tumour shallower, due to the nature of the dose falloff, and thus prescription to deeper points is possible without an unacceptable increase in dose to the surface of the rectal wall.
Clinical experience with endocavitary brachytherapy for definitive radiotherapy is largely limited to studies of medically inoperable patients. Vuong et al. have used brachytherapy boosts (30 Gy delivered in three treatment sessions) in addition to external beam radiotherapy (40 Gy in 16 fractions) for treatment of patients unfit for surgery, and have achieved local control in 73 % of patients at 2 years [47]. Marijnen et al. conducted a dose-finding study (the HERBERT trial [48]), where they combined 39 Gy in 13-fraction external beam radiotherapy with three brachytherapy boost treatments. The boost dose was escalated in consecutive patient cohorts from 5 to 8 Gy per fraction, with the optimal dose with respect to toxicity (grade ≥3 proctitis) identified as 7 Gy per fraction. Final results regarding oncological outcome have not yet been published, but the latest trial update at ESTRO 2015 reported 78 % tumour control at 1 year at the optimal boost level [49]. Finally, the results from the Danish watchful waiting trial, combining external and brachytherapy boosts, were discussed above. In general, available reports seem to indicate that a brachytherapy boost is optimally delivered at the end of or some weeks after completion of external beam radiotherapy, where tumour shrinkage ensures that boost treatment volumes will be minimised. Experience from other cancer sites does, however, call for cautions regarding the introduction of treatment gaps [50], and this may have to be taken into account in the design of treatment schedules.
Contact X-ray Therapy (Papillon Treatment)
The use of low-energy (50 kV) contact X-ray therapy for treatment of rectal cancer was popularised by Papillon from 1950 onwards [51], and this technique is consequently often referred to by his name. Treatment is delivered by positioning the tumour directly at the end of a cylindrical, endorectal collimator connected to an X-ray source, thus limiting irradiation to a circular disc corresponding to the distal opening of the collimator. The low-energy X-rays have very shallow penetration—fall-off to 50 % of surface dose is reached at ∼7-mm depth [52]—effectively limiting dose beyond the target. These characteristics require a somewhat different treatment approach than for external or brachytherapy treatment, as the entire tumour seldom can be irradiated to full dose in a single session. Instead, one treatment fraction is usually followed by a waiting period of a few weeks, whereby the surface portion of the tumour is allowed time to regress, before a second treatment is delivered to the underlying, newly exposed part of the tumour, and possibly later followed by a third or a fourth treatment. This does imply that reported doses from Papillon treatment—commonly two to four times 30 Gy, i.e. total doses often exceeding 100 Gy—cannot be directly compared to dose delivered with external beam or brachytherapy treatment.
One of the major challenges with the use of endocavitary contact X-ray treatment relates to limitations on collimator diameter, which effectively restricts the size and circumferential involvement of treatable tumours. Generally, tumours cannot be more than about 5 cm in diameter or involve more than half of the rectum circumference. Additionally, the rigid nature of the collimator means that only distal tumours can be reached, and for some tumour positions, it can be very hard to position the applicator opening sufficiently perpendicular to the rectal wall. This again limits the proportion of patients who can be treated using this technique.
Treatment planning and verification are based on visual guidance of the collimating tube, and thus no additional imaging or dose distribution calculations are available for individual patients. This may make the treatment quality highly operator dependent and may have contributed to the limited use of the treatment technique. Newer treatment units do provide some additional image guidance facilities compared to previous designs, but there is still no volumetric imaging or 3D dose calculation available. While the volume of normal tissue irradiated using contact X-ray treatment is substantially smaller than for the two previously discussed techniques, normal tissue toxicity still restricts the radiation doses that can safely be delivered, and as for brachytherapy treatment, the main limitation appears to be related to the tolerance of the rectal mucosa (see below).
The clinical experience with contact X-ray boost for rectal cancer is much more extensive than for any of the two previously discussed techniques. A number of publications by French, American and British groups have demonstrated the feasibility of using contact X-ray treatment either on its own or in combination with external beam radiotherapy for definitive treatment of rectal cancer [16, 53–56]. However, published studies are almost exclusively retrospective, single-institution series, with wide mix of patients and radiation doses, as well as many series using local excision for near-complete responders. This complicates any attempts at extrapolation from published studies to expected outcomes for patient groups treated with any specific contact X-ray regimen. In general, though, up towards 80 % of small and/or early tumours seem to be treatable with radiotherapy alone, when a combination of external beam and contact X-ray treatment is employed.
Conclusion
Watch-and-wait treatment strategies are emerging as feasible and safe treatment options for patients with rectal cancer who demonstrate clinical complete response after CRT. Reports of outcomes for patients treated with high-dose radiotherapy indicate that potentially half (or more) of small T1-3 cancers can be controlled locally using CRT alone. Consequently, upfront radiation dose escalation with the aim of non-surgical management is becoming attractive, especially for low tumours or for patients who are not candidates for surgery.
The published clinical experience with high-dose radiotherapy followed by watch-and-wait is still limited. Importantly, the limiting factor for dose escalation—and thus for the proportion of patients who can achieve tumour control with radiotherapy alone—is normal tissue toxicity, and very little information is available regarding late side effects and functional outcome after high-dose radiotherapy and non-surgical management. Maas et al. reported excellent functional outcome for patients followed with watch-and-wait after standard long-course CRT [13], and the Danish watchful waiting trial [17••] found this to be the case after high-dose CRT as well. Likely, irradiation of part of the rectum up to 60–70 Gy does not cause major functional problems, as long as the sphincter is not included in the treatment target. Published data from the Dutch and Danish studies are still very early, though, with functional outcome assessed only in the first couple of years after treatment. Considering the long timescale for development of fibrosis, deterioration might still happen with longer follow-up. Additionally, many of the studies utilizing high-dose radiotherapy report late rectal bleeding and proctitis—up to 30 % grade 2, and up to 10 % grade 3 [16, 17••, 47]. This observation is in accordance with clinical experience from other pelvic cancers, such as prostate cancer radiotherapy, where irradiation of large volumes of the rectum to excess of 60 Gy has been reported to increase the risk of late rectal bleeding [57]. It may be possible to optimize treatment techniques to limit this toxicity, for example by using balloon techniques for endorectal brachytherapy or by using contact X-ray treatment, but ultimately a certain amount of rectal mucosa will have to be included in the treatment volume. For contact X-ray treatment, there might also be a risk of induction of chronic ulcers, and the tolerance dose for this toxicity is not well-established. Overall, the presently available knowledge about the relationships between dose, volume and risk of late normal tissue toxicity after definitive radiotherapy is so limited that optimization of treatment techniques remains very challenging.
Comparing the three boost techniques discussed above, with the current evidence available, the choice of treatment technique will ultimately depend on the patient population treated as well as the feasibility of any given technique in the specific clinical practice. External beam boost is technically relatively simple and easily implemented in most departments. Additionally, it places no limits on the size or position of tumours to be treated. This may thus be the first choice for departments that want to increase the probability of non-surgical management for large fractions of the patient population undergoing standard neoadjuvant CRT. However, dose escalation will probably have to be constrained to 10–15 Gy (due to coincidental normal tissue irradiation, as discussed above), and there is therefore a limit to the expected proportion of patients who can archive tumour control with CRT alone. Brachytherapy is technically more complex, especially if one wants to deliver high-quality, image-guided treatments. On the other hand, the radiotherapy dose can likely be safely escalated to substantially higher doses than with external beam, and preliminary reports from treatment of cohorts of medically inoperable patients indicate a high proportion of patients with long-term tumour control. Boost dose is still limited by dose to the rectal mucosa, though, as demonstrated by the HERBERT dose-finding study, but brachytherapy nonetheless provides major advantages in terms of sparing of other normal tissue in the pelvis. Not all tumours are candidates for brachytherapy boost, e.g. very high tumours or tumours involving large parts of the circumference. Contact therapy provides for very conformal dose distributions, avoiding many of the previously mentioned problems with normal tissue toxicity, but can only be used upfront for relatively small tumours in specific parts of the rectum. Preceded by external beam radiotherapy, which can induce tumour shrinkage for patients with more advanced tumours, it may be an option for larger groups of patients. The specialised equipment needed for treatment, compared to widely available linear accelerators and HDR brachytherapy afterloaders, may limit its attractiveness for most radiotherapy centres, though.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marijnen CAM. Organ preservation in rectal cancer: have all questions been answered? Lancet Oncol. 2015;16:e13–22. Comprehensive overview of current evidence for organ-preserving treatment strategies for rectal cancer, including radiotherapy for non-surgical management.
Guren MG, Eriksen MT, Wiig JN, et al. Quality of life and functional outcome following anterior or abdominoperineal resection for rectal cancer. Eur J Surg Oncol. 2005;31:735–42.
Fazio VW, Zutshi M, Remzi FH, et al. A randomized multicenter trial to compare long-term functional outcome, quality of life, and complications of surgical procedures for low rectal cancers. Ann Surg. 2007;246:481–8. discussion 488–90.
Anderin C, Martling A, Hellborg H, Holm T. A population-based study on outcome in relation to the type of resection in low rectal cancer. Dis Colon Rectum. 2010;53:753–60.
Harrison JD, Solomon MJ, Young JM, et al. Patient and physician preferences for surgical and adjuvant treatment options for rectal cancer. Arch Surg. 2008;143:389–94.
Rutten HJT, den Dulk M, Lemmens VEPP, van de Velde CJH, Marijnen CAM. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol. 2008;9:494–501.
Smith FM, Rao C, Oliva Perez R, et al. Avoiding radical surgery improves early survival in elderly patients with rectal cancer, demonstrating complete clinical response after neoadjuvant therapy: results of a decision-analytic model. Dis Colon Rectum. 2015;58:159–71.
van Gijn W, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7. discussion 717–8.
Maas M, Beets-Tan RGH, Lambregts DMJ, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–40.
Perez RO, Habr-Gama A, Gama-Rodrigues J, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: Long-term results of a prospective trial (National Clinical Trial 00254683). Cancer. 2012;118:3501–11.
Smith JJ, Chow OS, Eaton A, et al. Organ preservation in patients with rectal cancer with clinical complete response after neoadjuvant therapy. J Clin Oncol 2015; 33 (suppl 3): abstr 509
Gerard J-P, Frin A-C, Doyen J, et al. Organ preservation in rectal adenocarcinoma (T1) T2-T3 Nx M0. Historical overview of the Lyon Sud – Nice experience using contact x-ray brachytherapy and external beam radiotherapy for 120 patients. Acta Oncol. 2015;54:545–51.
Appelt AL, Pløen J, Harling H, et al. Watchful Waiting: A prospective study of chemoradiotherapy as definitive treatment of low rectal cancer. Lancet Oncol. 2015. doi:10.1016/S1470-2045(15)00120-5. Prospective trial of high dose chemoradiotherapy as definitive treatment for low rectal cancer. Demonstrated that over half of all patients with T2-3N0-1 might potentially have local control with chemoradiotherapy alone.
Sanghera P, Wong DWY, McConkey CC, Geh JI, Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol). 2008;20:176–83.
Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84:949–54.
Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74–80. This study provided a quantitative estimate of the dose-response relationship for pathological tumour response of rectal cancer; this may inform the design of future treatment strategies for definitive radiotherapy.
Bonnetain F, Bosset JF, Gerard JP, et al. What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: surrogacy in question? Eur J Cancer. 2012;48:1781–90.
Appelt AL, Vogelius IR, Pløen J, et al. Long-term results of a randomized trial in locally advanced rectal cancer: No benefit from adding a brachytherapy boost. Int J Radiat Oncol Biol Phys. 2014;90:110–8.
Fokas E, Liersch T, Fietkau R, et al. Downstage migration after neoadjuvant chemoradiotherapy for rectal cancer: The reverse of the Will Rogers phenomenon? Cancer. 2015;121:1724–7.
Habr-Gama A, Perez RO, Nadalin W, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90–101.
Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Color Dis. 2006;8 Suppl 3:21–4.
Habr-Gama A, Perez RO, Proscurshim I, et al. Patterns of Failure and Survival for Nonoperative Treatment of Stage c0 Distal Rectal Cancer Following Neoadjuvant Chemoradiation Therapy. J Gastrointest Surg. 2006;10:1319–29.
Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, São Julião GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: Results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52:1927–34.
Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and Wait Approach Following Extended Neoadjuvant Chemoradiation for Distal Rectal Cancer: Are We Getting Closer to Anal Cancer Management? Dis Colon Rectum. 2013;56:1109–17. The authors from the Brazilian group demonstrated in this publication that a combination of high dose radiotherapy and additional chemotherapy after end of chemoradiotherapy resulted in a very high rate of clinical responders.
Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822–8. Newest publication from the Brazilian group; provides important details on late outcome for patients followed with watch-and-wait, including outcome after salvage therapies.
Dalton RS, Velineni R, Osborne ME, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Color Dis. 2012;14:567–71.
Gérard J-P, Chamorey E, Gourgou-Bourgade S, et al. Clinical complete response (cCR) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the ACCORD 12/PRODIGE 2 randomized trial. Radiother Oncol. 2015;115:246–52.
Vestermark LW, Jacobsen A, Qvortrup C, et al. Long-term results of a phase II trial of high-dose radiotherapy (60 Gy) and UFT/l-leucovorin in patients with non-resectable locally advanced rectal cancer (LARC). Acta Oncol. 2008;47:428–33.
Lee JH, Kim DY, Nam T-K, et al. Long-term follow-up of preoperative pelvic radiation therapy and concomitant boost irradiation in locally advanced rectal cancer patients: a multi-institutional phase II study (KROG 04-01). Int J Radiat Oncol Biol Phys. 2012;84:955–61.
Hernando-Requejo O, López M, Cubillo A, et al. Complete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiation. Strahlenther und Onkol. 2014;190:515–20.
Cilla S, Caravatta L, Picardi V, et al. Volumetric modulated arc therapy with simultaneous integrated boost for locally advanced rectal cancer. Clin Oncol (R Coll Radiol). 2012;24:261–8.
Gevaert T, Engels B, Garibaldi C, et al. Implementation of HybridArc treatment technique in preoperative radiotherapy of rectal cancer: dose patterns in target lesions and organs at risk as compared to helical Tomotherapy and RapidArc. Radiat Oncol. 2012;7:120.
Seierstad T, Hole KH, Saelen E, Ree AH, Flatmark K, Malinen E. MR-guided simultaneous integrated boost in preoperative radiotherapy of locally advanced rectal cancer following neoadjuvant chemotherapy. Radiother Oncol. 2009;93:279–84.
Raso R, Scalco E, Fiorino C, et al. Assessment and clinical validation of margins for adaptive simultaneous integrated boost in neo-adjuvant radiochemotherapy for rectal cancer. Phys Med. 2015;31:167–72.
Burbach JPM, Verkooijen HM, Intven M, et al. RandomizEd controlled trial for pre-operAtive dose-escaLation BOOST in locally advanced rectal cancer (RECTAL BOOST study): study protocol for a randomized controlled trial. Trials. 2015;16:58.
Hansen JW, Jakobsen A. The importance of applicator design for intraluminal brachytherapy of rectal cancer. Med Phys. 2006;33:3220–4.
Vuong T, Devic S, Podgorsak E. High dose rate endorectal brachytherapy as a neoadjuvant treatment for patients with resectable rectal cancer. Clin Oncol (R Coll Radiol). 2007;19:701–5.
Poon E, Reniers B, Devic S, Vuong T, Verhaegen F. Dosimetric characterization of a novel intracavitary mold applicator for 192Ir high dose rate endorectal brachytherapy treatment. Med Phys. 2006;33:4515–26.
Vuong T, Devic S, Moftah B, Evans M, Podgorsak EB. High-dose-rate endorectal brachytherapy in the treatment of locally advanced rectal carcinoma: technical aspects. Brachytherapy. 2005;4:230–5.
Webster MJ, Devic S, Vuong T, et al. HDR brachytherapy of rectal cancer using a novel grooved-shielding applicator design. Med Phys. 2013;40:091704.
Jakobsen A, Mortensen JP, Bisgaard C, Lindebjerg J, Hansen JW, Rafaelsen SR. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int J Radiat Oncol Biol Phys. 2006;64:461–5.
Appelt AL, Mortensen B, Pløen J, et al. MRI-guided brachytherapy for rectal cancer: A treatment planning study with a shieldable multicatheter applicator. Radiother Oncol. 2014;111 suppl 1:S419.
Vuong T, Niazi T, Devic S. Role of endoluminal brachytherapy for rectal cancer: current status and challenges. Radiother Oncol. 2015;115 suppl 1:S111.
Velema L, Cats A, Neelis K, Van der Linden Y, Nout R, Van Triest B, et al. Feasibility study of external beam radiotherapy followed by brachytherapy in inoperable rectal cancer patients. Radiother Oncol. 2014;111 suppl 1:S204.
Marijnen C. Endoluminal radiotherapy in rectal cancer: Which questions do we need to answer? Radiother Oncol. 2015;115 suppl 1:S112.
Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–61.
Lindegaard J, Gerard JP, Sun Myint A, Myerson R, Thomsen H, Laurberg S. Whither Papillon? - Future Directions for Contact Radiotherapy in Rectal Cancer. Clin Oncol. 2007;19:738–41.
Croce O, Hachem S, Franchisseur E, Marcié S, Gérard JP, Bordy JM. Contact radiotherapy using a 50kV X-ray system: Evaluation of relative dose distribution with the Monte Carlo code PENELOPE and comparison with measurements. Radiat Phys Chem. 2012;81:609–17.
Rauch P, Bey P, Peiffert D, Conroy T, Bresler L. Factors affecting local control and survival after treatment of carcinoma of the rectum by endocavitary radiation: A retrospective study of 97 cases. Int J Radiat Oncol Biol Phys. 2001;49:117–24.
Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: results for 199 patients with localized tumors. Int J Radiat Oncol. 2001;51:363–70.
Gerard J, Romestaing P, Chapet O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol. 2003;4:158–66.
Sun Myint A, Grieve RJ, McDonald AC, et al. Combined modality treatment of early rectal cancer: the UK experience. Clin Oncol (R Coll Radiol). 2007;19:674–81.
Ebert MA, Foo K, Haworth A, et al. Gastrointestinal dose-histogram effects in the context of dose-volume-constrained prostate radiation therapy: analysis of data from the RADAR prostate radiation therapy trial. Int J Radiat Oncol Biol Phys. 2015;91:595–603.
Compliance with Ethics Guidelines
Conflict of Interest
Ane L. Appelt and Anders Jakobsen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Localized Colorectal Cancer
Rights and permissions
About this article
Cite this article
Appelt, A.L., Jakobsen, A. Radiation Techniques for Increasing Local Control in the Non-Surgical Management of Rectal Cancer. Curr Colorectal Cancer Rep 11, 267–274 (2015). https://doi.org/10.1007/s11888-015-0284-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-015-0284-3