Abstract
Purpose of Review
Aim of the paper was to address all strengths and weakness of cardiac magnetic resonance (CMR) in arrhythmogenic cardiomyopathy, trying to highlight areas where further research and investigations should be carried out to fill current gaps in scientific knowledge.
Recent Findings
Arrhythmogenic cardiomyopathy represents a multifaceted clinical entity associated with arrhythmias and sudden death. Even though different diagnostic tools are available for appropriate identification and risk stratification, over the last few years cardiac magnetic resonance (CMR) has surfaced as an unmatched non-invasive imaging tool.
Summary
CMR is mandatory in the evaluation of arrhythmogenic cardiomyopathy. It is the only imaging technique providing the identification of myocardial fibrosis, particularly for left ventricular myocardium, as recent evidences demonstrated that left ventricular involvement in arrhythmogenic cardiomyopathy is associated with greater risk of sudden death than lone right ventricular involvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sudden cardiac death (SCD) is an uncommon yet devastating and unexpected conclusion of a cardiac condition previously unknown or overlooked. Among the various cardiac diseases on the basis of SCD, arrhythmogenic right ventricular cardiomyopathy (ARVC) may be frequently found, especially among young subjects. ARVC is defined as a cardiomyopathy characterized by a progressive fibrofatty infiltration/metaplasia of the ventricular walls causing dilatation, global or regional dysfunction acting as substrate for malignant arrhythmias and death, portending therefore a negative prognosis. Even though in the past years arrhythmogenic cardiomyopathy was considered to be more likely to affect the right ventricle, hence, the term ARVC, nowadays scientific evidence has described this pathophysiological phenomenon and its subsequent consequences in both ventricles or even isolated in the left one (this form often named as arrhythmogenic left ventricular cardiomyopathy, ALVC).
Given these premises and the psychosocial consequences of the loss of patients with ARVC, it is clear why over the last years scientific research is active on trying to identify diagnostic methods for an accurate and early identification of these patients. Moreover, the prognostic significance of these findings as wells as the ability to differentiate among various phenocopies are still necessary. Cardiac magnetic resonance (CMR) is universally recognized as the gold-standard non-invasive imaging technique in all patients with clinical suspicion of ARVC. CMR has a unique ability to adequately image both the left and right ventricle as well as accurately measure ventricular volumes and systolic function. Moreover, it provides detailed myocardial tissue characterization in terms of the presence and extent of fat infiltration and myocardial fibrosis by means of late gadolinium enhancement (LGE).
CMR and Echocardiography
CMR is a key diagnostic tool in the 2010 revised Task Force Criteria (TFC) both for the qualitative assessment of right ventricular (RV) regional wall motion abnormalities (RWMA) as well as for quantitative assessment of RV volumes and global systolic function quantification. Its value has been validated when compared with the TFC or genotype positive patients [1•]. Useful information may as well be obtained with different non-invasive imaging modalities such as echocardiography that is included as well in the TFC. Even though echocardiography is by all means a useful diagnostic technique given the low cost, availability and easiness in performance [2], some significant differences and limitations need to be acknowledged. Protonotarios et al. showed how in affected families with desmosome mutations, on the basis of the TFC, serial ECGs showed better diagnostic utility particularly in early stages than two-dimensional echocardiography [3•]. Moreover, the specific comparison of echocardiography versus CMR showed some interesting data. When the impact of the discrepancy between the 2 techniques on the clinical diagnosis of ARVC was evaluated, Borgquist et al. found how a significant proportion of patients with imaging-positive ARVC by CMR did not fulfil echocardiographic TFC criteria. The authors concluded that the subtle structural changes in the RV may not be reliably assessed by echocardiography and this should be reflected in the guidelines [4].
Traditional and Novel CMR Techniques for ARVC Diagnosis
CMR has the ability to correct and reproducibly assess biventricular volumes and function [5] and to detect the localization, pattern and extent of fatty infiltration/metaplasia by routinely performed cine images [6] [7] or dedicated T1-weighted fast spin echo sequences. The usefulness of combining a functional CMR assessment with tissue characterization has been described and confirmed. When all possible CMR criteria are used, the best diagnostic accuracy (98%) may be achieved by the combined evaluation of any RV-RWMA (excluding hypokinesia) with any left ventricle (LV) or RV signal abnormality yielding a 100% specificity and 96% sensitivity [8•]. Moreover, and in the specific scenario of ARVC, LGE in the RV may be found in a significant number of patients, up to 67% [9]. The abovementioned findings on the additional diagnostic strength of tissue characterization have not been confirmed in paediatric patients. Etoom et al. [10] in their single-centre retrospective study on children and adolescents with ARVC concluded that CMR is important for diagnosis but without additional values of specific sequences for fatty infiltration and myocardial fibrosis. A possible explanation of these discordant findings between adult and paediatric populations are likely connected to the fact that in the abovementioned [10] study, tissue characterization findings were assessed and deemed not useful exclusively for the RV. It is known that RV is a few millimetre thick in adult patients and even more thinner in children. It is likely therefore that the lack of additional diagnostic information is due to the intrinsic limitations of the spatial resolution of CMR. By applying the same analysis in bigger hearts of adult subjects and by addressing tissue abnormalities also of the thicker LV, a cardiac pathological substrate is likely to be found more often.
Other than the commonly used sequences in the CMR field, recently novel techniques begin to show potential use for diagnostic purposes in the ARVC area. One single-centre small study has shown promising results of native T1 mapping of the LV that according to the authors could help differentiate patients with overt ARVC and at-risk relatives from control subjects, and it may have the potential to detect early ARVC [11]. Given major methodological limitations and lack of bigger studies in this field, we advise caution before translating these findings into clinical practice. Moving forward and given the need to assess subtle changes in the RV especially for early forms of ARVC, strain imaging has been also shown to be potentially useful. When ARVC patients were compared with highly trained athletes, RVEF and RV strain analysis was able to allow a distinction between these 2 entities [12]. Regional strain in ARVC was found to be superior to LGE in detecting arrhythmogenic ventricular tachycardia substrate in ARVC, facilitating therefore the planning of ablation procedures [13].
Differential Diagnosis
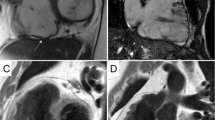
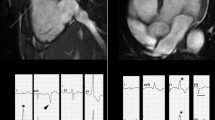
It is clear that CMR is able to offer important diagnostic aid in the search of ARVC. An additional point of strength that has been adequately investigated over the past years is the ability to offer a differential diagnosis when CMR pathological findings are compared between different cardiac conditions with a similar clinical presentation or a similar phenotypical appearance (Figs. 1 and 2). It has been described that CMR when performed with an ARVC query may offer a differential diagnosis in almost 10% of cases [14].
A case of a patient with biventricular arrhythmogenic cardiomyopathy. In the 4-chamber cine image (left panel), regions of fat infiltration are showed as intramyocardial “India ink” sign of left ventricular lateral and apical wall (arrows). Wall thinning of right ventricle is also evident. In post-contrast images (middle and right panels), biventricular areas of late enhancement (arrows) is showed in both 4-chamber and short axis view
One of the most troublesome differential diagnoses in everyday clinical practice is between subtle early ARVC and athlete’s heart with or without ventricular ectopic beats (VEB). The distinction between the two entities is not always straightforward, and a number of parameters may be necessary to provide a useful conclusion trying to navigate between the overlapping features between the 2 forms. The best currently available way to do so is by identifying RV RWMA or low RVEF that are not to be found in healthy athletes whereas RV end-diastolic volume index is not a valid parameter [15]. Moreover, a balanced dilatation between LV and RV as a normal adaptation seen in athletes needs to be considered [16]. Apart from differentiating between healthy and unhealthy conditions, CMR is capable of differentiating among other pathological cardiac conditions that may share some clinical/echocardiographic features. Cardiac sarcoidosis may mimic ARVC and even share many of the TFC allowing to incorrectly classify cases of cardiac sarcoidosis as definite ARVC [17]. Being able to correctly and timely identify patients with cardiac sarcoidosis may have serious implications regarding immunosuppressive therapy and/or cardiac screening. High clinical suspicion is helpful, since some elements allow to differentiate between the two forms. Cardiac sarcoidosis patients are generally older, show more LV LGE and more frequently located in the septum. Extracardiac findings such as mediastinal lymphadenopathy are frequently found in cardiac sarcoidosis as well. On the contrary, ARVC patients may have a family history of SCD and have more frequently involvement of the RV in terms of low RVEF and high RV volumes [18].
A differential diagnosis between ALVC and dilated cardiomyopathy is very challenging. In dilated cardiomyopathy, the most characteristic features are the LV dysfunction and dilation, and LGE is present approximately in only 25% of patients [19]. On contrast, for the diagnosis of ALVC, LV dysfunction and dilation are not necessary criteria, but non-ischemic LGE (or fat infiltration) is the most important diagnostic feature. Then, in case of LV dysfunction and dilation with negative LGE, the diagnosis of dilated cardiomyopathy should be suspected. On contrast, the presence of non-ischemic LGE with preserved LV function or even with mild dysfunction should suggest ALVC. However, the identification of desmosomal mutation by genetic evaluation is often mandatory for the diagnosis of ALVC. Finally, genetic evaluation is also useful to distinguish between ALVC, which may present signs of myocardial inflammation and myocarditis.
Prognosis
CMR’s ability to appropriately diagnose ARVC in an early or late stage has been abundantly documented and established. Over the last years, attention has shifted towards the prognostic implications of CMR findings in patients with ARVC. More importantly, scientific evidence has addressed not only the role of CMR as a prognostic tool but also tried to identify the markers that could act as prognosticators in this cohort of patients.
The prognostic role of CMR was first investigated in patients with VEB originating from the RV regardless of the definitive diagnosis. When more than 1000 RV VEBs are present, the evidence of RV abnormalities were associated with worse prognosis [20]. Based on this preliminary evidence, the same question was addressed in the specific setting of ARVC [21]. By comparing CMR findings in 175 patients with definite, borderline and possible diagnosis of ARVC and during the subsequent follow-up, 35 patients experienced hard cardiac events such as SCD and appropriate ICD shock. Thirty-four patients with hard cardiac events had an abnormal CMR (negative predictive value = 96.9%) with LV involvement either as fat infiltration or LGE at CMR. Tissue abnormalities and non-sustained ventricular tachycardia were the only independent predictors of cardiac events in the whole population and in the specific group of patients with definite ARVC. Moving forward from these findings, a multicentre study sought to assess the prognostic role of CMR in consecutive patients with ARVC and to evaluate the efficacy of the novel 5-year ARVC risk score to predict cardiac events in different CMR presentations [22••]. ARVC risk score recently proposed to predict the 5-year risk of malignant ventricular arrhythmias in patients with ARVC. Authors of the study performed CMR in 140 patients with definite ARVC and retrospectively calculated the ARVC risk score. After a median follow-up of 5 years (2 to 8 years), the combined endpoint of SCD, appropriate implantable cardioverter-defibrillator intervention and aborted cardiac arrest were considered. Forty-eight patients (34%) experienced a major event during the follow-up period. None of the patients with a completely negative CMR had a cardiac event. Patients with a definite ARVC diagnosis had different prognosis based on the different CMR presentation. Moreover, patients with LV involvement (LV dominant and biventricular) had a worse prognosis than those with lone RV. The independent predictors of major cardiac events were the presence of LV involvement and the 5-year ARVC risk score. Interestingly enough, however, the estimated 5-year risk was able to predict accurately the observed risk only in the cohort of patients with lone RV, underestimating the risk in those with LV involvement.
CMR Limitations
Caution must be advised when CMR is performed by non-experienced CMR personnel in order to try not to over-diagnose RV findings and to balance CMR findings with their clinical weight. Especially for RWMA, there is a real risk of describing findings with no clinical significance [23]. By combining qualitative criteria such as RWM with quantification of RV volumes and systolic function specificity increases at the expense of a lower sensitivity [24].
Unfortunately, currently available criteria in order to avoid false positive cases fail to consider tissue characterization criteria, reducing the potential information deriving from CMR to a mere volume and function assessment. Even though ARVC is defined as a cardiomyopathy with fibro-fatty replacement and despite the pluri-demonstrated CMR’s ability to adequately identify fat and fibrosis [25], this is yet to be translated into clinical practice. It is likely that by combining all available potential information, diagnostic accuracy will increase without the risk of significantly increasing false positive cases. This is our personal experience, when CMR is performed in high volume centres by qualified CMR experienced readers.
Conclusions
CMR is a powerful tool in the ARVC clinician’s armamentarium. Given its superiority compared with other non-invasive and invasive diagnostic options, CMR is, in our opinion, to be performed in all cases with a clinical suspicion of ARVC. When performed in high volumes centres by experienced diagnosticians, it allows to correctly diagnose this particular yet potentially fatal condition, differentiate it from other phenocopies and predict its evolution towards hard cardiac events.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Sen-Chowdhry S, Prasad SK, Syrris P, Wage R, Ward D, Merrifield R, et al. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol. 2006;48:2132–40. This study was the first showing the role of magnetic resonance in arrhythmogenic cardiomyopathy.
Gotschy A, Saguner AM, Niemann M, Hamada S, Akdis D, Yoon J-N, et al. Right ventricular outflow tract dimensions in arrhythmogenic right ventricular cardiomyopathy/dysplasia-a multicentre study comparing echocardiography and cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2018;19:516–23.
• Protonotarios N, Anastasakis A, Antoniades L, Chlouverakis G, Syrris P, Basso C, et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia on the basis of the revised diagnostic criteria in affected families with desmosomal mutations. Eur Heart J. 2011, 32:1097–104. This study demonstrated the evolving characteristics of ARVD.
Borgquist R, Haugaa KH, Gilljam T, Bundgaard H, Hansen J, Eschen O, et al. The diagnostic performance of imaging methods in ARVC using the 2010 Task Force criteria. Eur Heart J Cardiovasc Imaging. 2014;15:1219–25.
Aquaro GD, Camastra G, Monti L, Lombardi M, Pepe A, Castelletti S, et al. Reference values of cardiac volumes, dimensions, and new functional parameters by MR: a multicenter, multivendor study. J Magn Reson Imaging. 2017;45:1055–67.
Aquaro GD, Todiere G, Strata E, Barison A, Di Bella G, Lombardi M. Usefulness of India ink artifact in steady-state free precession pulse sequences for detection and quantification of intramyocardial fat. J Magn Reson Imaging. 2014;40:126–32.
Aquaro GD, Nucifora G, Pederzoli L, Strata E, De Marchi D, Todiere G, et al. Fat in left ventricular myocardium assessed by steady-state free precession pulse sequences. Int J Card Imaging. 2012;28:813–21.
• Aquaro GD, Barison A, Todiere G, Grigoratos C, Ait Ali L, Di Bella G, et al. Usefulness of combined functional assessment by cardiac magnetic resonance and tissue characterization versus Task Force Criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2016;118:1730–6. This study demonstrated the diagnostic role of tissue characterization by cardiac magnetic resonance.
Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103.
Etoom Y, Govindapillai S, Hamilton R, Manlhiot C, Yoo S-J, Farhan M, et al. Importance of CMR within the Task Force Criteria for the diagnosis of ARVC in children and adolescents. J Am Coll Cardiol. 2015;65:987–95.
Bourfiss M, Prakken NHJ, van der Heijden JF, Kamel I, Zimmerman SL, Asselbergs FW, et al. Diagnostic value of native T1 mapping in arrhythmogenic right ventricular cardiomyopathy. JACC Cardiovasc Imaging. 2019;12:1580–2.
Czimbalmos C, Csecs I, Dohy Z, Toth A, Suhai FI, Müssigbrodt A, et al. Cardiac magnetic resonance based deformation imaging: role of feature tracking in athletes with suspected arrhythmogenic right ventricular cardiomyopathy. Int J Card Imaging. 2019;35:529–38.
Zghaib T, Ghasabeh MA, Assis FR, Chrispin J, Keramati A, Misra S, et al. Regional strain by cardiac magnetic resonance imaging improves detection of right ventricular scar compared with late gadolinium enhancement on a multimodality scar evaluation in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2018;11:e007546.
Liu T, Pursnani A, Sharma UC, Vorasettakarnkij Y, Verdini D, Deeprasertkul P, et al. Effect of the 2010 task force criteria on reclassification of cardiovascular magnetic resonance criteria for arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Magn Reson. 2014;16:47.
D’Ascenzi F, Solari M, Corrado D, Zorzi A, Mondillo S. Diagnostic differentiation between arrhythmogenic cardiomyopathy and athlete’s heart by using imaging. JACC Cardiovasc Imaging. 2018;11:1327–39.
Luijkx T, Velthuis BK, Prakken NHJ, Cox MGPJ, Bots ML, Mali WPTM, et al. Impact of revised Task Force Criteria: distinguishing the athlete’s heart from ARVC/D using cardiac magnetic resonance imaging. Eur J Prev Cardiol. 2012;19:885–91.
Hauer RNW. Cardiac sarcoidosis mimicking definite arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;
Steckman DA, Schneider PM, Schuller JL, Aleong RG, Nguyen DT, Sinagra G, et al. Utility of cardiac magnetic resonance imaging to differentiate cardiac sarcoidosis from arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2012;110:575–9.
Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin S, et al. Arrhythmogenic right ventricular cardiomyopathy: characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J Am Heart Assoc. 2020;9:e014628.
Aquaro GD, Pingitore A, Strata E, Di Bella G, Molinaro S, Lombardi M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J Am Coll Cardiol. 2010;56:1235–43.
Aquaro GD, Pingitore A, Di Bella G, Piaggi P, Gaeta R, Grigoratos C, et al. Prognostic role of cardiac magnetic resonance in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2018;122:1745–53.
• Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2020;75:2753–65. This is the first study demonstrating that left ventricular involvement in ARVC is more arrhythmogenic than lone right ventricular involvement.
Rastegar N, Burt JR, Corona-Villalobos CP, Te Riele AS, James CA, Murray B, et al. Cardiac MR findings and potential diagnostic pitfalls in patients evaluated for arrhythmogenic right ventricular cardiomyopathy. Radiographics. 2014;34:1553–70.
Cox MGPJ, van der Smagt JJ, Noorman M, Wiesfeld AC, Volders PGA, van Langen IM, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy diagnostic task force criteria: impact of new task force criteria. Circ Arrhythm Electrophysiol. 2010;3:126–33.
Klopotowski M, Kukula K, Malek LA, Spiewak M, Polanska-Skrzypczyk M, Jamiolkowski J, et al. The value of cardiac magnetic resonance and distribution of late gadolinium enhancement for risk stratification of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiol. 2016;68:49–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article is a review paper and does not contain new research studies with human or animal subjects.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Myocardial Disease
Rights and permissions
About this article
Cite this article
Grigoratos, C., Aquaro, G.D. The Role of Cardiovascular Magnetic Resonance in ARVC. Curr Cardiol Rep 23, 56 (2021). https://doi.org/10.1007/s11886-021-01488-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01488-1