Abstract
Purpose of Review
This review discusses the basic and evolving echocardiographic and cardiac magnetic resonance (CMR) approaches in the diagnosis and management of patients with hypertrophic cardiomyopathy (HCM).
Recent Findings
Newer imaging technologies and techniques in both echocardiography and CMR have proved to add incremental value to our understanding of HCM. 3D reconstruction in echocardiography and CMR allows for more accurate morphological and volumetric assessment of the left ventricle. Echocardiographic and CMR-based left atrial assessment, including for its mechanical properties, has been shown to be correlated to outcomes and development of atrial fibrillation. Tissue characterization and scar burden quantification by late gadolinium enhancement on CMR has revolutionized our understanding of fibrotic processes in HCM and their contribution to disease severity and clinical outcomes.
Summary
Cardiac imaging plays a crucial role in HCM patients. Using echocardiography and CMR as complementary modalities allows for improved diagnostics, optimization of treatment, and better prognostication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disorder, and its prevalence is thought to be 1:500 [1,2,3, 4••]. The definition of HCM includes the presence of left ventricular (LV) hypertrophy with maximal myocardial wall thickness ≥ 15 mm, in the absence of loading conditions that could induce the same extent of hypertrophy, such as aortic stenosis or longstanding hypertension [5, 6]. While HCM genotypic expression has autosomally dominant inheritance, its phenotypic expression can be extremely variable, even between patients with the same pathogenic variant, in terms of age of clinical presentation, severity, presence of left ventricular outflow tract (LVOT) obstruction, as well as prognosis. Most patients with HCM will have a normal life with a near-normal life span. However, some patients will develop symptoms necessitating therapeutic interventions, including pharmacotherapy, alcohol septal ablation, surgical myectomy, or even heart transplantation. Choosing the most suitable treatment relies on the pathophysiology behind the clinical presentation. This could be multifactorial and includes HCM-related mechanisms such as diastolic or systolic dysfunction, LVOT obstruction, myocardial ischemia due to microvascular dysfunction, arrhythmia, or mitral valve regurgitation, as well as non-HCM-related mechanisms, including coronary artery disease, primary valvular abnormalities, or pulmonary disease.
Cardiac imaging plays a crucial role in the diagnosis and management of HCM patients. Although clues for HCM can be found on the electrocardiogram, the gold standards for HCM diagnosis, assessment of treatment efficiency, and prognostication remain transthoracic echocardiography and cardiac magnetic resonance imaging (CMR).
This review will describe the essential and complementary role of echocardiography and cardiac magnetic resonance in the diagnosis and management of patients with HCM.
CMR
CMR has emerged as an invaluable imaging modality in the assessment of HCM patients. CMR has been proven to be optimized to the diverse phenotypic expression of HCM, given its high spatial and temporal resolution, the ability to clearly delineate the endocardial and epicardial borders, and the capability of tomographic cardiac reconstruction with excellent visualization of all LV segments. Furthermore, images are independent of factors that could impede obtainment of proper echocardiographic acoustic windows, such as body habitus, chest wall geometry, and pulmonary parenchymal disease.
While CMR provides the ability to accurately measure LV wall thickness, LV mass, and LV ejection fraction, it is the gold standard method for tissue characterization and volumetric assessment. Despite the aforementioned CMR advantages over echocardiography, it has certain drawbacks. Image quality in CMR is dependent upon cardiac and respiratory gating. Lack of patient cooperation, arrhythmias, limited availability, lack of portability, and its high cost are among the well-known CMR limitations. Furthermore, gadolinium-based contrast is contraindicated in advanced renal failure as it may lead to nephrogenic systemic sclerosis.
Morphological Assessment
Myocardial Wall Thickness
As previously detailed, noninvasive diagnosis of HCM is morphologically based on LV wall thickness ≥ 15 mm at end-diastole in general (or ≥ 13 mm in patients with a known family history of HCM), in a nondilated LV [6, 7]. The magnitude of LV hypertrophy has been shown to strongly correlate linearly with the risk of sudden cardiac death (SCD), and HCM patients with massive hypertrophy (i.e., thickness ≥ 30 mm) are considered at the highest risk [6, 8••, 9]. This underscores the importance of reliable and accurate measurement of LV wall thickness.
Despite the fact that the ventricular septum is predominantly involved in HCM, any myocardial segment can be affected, including the LV free wall and LV apex (Fig. 1). Maron et al. [10••] described different patterns of left ventricular hypertrophy (LVH) in 333 HCM patients who underwent CMR examination and have shown that the predominant area of LV wall thickening in HCM involved the continuum of the basal anterior free wall with the basal anterior interventricular septum. The next most common area for hypertrophy was the mid-ventricular posterior septum. Due to its lower spatial resolution and the effect of adjacent organs (such as chest wall or lung parenchyma) on image quality, echocardiography can miss the diagnosis of HCM when the hypertrophy is confined to the anterolateral wall [11] or the posterior septum in the area of insertion of the right ventricle (RV) free wall [10••]. CMR is not limited by such constraints, due to its higher spatial resolution and tomographic imaging capabilities, and it is the preferred method for assessment of myocardial hypertrophy in the anterior wall, posterior septum, and apex of the heart. Moreover, it has been showed that in up to 10% of HCM patients, hypertrophy is confined to only 1 or 2 LV segments [10••]. These localized hypertrophic segments could be missed in routine echocardiography due to its limited echocardiographic imaging planes [12].
a–g HCM morphologies in CMR (first row) and echocardiography (second row). a Reverse curvature. b Sigmoid septum. c Neutral septum with basal to apical septal hypertrophy. d Extreme LV septal hypertrophy (42 mm). e Apical hypertrophy. f Apical aneurysm. g Basal inferior wall crypt (shown by asterisk). h Septal-apical muscle bundle (shown by asterisk). LV left ventricle, RV right ventricle, LA left atrium, RA right atrium, Ao aorta, An aneurysm
The extent of hypertrophy could be overestimated in echocardiography due to inclusion in the LV wall measurement process of the right ventricular myocardium [13, 14], LV trabeculations, papillary muscles, or apical septal bundle [15•]. Hindieh et al. compared maximal LV wall thickness in CMR and TTE and reported that almost half of the patients were identified to have intermodal measurement discrepancies ≥ 10% even when reported by readers experienced in HCM studies. In 15.9% of patients, measurement discrepancy occurred at diagnostic or prognostic cutoffs, underscoring the clinical importance of accurate LV wall thickness measurement [15•]. Another study by Bois et al. [16•] in 618 patients showed significant differences between CMR and echocardiography in the assessment of maximal wall thickness, with a median difference of 3 mm, while exact agreement between the two studies occurred in only 12% of patients.
Administration of contrast during TTE image acquisition can help improve endocardial definition and myocardial delineation and can prove very useful in assessment of the apical form of HCM, as will be detailed later (Fig. 2).
Contrast echocardiography (middle row) and cardiac magnetic resonance (CMR, lower row) in patients with technically difficult echocardiographic studies with limited views (upper row). a Apical HCM which was not well-visualized in the 2D echocardiogram but demonstrated in contrast echocardiogram and CMR. b Small apical aneurysm not seen in the 2D echocardiogram but revealed with the use of contrast and in CMR (white arrows). c Difficult to assess basal antero-septal hypertrophy due to lack of RV side visualization. RV chamber is well demonstrated with the use of contrast echocardiography and in CMR, allowing for accurate measurement of the interventricular septum (40 mm). LV left ventricle, RV right ventricle, LA left atrium, RA right atrium
Left Ventricular Mass and Volume
CMR is the most accurate and reproducible method of quantifying LV mass [17]. While echocardiographic assessment of LV mass and volumes in HCM is limited by the heterogeneity of the LV geometry, CMR-based LV assessment is not reliant upon the geometric assumptions used in echocardiography, as it is performed by direct tracing of myocardial borders. However, real-time 3D echocardiographic measurements of volumes and mass have been shown to be correlated closely to CMR-based measurements [18].
LV mass in patients with limited and focal hypertrophy can be normal [19], explaining why the diagnosis of HCM relies on the presence of increased segmental wall thickness and not LV mass. This fact may explain why LV mass lacks specificity as an outcome predictor. On the other hand, it has been shown that LV mass indexed to body surface area is more sensitive than maximal LV wall thickness in predicting HCM-related mortality [19], and that a CMR-derived indexed LV mass more than two standard deviations above a healthy control cohort is a sensitive predictor of clinical outcomes in HCM [20]. However, the relevance of LV mass as an independent marker for predicting adverse outcomes in HCM is still not well defined.
LV Apex
Apical HCM has been long thought to have a benign clinical course with low rate of cardiovascular mortality, compared with other morphological forms of HCM. However, severe clinical manifestations, including arrhythmias and apical infarction leading to aneurysm formation, have been increasingly recognized.
Accurate assessment and characterization of the LV apex, as well as the development of apical aneurysm, are pivotal for prognostication, as apical aneurysms are associated with substantial cardiovascular morbidity and mortality [21, 22, 23••]. Furthermore, thrombus can develop in the dyskinetic/akinetic apical aneurysm [21, 23••, 24, 25], putting patients at risk of thromboembolic complications [26] and sometimes may be the presenting symptom.
Rowin et al. showed that in a cohort of 1940 HCM patients, apical aneurysm was evident in 4.8% [23••]. However, in the apical form of HCM, the incidence of apical aneurysms has been reported to be much higher, ranging from 10 to 30% [27, 28].
CMR has been found to be superior to echocardiography in detecting apical segment hypertrophy (Fig. 2) [12, 28, 29]. In a large cohort of apical HCM patients, 9 of 105 patients were initially reported to have negative echocardiograms, which on review after CMR were positive [30].
Small to moderate sized apical aneurysms may not be reliably detected by echocardiography for the same reasons that apical hypertrophy can also be missed (Fig. 2) [28], and CMR has been shown to be the preferred modality for apical aneurysm detection due to its high spatial and contrast resolution. However, the use of contrast agents can improve TTE characterization of the LV apex and should be routinely used in the evaluation of suspected apical HCM [6, 31], as well as confirmed apical HCM for the presence of thrombus (Fig. 2).
Right Ventricle
Assessment of the RV by echocardiography is limited due to its retrosternal position, making CMR the gold standard for RV characterization.
Right ventricular involvement in HCM occurs in approximately 18% of all cases, most commonly involving the mid to apical portion of the right ventricle [32, 33]. Maron et al. [13] showed that maximum RV wall thickness was increased to ≥ 8 mm in one third of patients with HCM compared to reference controls. Most commonly, the RV areas that are involved in hypertrophy are confined to the insertion of the RV wall into the anterior and posterior septum.
The prognostic significance of RV involvement in HCM is not yet known.
Tissue Characterization
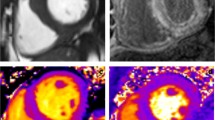
Tissue characterization in HCM is perhaps the most important advantage of CMR over echocardiography, offering an invaluable incremental value in HCM assessment (Fig. 3). CMR allows noninvasive assessment of the presence and extent of myocardial fibrosis by late gadolinium enhancement (LGE) [34, 35•, 36,37,38]. A typical protocol involves CMR image acquisition 5–20 min following gadolinium administration [39]. LGE is based on the kinetic behavior of gadolinium that washes out slower from diseased myocardium (due to fibrosis or infarction), compared with the healthy myocardium [40].
Contrast cardiac magnetic resonance images showing areas of late gadolinium hyperenhancement (LGE) consistent with myocardial fibrosis (white arrows). a Dense LGE in hypertrophic basal septum. b LGE in scarred apical aneurysm (shown by asterisk). c Diffuse extensive LGE in end-stage, burnt out, phase of HCM (short axis, and apical four-chamber, three-chamber, and two-chamber views). Note the dilated left ventricle with relatively thin myocardial walls. LV ejection fraction in this patient is 36%. LV left ventricle, RV right ventricle, LA left atrium, RA right atrium, Ao aorta
CMR-based scar burden assessment by LGE has been shown to correlate closely with histopathological findings in HCM [35•, 38, 41], with approximately half to two thirds of patients with HCM showing some degree of myocardial fibrosis [42]. LGE burden can be quantified by mass and as a percentage of total LV mass [43••]. LGE is typically patchy, located in the mid-wall, and involves areas of myocardial hypertrophy and RV insertion points [34, 42]. An LGE pattern that is transmural and diffusely distributed is characteristic of end-stage heart failure and systolic dysfunction (Fig. 3).
The extent of myocardial scarring on CMR has been shown to predict HCM-related adverse events, including progressive heart failure and sudden death [43••, 44,45,46,47, 48••, 49], and therefore, its quantification has gained great importance. A large multicenter study by Chan et al. involving 1293 HCM patients found a linear correlation between risk of sudden cardiac death and amount of LGE, even after adjustment for other disease variables, including age and LVEF [43••]. On the other hand, in another study by Ismail et al., LGE extent was predictive of sudden cardiac death on univariate analysis, but this relationship was not maintained after adjusting for LVEF [50]. Mentias et al. have shown in 1423 adult HCM patients with low/intermediate ESC SCD risk and normal LV systolic function that LGE can provide incremental prognostic utility over ESC SCD risk score, and that LGE percentage was directly and significantly associated with the rate of the composite endpoint (SCD and appropriate ICD discharge) [51••].
Myocardial fibrosis may play a pivotal role in the genesis of arrhythmias by promoting dispersion of electrical activity, resulting in re-entry and ventricular arrhythmias. It has been shown that myocardial fibrosis, as assessed by CMR, is independently associated with NSVT, both in frequency and duration, in HCM [37, 52,53,54,55, 56••, 57, 58].
Currently, the ACCF/AHA guidelines recommend considering using LGE as an arbitrator towards the decision for ICD implantation in those patients in whom sudden cardiac death risk remains ambiguous after considering conventional risk factors (class IIb, level of evidence C) [5]. However, the ESC-SCD Risk Calculator does not incorporate LGE in its risk prediction model [6, 59••].
T1 mapping is a CMR technique whereby the value of native and post-contrast T1 relaxation can be quantified and potentially used to assess myocardial fibrosis. T1 mapping shows promise in discriminating HCM from athlete’s heart [60], hypertensive heart disease [61], amyloidosis [62], and Anderson–Fabry disease [63], and may hold promise for the assessment of SCD risk in the future.
Mitral Valve Apparatus
The mitral valve plays an important role in HCM. More than 50% of HCM patients show some degree of mitral valve abnormality, mostly involving elongation of one or both leaflets [64, 65•]. The abnormal mitral valve plays an important role in the pathophysiology of LVOT obstruction. Drag forces and Venturi effect can cause the anterior mitral leaflet to be pushed towards the septum, leading to the development of dynamic LVOT obstruction, often with high gradients, as well as the typical posteriorly directed mitral regurgitation (MR) jet (Fig. 4).
SAM and Dynamic LVOT obstruction before and after surgical myectomy. a–c The mechanism of LVOT obstruction as seen in transthoracic echocardiography and CMR. In early and mid-systole, both anterior and posterior mitral valve leaflets are moving towards the septum (asterisk in a and c). Septal movement of the anterior leaflet causes narrowing of the LVOT with turbulent blood flow, as well as malcoaptation of the mitral valve leaflets which results in posteriorly directed MR (b). d–f Post myectomy the mitral leaflets do not move towards the septum, the LVOT is not obstructed, and there is laminar blood flow through the LVOT. There is normal coaptation of the leaflets and therefore no signs of MR.
Echocardiographic assessment of the mitral valve structure and function can confirm the presence of LVOT obstruction or can direct our attention to other pathologies. LVOT gradient without concomitant MR can indicate the presence of aortic valve pathology or subaortic membrane. When the MR jet is central or anteriorly directed, mechanisms other than SAM should be sought after, such as mitral valve prolapse, primary mitral pathology, mitral annular calcification, or chordal rupture. The presence of more than one jet of MR can indicate a mixed-pathology mechanism. Trans-esophageal echocardiography (TEE) and 3D echocardiography can be of extreme utility in clearly demonstrating the number of MR jets and their dynamics, as well as the mitral apparatus—including mitral leaflets and their mechanism of malcoaptation—the chords, and the papillary muscles.
Demonstrating the presence of MR, as well as determination of its exact mechanism and severity, are of utmost importance in the assessment of symptomatic HCM patients and treatment planning. In patients in whom the degree or mechanism of MR remain undetermined by resting echocardiography, an exercise stress echo should be considered, as it may unveil latent MR due to its reliance on LV preload and afterload.
Despite the increasing use of CMR in the diagnosis and management of HCM patients, echocardiography remains the modality of choice for imaging of the mitral valve and assessment of LVOT gradients and obstruction. However, CMR is still of value in determining the severity of MR by measuring regurgitant volume and fraction [66]. Due to its spatial resolution and tomographic imaging capabilities, CMR can be useful in the diagnosis of papillary muscle abnormalities, including papillary muscle hypertrophy, antero-apical displacement, presence of multiple accessory papillary muscles, and anomalous papillary muscle insertion into the anterior mitral valve leaflet. All these pathologies can contribute to the obstruction mechanism, with some necessitating papillary muscle reorientation as part of a surgical myectomy [67, 68].
Left Atrial Assessment
Atrial fibrillation (AF) is the most common sustained arrhythmia in HCM [69, 70, 71•, 72•], and atrial remodeling is thought to contribute significantly to its pathogenesis [73•, 74]. Hemodynamic loading conditions can lead to a process in which there is an increase in LA volume, reduction in LA ejection fraction, and worsening of LA strain [74]. However, the possibility of a primary atrial myopathy has not been fully excluded [71•, 72•, 75]. Several studies have demonstrated an association between LA size and AF development [76], AF-related hospitalization, stroke, and death [77, 78, 79•], as well as HCM severity [80].
Routine echocardiographic LA evaluation includes assessment of LA size through quantification of LA diameter, area, or volume. As with LV volumetric assessment, LA volume quantification by CMR is considered the gold standard [81].
Strain analysis by the means of speckle-tracking echocardiography or feature-tracking on CMR has gained popularity recently for the assessment and characterization of LA phasic function (reservoir, conduit, and booster functions). Abnormalities in LA reservoir and conduit function, as well as depression of LA contractility (booster function), have been reported in HCM patients [75, 82, 83], and shown to be correlated with adverse cardiovascular outcomes [75, 84, 85] and atrial fibrillation development [73•].
CMR-based study conducted by Sivalokanathan et al. demonstrated evidence of left atrial fibrosis (by LGE) in all 45 HCM patients recruited for the study [86]. In HCM patients with paroxysmal atrial fibrillation, there was a greater degree of LA structural remodeling, lower LA ejection fraction, as well as higher left atrial fibrosis burden.
Functional Assessment
LV Systolic Function
Most HCM patients have normal to hyperdynamic LV systolic function, and only a minority of them will develop end-stage LV systolic dysfunction (LV ejection fraction < 50%). Diagnosis of patients with LV systolic dysfunction (LVSD) is important as they are at higher risk of SCD and therefore require appropriate and unique management [87]. In TTE, LV systolic function is usually measured using the Simpson’s biplane method, which relies on measurements of end-systolic and end-diastolic LV volumes to calculate stroke volume and LV ejection fraction (LVEF). However, these measurements are not accurate in patients with LVH, as they tend to overestimate LVEF due to preserved and even augmented radial wall thickening seen in HCM (in order to compensate for the reduced longitudinal contraction) [88]. Studies demonstrated that the use of tissue Doppler imaging (TDI) and 2D speckle-tracking echocardiography (STE) can detect LV systolic dysfunction more accurately and in earlier stages [89,90,91]. HCM patients were found to have low systolic velocities and increased systolic asynchrony on TDI, and Systolic (s′) velocity of < 4 cm/s at the lateral mitral annulus was found to be associated with LV dysfunction and disease progression [91]. 2D-STE can be used to detect reduction in regional and global longitudinal strain before reduction in LVEF is evident.
HCM patients often present with decreased longitudinal, circumferential, and radial strains even in the absence of fibrosis. Myocardial fibrosis was found to be associated with reduced segmental longitudinal strain, and both global longitudinal strain measured by echocardiography and LGE obtained from CMR were found to be markers of ventricular arrhythmia in HCM patients [92, 93].
Although echocardiography is a useful tool in LV systolic assessment, CMR is still the gold standard in obtaining an accurate LVEF. It can also be used for strain analysis and LGE quantification, which are both, as mentioned, of prognostic significance [93].
LV Diastolic Function
Diastolic dysfunction is present in almost all patients with HCM. Contributors include LV hypertrophy, nonuniformity of ventricular contraction and relaxation, abnormal intracellular calcium handling, diffuse myocardial ischemia, fibrosis, and LVOT obstruction [5].
Echocardiography is the imaging modality of choice for the assessment of diastolic function in HCM. However, accurate classification of the grade of diastolic function presents a challenge owing to the variability of its presentation (in terms of phenotype, amount of myocardial scarring and fiber disarray, magnitude of myocardial mass, and obstructive versus nonobstructive physiology), and the multifactorial nature of LV diastolic dysfunction in HCM.
Whereas echocardiography has been successfully utilized for estimation of diastolic function and left ventricular filling pressures in various cardiac disorders [94,95,96,97], several studies have shown that conventional mitral inflow and pulmonary venous flow velocities are poorly predictive of LV filling pressures in hypertrophic cardiomyopathy, and these should not be used alone to assess diastolic function in HCM [98, 99]. In accordance with the recent EAE/ASE recommendations [100], an integrated four-criteria approach to assess high LV filling pressures in HCM is recommended and should take into consideration the following variables: (1) average E/e′ ratio > 14, (2) LA volume index (> 34 mL/m2), (3) Ar-A duration ≥ 30 ms, and (4) peak velocity of TR jet by CW Doppler > 2.8 m/s. As individual variables have weak correlations with LV filling pressures when used alone in patients with HCM, a comprehensive approach is recommended for assessment of LV diastolic function in this population [98, 99, 101, 102].
Presently, use of CMR for diastolic evaluation has not been validated in HCM and its use for this purpose requires acquisition of special pulse sequences [103].
Left Ventricular Outflow Tract Obstruction
Dynamic LVOT obstruction is a common manifestation in patients with HCM and is defined by an LVOT gradient ≥ 30 mmHg at rest or post-provocation [5, 6]. It is present in up to 70% of HCM patients at rest or with provocation and can cause significant symptoms such as reduced exercise capacity, exertional dyspnea, chest pain, pre-syncope, and syncope. Presence of significant LVOT obstruction is thought to be related to adverse prognosis and is considered a risk factor for heart failure and sudden cardiac death [104].
Three main mechanisms play a causative role in the pathophysiology of LVOT obstruction. These include LV outflow tract narrowing secondary to localized hypertrophy, rapid LV ejection caused by LV hyper-contractility, and elongated and anteriorly displaced mitral leaflets. These result in creating drag forces and a Venturi effect that move the mitral leaflet towards the septum during systole, thus causing systolic anterior motion (SAM) of the mitral valve leaflet (Fig. 4) [105]. This movement creates contact between the septum and the mitral leaflet, causing LVOT obstruction and elevated gradients. Through interruption of the normal coaptation of the mitral valve leaflets, SAM can result also in the typical posteriorly directed mitral regurgitation. The duration of the septum-mitral leaflet contact determines the degree of obstruction and MR severity.
Accurate assessment and diagnosis of LVOT obstruction has important implications on symptom evaluation and management, as well as the prognostication process. A high degree of caution should be addressed when evaluating the degree of the obstruction and its mechanism. The most effective noninvasive way to evaluate the exact location of the LV gradient and its severity is by transthoracic echocardiography (TTE). The LVOT gradient can be obtained by measuring LVOT velocity using continuous-wave Doppler, which results in a typical dagger-shaped wave (Fig. 5). This shape is typical of LVOT obstruction as the wave starts at early systole and the tip of the dagger represents the timing of contact between the mitral valve leaflet and the septum. This allows us to differentiate LVOT gradient from mid-ventricular gradient or intracavitary obliteration, both occurring later in systole (Fig. 5).
Continuous-wave Doppler measurements in hypertrophic obstructive cardiomyopathy. a, b Typical dagger-shaped Doppler signal of LVOT gradient in latent LVOT obstruction. Resting LVOT gradient is only 19 mmHg (a) with no significant obstruction. After provocation using Valsalva maneuver (b), the gradient increased to 63 mmHg indicating LVOT obstruction. c MR contamination of the LVOT gradient. The first beat (*) demonstrates MR superimposing the LVOT gradient; the jet is almost 6 m per second and does not have a dagger shape. After slight adjustment of the transducer, we can see the previously concealed dagger-shaped jet of the LVOT gradient in the third beat (**). d The typical jet of mid-ventricular obstruction, peaking in late systole
The dagger-shaped Doppler recording is also useful in distinguishing an LVOT gradient from MR, which typically begins earlier in systole and has a different jet shape and a higher peak velocity. The MR Doppler may contaminate LVOT measurements, leading to falsely high gradients, and underscoring the need for a cautious and accurate assessment (Fig. 5).
Another important distinction is between dynamic LVOTO obstruction and a subaortic membrane, both capable of causing similar symptoms and abnormal LVOT gradients from continuous-wave Doppler. However, the absence of SAM, the lack of the typical dagger-shaped continuous-wave Doppler signal, and the lack of a posteriorly directed MR jet, all point to fixed obstruction originating from a possible subaortic membrane rather than SAM-induced LVOT obstruction.
Approximately half of the patients with LVOT obstruction will have LVOT gradients above 30 mmHg at rest, while the other half will demonstrate obstructive physiology only after provocative maneuvers that induce an increase in LV contractility, a decrease in LV afterload, or a decrease in LV volume (latent LVOT obstruction).
Evaluation of LVOT maximal gradients should routinely be obtained both at rest and post- provocation, usually by using the Valsalva maneuver [5, 6]. Stress echocardiography should be considered in symptomatic patients in whom abnormal LVOT gradients could not be assessed or documented with routine maneuvers such as Valsalva or nitrite supplementation [5, 6]. Echocardiography is also the preferred modality to assess the response to treatment in patients with LVOT obstruction, either after implementation of pharmacotherapy or surgical myectomy.
Periprocedural Assessment
As previously described, both echocardiography and CMR have a role in evaluation of symptomatic patients. The appropriate treatment, whether invasive or noninvasive, should be determined only following identifying the main etiology of symptoms and the contributing factors.
Septal reduction therapy is indicated in symptomatic patients with LVOTO ≥ 50 mmHg despite maximally tolerated medical therapy [5, 6]. The two invasive procedures used for septal reduction are surgical myectomy and alcohol septal ablation.
While septal alcohol ablation results only in reduction in the size of the basal septum, surgical myectomy can be more extensive, and may be expanded to include papillary muscle reorientation, aortoplasty, and MV repair. Both echocardiography and CMR have a role in preoperative assessment, thus influencing decision making towards the appropriate procedure and its extent.
Performing a successful surgical myectomy relies on resecting enough myocardial tissue, so the resultant septal thickness remains at 8–10 mm, with the resection extending at least 1 cm below the site of septal-leaflet contact. As the effect of surgical myectomy on LVOT obstruction mechanism is immediate, intraoperative TEE is essential to confirm obtaining good surgical results, e.g., reduced LVOTO and MR, while ruling out possible complications such as ventricular septal defect (VSD) or aortic regurgitation.
Alcohol septal ablation (ASA) involves injection of ethanol into one or more septal perforator arteries, resulting in a controlled infarct in the basal septum and reduction of LVOT gradients. This method requires preprocedural assessment in order to detect proper septal perforator arteries by coronary angiography. In addition, in order to control the location and size of the infarct as much as possible, a contrast agent is injected into the target septal perforator artery and echocardiography is used to visualize the myocardial tissue supplied by that artery. This allows for more accurate estimation of the affected area in order to predict the success of the procedure and avoid possible complications such as extensive infarct involving the RV or papillary muscles. TTE is often used for myocardial imaging, though TEE can be used if needed to optimize imaging. Using echocardiography in order to demonstrate LVOT obstruction alleviation during ASA is of less benefit than in surgical myectomy, as the mechanism of the immediate LVOT gradient reduction during the procedure is probably related to transient decreased global LV systolic function. However, LV function recovers gradually in the early postprocedural period, followed by gradual localized decrease during the 6 months post-procedure during which the infarcted scar tissue is formed [106].
Family Screening
HCM has an autosomal dominant inheritance pattern, and therefore, family members of patients with HCM have a 50% chance to inherit it. In the last decade, the use of genetic testing for family screening has become more common. Progression in genome sequencing methods has resulted in cheaper, more efficient tests. The yield of genetic testing for HCM-causing mutations in patients with HCM ranges between 30 and 50% [107, 108] depending on the characteristics of the population tested. Patients with focal basal septal hypertrophy and those with apical hypertrophy have the lowest yield.
In HCM patients in whom a pathogenic genetic variant is not detected, family screening for first-degree family members should be done every 3–5 years by the means of echocardiography, ECG, and physical examination. On the other hand, if a pathogenic genetic variant is known, family screening can be performed by a simple blood test for the known familial variant. In these cases, echocardiography and MRI imaging still have an important role, as not all patients who carry the pathogenic variant will develop phenotypic expression, due to incomplete penetrance [109]. Given this, family members who are geno-positive should undergo initial echocardiographic evaluation in order to determine if they demonstrate the hypertrophic features (pheno-positive) or not (pheno-negative).
Although CMR is not considered a part of routine family screening, it is of use in cases of nonconclusive diagnosis. In addition to more accurate evaluation of LV wall thickness and morphology, CMR can detect other structural features that may favor HCM diagnosis such as apical-septal bundles [110•], infero-septal crypts [111•], and abnormal late gadolinium enhancement.
Preclinical Diagnosis
In geno-positive pheno-negative patients, a yearly follow-up with ECG and echocardiography is recommended in order to detect signs of LV hypertrophy, as an HCM phenotype may develop with time [5]. In patients who are geno-negative or patients who did not perform genetic testing, echocardiography, alongside physical examination and ECG, remains the imaging of choice for screening and follow-up.
HCM Phenocopies
Diagnosis of HCM is based on the demonstration of LVH, with maximal left ventricular wall thickness ≥ 15 mm. However, hypertrophic cardiomyopathy is not the only condition in which LVH is present. LVH that is not HCM-induced is mostly related to abnormal loading condition such as hypertension, aortic stenosis, or morbid obesity. Athlete’s heart can mimic HCM, and the differentiation between the two entities can prove to be challenging. In athlete’s heart, in contrast to HCM, LV hypertrophy is often accompanied by LV cavity enlargement. A period of deconditioning can assist in clarifying the LVH mechanism, as in athlete’s heart, this will often result in decreased LV mass or wall thickness [112].
It is more challenging, however, to distinguish between HCM and other, more rare, inherited syndromes causing LVH. Diagnosis of these syndromes is of clinical importance as some phenocopies have specific treatment or prognostic implications. Some phenocopies can involve multiple systems, such as Fabry’s disease, which can cause renal dysfunction, typical cutaneous lesions, peripheral neuropathy, and hypertension. Another example is amyloidosis, which can cause LVH, progressive neuropathy, and kidney disease. Other phenocopies, like PRKAG2-associated cardiomyopathy, can manifest merely in the heart, making it more challenging to diagnose. Diagnosis of HCM phenocopies can be done by genetic testing with panels containing the common HCM genocopies. However, these are expensive and not always available. Apart from a thorough clinical history and physical examination, cardiac imaging is a useful tool for differentiation of the various HCM phenocopies. Most phenocopies are difficult to diagnose by echocardiography alone, although it may provide us with clues for a specific diagnosis such as the extent and localization of hypertrophy. While in some phenocopies, like Fabry’s disease, the hypertrophy can be either asymmetric or concentric [113], in others, like amyloidosis, concentric hypertrophy is more common [114]. Echocardiographic assessment of longitudinal strain can also help identifying cardiac amyloidosis, which has a distinct pattern of apical sparing [114]. CMR, given its superior morphological assessment over echocardiography, as well as the ability to characterize the myocardial tissue as detailed earlier, can be of extreme aid in the case of suspected HCM phenocopy presence. For example, while in HCM, the LGE pattern is mid-myocardial and often occurs in the hypertrophied segments, in Fabry’s disease, the LGE pattern is typically localized in the basal-inferior segment [113].
Conclusion
Noninvasive cardiac imaging has an undeniable role in the evaluation of HCM patients, both from the diagnostic and therapeutic aspects. While echocardiography remains the first-line modality for HCM evaluation given its availability, cost, and simplicity, CMR has emerged as the gold standard image modality for tissue characterization and structural and volumetric assessment. However, as echocardiography has certain advantages over CMR, particularly in the assessment of valvular severity, as well as hemodynamic evaluation, an integrated approach combining both modalities will add to a more accurate HCM diagnosis and better management.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–9.
Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64(1):83–99. https://doi.org/10.1016/j.jacc.2014.05.003.
Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249–54. https://doi.org/10.1016/j.jacc.2015.01.019.
•• Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(7):655–68. https://doi.org/10.1056/NEJMra1710575 This is an important comprehensive review of HCM from an epidemiological, diagnostic, and therapeutic perspectives.
American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American Society of E, American Society of Nuclear C, Heart Failure Society of A, Heart Rhythm S et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2011;142(6):1303–1338. doi:https://doi.org/10.1016/j.jtcvs.2011.10.019.
Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014. 2014;35(39):2733–79. https://doi.org/10.1093/eurheartj/ehu284.
Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24(5):473–98. https://doi.org/10.1016/j.echo.2011.03.006.
•• Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–85. https://doi.org/10.1056/NEJM200006153422403 This study showed a correlation between the severity of LV hypertrophy and risk of sudden death.
Olivotto I, Gistri R, Petrone P, Pedemonte E, Vargiu D, Cecchi F. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41(2):315–21.
•• Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54(3):220–8. https://doi.org/10.1016/j.jacc.2009.05.006 This study emphasized the emerging role of CMR in characterizeing the diverse patterns of LV hypertrophy in HCM.
Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112(6):855–61. https://doi.org/10.1161/CIRCULATIONAHA.104.507723.
Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90(6):645–9.
Maron MS, Hauser TH, Dubrow E, Horst TA, Kissinger KV, Udelson JE, et al. Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol. 2007;100(8):1293–8. https://doi.org/10.1016/j.amjcard.2007.05.061.
Keeling AN, Carr JC, Choudhury L. Right ventricular hypertrophy and scarring in mutation positive hypertrophic cardiomyopathy. Eur Heart J. 2010;31(3):381. https://doi.org/10.1093/eurheartj/ehp528.
• Hindieh W, Weissler-Snir A, Hammer H, Adler A, Rakowski H, Chan RH. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10(8). https://doi.org/10.1161/CIRCIMAGING.117.006309 This study highlights the discrepancy in maximal wall thickness between CMR and echocardiography.
• Bois JP, Geske JB, Foley TA, Ommen SR, Pellikka PA. Comparison of maximal wall thickness in hypertrophic cardiomyopathy differs between magnetic resonance imaging and transthoracic echocardiography. Am J Cardiol. 2017;119(4):643–50. https://doi.org/10.1016/j.amjcard.2016.11.010 This study highlights the discrepancy in maximal wall thickness between CMR and echocardiography.
Pennell DJ. Ventricular volume and mass by CMR. J Cardiovasc Magn Reson. 2002;4(4):507–13.
Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, et al. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114(7):654–61. https://doi.org/10.1161/CIRCULATIONAHA.106.626143.
Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52(7):559–66. https://doi.org/10.1016/j.jacc.2008.04.047.
Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357(9254):420–4. https://doi.org/10.1016/S0140-6736(00)04005-8.
Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, et al. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118(15):1541–9. https://doi.org/10.1161/CIRCULATIONAHA.108.781401.
Rowin EJ, Maron BJ, Chokshi A, Maron MS. Left ventricular apical aneurysm in hypertrophic cardiomyopathy as a risk factor for sudden death at any age. Pacing Clin Electrophysiol. 2018;41:1031–3. https://doi.org/10.1111/pace.13413.
•• Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, et al. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol. 2017;69(7):761–73. https://doi.org/10.1016/j.jacc.2016.11.063 This study shows a strong correlation between apical aneurysm in HCM and adverse outcomes.
Holloway CJ, Betts TR, Neubauer S, Myerson SG. Hypertrophic cardiomyopathy complicated by large apical aneurysm and thrombus, presenting as ventricular tachycardia. J Am Coll Cardiol. 2010;56(23):1961. https://doi.org/10.1016/j.jacc.2010.01.078.
Raza M, Chalfoun N, Wissam A, Hashmi H, McNamara R. Hypertrophic cardiomyopathy with a large apical ventricular aneurysm and mural thrombus. Glob Cardiol Sci Pract. 2018;2018(1):9. https://doi.org/10.21542/gcsp.2018.9.
Kalra A, Maron MS, Rowin EJ, Colgan TK, Lesser JR, Maron BJ. Coronary embolization in hypertrophic cardiomyopathy with left ventricular apical aneurysm. Am J Cardiol. 2015;115(9):1318–9. https://doi.org/10.1016/j.amjcard.2015.02.016.
Matsubara K, Nakamura T, Kuribayashi T, Azuma A, Nakagawa M. Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;42(2):288–95.
Fattori R, Biagini E, Lorenzini M, Buttazzi K, Lovato L, Rapezzi C. Significance of magnetic resonance imaging in apical hypertrophic cardiomyopathy. Am J Cardiol. 2010;105(11):1592–6. https://doi.org/10.1016/j.amjcard.2010.01.020.
Pons-Llado G, Carreras F, Borras X, Palmer J, Llauger J, Bayes de Luna A. Comparison of morphologic assessment of hypertrophic cardiomyopathy by magnetic resonance versus echocardiographic imaging. Am J Cardiol. 1997;79(12):1651–6.
Eriksson MJ, Sonnenberg B, Woo A, Rakowski P, Parker TG, Wigle ED, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39(4):638–45.
Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, et al. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21(11):1179–201; quiz 281. https://doi.org/10.1016/j.echo.2008.09.009.
Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44(5):412–27. https://doi.org/10.1111/j.1365-2559.2004.01835.x.
Mozaffarian D, Caldwell JH. Right ventricular involvement in hypertrophic cardiomyopathy: a case report and literature review. Clin Cardiol. 2001;24(1):2–8.
Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43(12):2260–4. https://doi.org/10.1016/j.jacc.2004.03.035.
• Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph-Edwards A, et al. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. 2013;6(5):587–96. https://doi.org/10.1016/j.jcmg.2012.09.018 This study shows the correlation between LGE quantification and histopathological findings in CMR.
Kim RJ, Judd RM. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol. 2003;41(9):1568–72.
Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. 2009;54(3):242–9. https://doi.org/10.1016/j.jacc.2009.04.026.
Papavassiliu T, Schnabel P, Schroder M, Borggrefe M. CMR scarring in a patient with hypertrophic cardiomyopathy correlates well with histological findings of fibrosis. Eur Heart J. 2005;26(22):2395. https://doi.org/10.1093/eurheartj/ehi518.
Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D'Andrea A, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16(3):280. https://doi.org/10.1093/ehjci/jeu291.
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002.
Maron MS. Contrast-enhanced CMR in HCM: what lies behind the bright light of LGE and why it now matters. JACC Cardiovasc Imaging. 2013;6(5):597–9. https://doi.org/10.1016/j.jcmg.2012.10.028.
Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53(3):284–91. https://doi.org/10.1016/j.jacc.2008.08.064.
•• Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130(6):484–95. https://doi.org/10.1161/CIRCULATIONAHA.113.007094 This study examined LGE in 1293 HCM patients and showed a linear correlation between risk of sudden cardiac death and amount of LGE, even after adjustment for other disease variables.
O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):867–74. https://doi.org/10.1016/j.jacc.2010.05.010.
Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):875–87. https://doi.org/10.1016/j.jacc.2010.05.007.
Neilan TG, Farhad H, Mayrhofer T, Shah RV, Dodson JA, Abbasi SA, et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging. 2015;8(4):414–23. https://doi.org/10.1016/j.jcmg.2014.11.017.
Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5(4):370–7. https://doi.org/10.1016/j.jcmg.2011.11.021.
•• Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging. 2016;9(12):1392–402. https://doi.org/10.1016/j.jcmg.2016.02.031 This meta-analysis consisted of seven study, involving 2993 HCM patients, and showed the prognostic value of LGE in SCD prediction.
Ismail TF, Jabbour A, Gulati A, Mallorie A, Raza S, Cowling TE, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100(23):1851–8. https://doi.org/10.1136/heartjnl-2013-305471.
Ismail TF, Prasad SK, Pennell DJ. Prognostic importance of late gadolinium enhancement cardiovascular magnetic resonance in cardiomyopathy. Heart. 2012;98(6):438–42. https://doi.org/10.1136/heartjnl-2011-300814.
•• Mentias A, Raeisi-Giglou P, Smedira NG, Feng K, Sato K, Wazni O, et al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol. 2018;72(8):857–70. https://doi.org/10.1016/j.jacc.2018.05.060 This study shows the incremental prognostic utility of LGE extent in low to intermediate-risk HCM patients.
Kwon DH, Setser RM, Popovic ZB, Thamilarasan M, Sola S, Schoenhagen P, et al. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging. 2008;24(6):617–25. https://doi.org/10.1007/s10554-008-9292-6.
Fluechter S, Kuschyk J, Wolpert C, Doesch C, Veltmann C, Haghi D, et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2010;12:30. https://doi.org/10.1186/1532-429X-12-30.
Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(14):1369–74. https://doi.org/10.1016/j.jacc.2007.11.071.
Appelbaum E, Maron BJ, Adabag S, Hauser TH, Lesser JR, Haas TS, et al. Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5(1):78–85. https://doi.org/10.1161/CIRCIMAGING.111.963819.
•• Mc LA, Ellims AH, Prabhu S, Voskoboinik A, Iles LM, Hare JL, et al. Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27(5):571–80. https://doi.org/10.1111/jce.12948 This study shows an association between LGE and T1-mapping for LGE extent and ventricular arrhythmia burden in HCM.
Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3(1):51–8. https://doi.org/10.1161/CIRCHEARTFAILURE.109.854026.
Weissler-Snir A, Hindieh W, Spears DA, Adler A, Rakowski H, Chan RH. The relationship between the quantitative extent of late gadolinium enhancement and burden of nonsustained ventricular tachycardia in hypertrophic cardiomyopathy: a delayed contrast-enhanced magnetic resonance study. J Cardiovasc Electrophysiol. 2019;30:651–7. https://doi.org/10.1111/jce.13855.
•• O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014;35(30):2010–20. https://doi.org/10.1093/eurheartj/eht439 This study led to the development of a novel sudden cardiac death risk calculator which has been encorporated in the ESC guidelines.
Swoboda PP, McDiarmid AK, Erhayiem B, Broadbent DA, Dobson LE, Garg P, et al. Assessing myocardial extracellular volume by T1 mapping to distinguish hypertrophic cardiomyopathy from athlete’s heart. J Am Coll Cardiol. 2016;67(18):2189–90. https://doi.org/10.1016/j.jacc.2016.02.054.
Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, et al. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8(12). https://doi.org/10.1161/CIRCIMAGING.115.003285.
Nam BD, Kim SM, Jung HN, Kim Y, Choe YH. Comparison of quantitative imaging parameters using cardiovascular magnetic resonance between cardiac amyloidosis and hypertrophic cardiomyopathy: inversion time scout versus T1 mapping. Int J Cardiovasc Imaging. 2018;34(11):1769–77. https://doi.org/10.1007/s10554-018-1385-2.
Karur GR, Robison S, Iwanochko RM, Morel CF, Crean AM, Thavendiranathan P, et al. Use of myocardial T1 mapping at 3.0 T to differentiate Anderson-Fabry disease from hypertrophic cardiomyopathy. Radiology. 2018;288(2):398–406. https://doi.org/10.1148/radiol.2018172613.
Klues HG, Maron BJ, Dollar AL, Roberts WC. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation. 1992;85(5):1651–60.
• Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124(1):40–7. https://doi.org/10.1161/CIRCULATIONAHA.110.985812 This study shows the CMR-based various mitral valve morphologies and abnormalities in HCM patients.
Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65(11):1078–88. https://doi.org/10.1016/j.jacc.2014.12.047.
Patel P, Dhillon A, Popovic ZB, Smedira NG, Rizzo J, Thamilarasan M, et al. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging. 2015;8(7):e003132. https://doi.org/10.1161/CIRCIMAGING.115.003132.
Kwon DH, Smedira NG, Thamilarasan M, Lytle BW, Lever H, Desai MY. Characteristics and surgical outcomes of symptomatic patients with hypertrophic cardiomyopathy with abnormal papillary muscle morphology undergoing papillary muscle reorientation. J Thorac Cardiovasc Surg. 2010;140(2):317–24. https://doi.org/10.1016/j.jtcvs.2009.10.045.
Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517–24.
Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014;100(6):465–72. https://doi.org/10.1136/heartjnl-2013-304276.
• Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, et al. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136(25):2420–36. https://doi.org/10.1161/CIRCULATIONAHA.117.029267 This study shows the prevalence and clinical importnace of atrial fibrillation in patietns with HCM, associated with a low disease-related mortality.
• Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc. 2014;3(3):e001002. https://doi.org/10.1161/JAHA.114.001002 This study showed that atrial fibrillation is a strong predictor of mortality, even after adjustment to other risk facotrs.
• Maron BJ, Haas TS, Maron MS, Lesser JR, Browning JA, Chan RH, et al. Left atrial remodeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am J Cardiol. 2014;113(8):1394–400. https://doi.org/10.1016/j.amjcard.2013.12.045 This study assessed the left atrial function, morphology, and mechanics by CMR, and correlated this to atrial fibrillation development.
Kim KJ, Choi HM, Yoon YE, Kim HL, Lee SP, Kim HK, et al. Left atrial mechanical function and global strain in hypertrophic cardiomyopathy. PLoS One. 2016;11(6):e0157433. https://doi.org/10.1371/journal.pone.0157433.
Vasquez N, Ostrander BT, Lu DY, Ventoulis I, Haileselassie B, Goyal S, et al. Low left atrial strain is associated with adverse outcomes in hypertrophic cardiomyopathy patients. J Am Soc Echocardiogr. 2019;32:593–603.e1. https://doi.org/10.1016/j.echo.2019.01.007.
Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, et al. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10(2). https://doi.org/10.1161/CIRCEP.116.004052.
Yang WI, Shim CY, Kim YJ, Kim SA, Rhee SJ, Choi EY, et al. Left atrial volume index: a predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22(12):1338–43. https://doi.org/10.1016/j.echo.2009.09.016.
Tani T, Yagi T, Kitai T, Kim K, Nakamura H, Konda T, et al. Left atrial volume predicts adverse cardiac and cerebrovascular events in patients with hypertrophic cardiomyopathy. Cardiovasc Ultrasound. 2011;9:34. https://doi.org/10.1186/1476-7120-9-34.
• Hiemstra YL, Debonnaire P, Bootsma M, van Zwet EW, Delgado V, Schalij MJ, et al. Global longitudinal strain and left atrial volume index provide incremental prognostic value in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10(7). https://doi.org/10.1161/CIRCIMAGING.116.005706 This study shows that reduced LV strain, as well as large LA volumes are associated with adverse evets in HCM patients.
Yang H, Woo A, Monakier D, Jamorski M, Fedwick K, Wigle ED, et al. Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr. 2005;18(10):1074–82. https://doi.org/10.1016/j.echo.2005.06.011.
Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. https://doi.org/10.1186/1532-429X-12-65.
Rosca M, Popescu BA, Beladan CC, Calin A, Muraru D, Popa EC, et al. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2010;23(10):1090–8. https://doi.org/10.1016/j.echo.2010.07.016.
Paraskevaidis IA, Panou F, Papadopoulos C, Farmakis D, Parissis J, Ikonomidis I, et al. Evaluation of left atrial longitudinal function in patients with hypertrophic cardiomyopathy: a tissue Doppler imaging and two-dimensional strain study. Heart. 2009;95(6):483–9. https://doi.org/10.1136/hrt.2008.146548.
Fujimoto K, Inoue K, Saito M, Higashi H, Kono T, Uetani T, et al. Incremental value of left atrial active function measured by speckle tracking echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography. 2018;35(8):1138–48. https://doi.org/10.1111/echo.13886.
Hinojar R, Zamorano JL, Fernandez-Mendez M, Esteban A, Plaza-Martin M, Gonzalez-Gomez A, et al. Prognostic value of left atrial function by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2019;35:1055–65. https://doi.org/10.1007/s10554-019-01534-8.
Sivalokanathan S, Zghaib T, Greenland GV, Vasquez N, Kudchadkar SM, Kontari E, et al. Hypertrophic cardiomyopathy patients with paroxysmal atrial fibrillation have a high burden of left atrial fibrosis by cardiac magnetic resonance imaging. JACC Clin Electrophysiol. 2019;5(3):364–75. https://doi.org/10.1016/j.jacep.2018.10.016.
Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114(3):216–25. https://doi.org/10.1161/CIRCULATIONAHA.105.583500.
Maciver DH. A new method for quantification of left ventricular systolic function using a corrected ejection fraction. Eur J Echocardiogr. 2011;12(3):228–34. https://doi.org/10.1093/ejechocard/jeq185.
Cardim N, Longo S, Ferreira T, Pereira A, Gouveia A, Reis RP, et al. Tissue Doppler imaging assessment of long axis left ventricular function in hypertensive patients with concentric left ventricular hypertrophy: differential diagnosis with hypertrophic cardiomyopathy. Rev Port Cardiol. 2002;21(6):709–40.
Oki T, Mishiro Y, Yamada H, Onose Y, Matsuoka M, Wakatsuki T, et al. Detection of left ventricular regional relaxation abnormalities and asynchrony in patients with hypertrophic cardiomyopathy with the use of tissue Doppler imaging. Am Heart J. 2000;139(3):497–502.
Bayrak F, Kahveci G, Mutlu B, Sonmez K, Degertekin M. Tissue Doppler imaging to predict clinical course of patients with hypertrophic cardiomyopathy. Eur J Echocardiogr. 2008;9(2):278–83. https://doi.org/10.1093/ejechocard/jen049.
Popovic ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, et al. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21(12):1299–305. https://doi.org/10.1016/j.echo.2008.09.011.
Haland TF, Almaas VM, Hasselberg NE, Saberniak J, Leren IS, Hopp E, et al. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17(6):613–21. https://doi.org/10.1093/ehjci/jew005.
Rossvoll O, Hatle LK. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures. J Am Coll Cardiol. 1993;21(7):1687–96.
Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22(7):1972–82.
Vanoverschelde JL, Robert AR, Gerbaux A, Michel X, Hanet C, Wijns W. Noninvasive estimation of pulmonary arterial wedge pressure with Doppler transmitral flow velocity pattern in patients with known heart disease. Am J Cardiol. 1995;75(5):383–9.
Pozzoli M, Capomolla S, Pinna G, Cobelli F, Tavazzi L. Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure with and without mitral regurgitation. J Am Coll Cardiol. 1996;27(4):883–93.
Nishimura RA, Appleton CP, Redfield MM, Ilstrup DM, Holmes DR Jr, Tajik AJ. Noninvasive doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol. 1996;28(5):1226–33. https://doi.org/10.1016/S0735-1097(96)00315-4.
Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH 3rd, Zoghbi WA, Quinones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99(2):254–61.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60. https://doi.org/10.1093/ehjci/jew082.
Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116(23):2702–8. https://doi.org/10.1161/CIRCULATIONAHA.107.698985.
Aquaro GD, Pizzino F, Terrizzi A, Carerj S, Khandheria BK, Di Bella G. Diastolic dysfunction evaluated by cardiac magnetic resonance: the value of the combined assessment of atrial and ventricular function. Eur Radiol. 2019;29(3):1555–64. https://doi.org/10.1007/s00330-018-5571-3.
Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303. https://doi.org/10.1056/NEJMoa021332.
Sherrid MV, Gunsburg DZ, Moldenhauer S, Pearle G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000;36(4):1344–54.
Carasso S, Woo A, Yang H, Schwartz L, Vannan MA, Jamorski M, et al. Myocardial mechanics explains the time course of benefit for septal ethanol ablation for hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2008;21(5):493–9. https://doi.org/10.1016/j.echo.2007.08.020.
Millat G, Bouvagnet P, Chevalier P, Dauphin C, Jouk PS, Da Costa A, et al. Prevalence and spectrum of mutations in a cohort of 192 unrelated patients with hypertrophic cardiomyopathy. Eur J Med Genet. 2010;53(5):261–7. https://doi.org/10.1016/j.ejmg.2010.07.007.
Alfares AA, Kelly MA, McDermott G, Funke BH, Lebo MS, Baxter SB, et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet Med. 2015;17(11):880–8. https://doi.org/10.1038/gim.2014.205.
Golbus JR, Puckelwartz MJ, Fahrenbach JP, Dellefave-Castillo LM, Wolfgeher D, McNally EM. Population-based variation in cardiomyopathy genes. Circ Cardiovasc Genet. 2012;5(4):391–9. https://doi.org/10.1161/CIRCGENETICS.112.962928.
• Gruner C, Chan RH, Crean A, Rakowski H, Rowin EJ, Care M, et al. Significance of left ventricular apical-basal muscle bundle identified by cardiovascular magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Eur Heart J. 2014;35(39):2706–13. https://doi.org/10.1093/eurheartj/ehu154 This study highlights the role of CMR in identifying apical-septal bundles as a part of HCM phenotype.
• Maron MS, Rowin EJ, Lin D, Appelbaum E, Chan RH, Gibson CM, et al. Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5(4):441–7. https://doi.org/10.1161/CIRCIMAGING.112.972760 This study highlights the role of CMR in identifying myocardial crypts as a part of HCM phenotype.
Caselli S, Maron MS, Urbano-Moral JA, Pandian NG, Maron BJ, Pelliccia A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2014;114(9):1383–9. https://doi.org/10.1016/j.amjcard.2014.07.070.
Deva DP, Hanneman K, Li Q, Ng MY, Wasim S, Morel C, et al. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-Fabry disease. J Cardiovasc Magn Reson. 2016;18:14. https://doi.org/10.1186/s12968-016-0233-6.
Falk RH, Quarta CC, Dorbala S. How to image cardiac amyloidosis. Circ Cardiovasc Imaging. 2014;7(3):552–62. https://doi.org/10.1161/CIRCIMAGING.113.001396.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Manhal Habib, Sara Hoss, and Harry Rakowski declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Echocardiography
Rights and permissions
About this article
Cite this article
Habib, M., Hoss, S. & Rakowski, H. Evaluation of Hypertrophic Cardiomyopathy: Newer Echo and MRI Approaches. Curr Cardiol Rep 21, 75 (2019). https://doi.org/10.1007/s11886-019-1173-1
Published:
DOI: https://doi.org/10.1007/s11886-019-1173-1