Abstract
Purpose of Review
Evidence has clearly demonstrated the importance of lifestyle factors (e.g., diet, physical activity, smoking) in the development of cardiovascular disease (CVD). Interventions targeting these behaviors may improve outcomes for CVD patients. The aim of this review is to summarize the effects of lifestyle interventions in individuals with established CVD.
Recent Findings
Most recent trials focused on diet, physical activity, stress reduction, or a combination of these. Findings were mixed, but most interventions improved at least some markers of cardiovascular risk. Few studies measured long-term clinical outcomes, but some suggested a possible benefit of stress reduction and multifaceted interventions on cardiovascular events.
Summary
The benefits of lifestyle change for CVD patients have been established by decades of evidence. However, further research is needed to determine the optimal intensity, duration, and mode of delivery for interventions. Additional studies with long-term follow-up and measurement of clinical outcomes are also needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the USA, though cancer has now overtaken cardiovascular disease as the number one cause of death among women [1]. Trans-cultural studies, such as the Seven Countries Study [2,3,4] and in particular migration studies such as Ni-Hon-San [5,6,7], have long since established the powerful role of environmental, cultural, and lifestyle factors in the epidemiology of heart disease. Lifestyle factors best known to influence cardiovascular disease risk include diet [8], physical activity [9, 10], and smoking [11]. Alcohol intake [12], psychological stress, [13], sleep [14], and social connections [15] are also associated with cardiovascular risk. These factors interact in complex ways, inviting a holistic view of lifestyle.
Diet is arguably the most complex of the lifestyle components and influences the pathogenesis of coronary artery disease in a variety of ways. Once coronary artery atherosclerosis is established, diet plays a role in determining both progression of plaque deposition and the reactivity of the endothelium, both of which may be predictive of cardiac events [16,17,18]. Epidemiologic studies have demonstrated reliable associations between dietary factors and cardiovascular morbidity and mortality. [8, 19] In particular, high intake of sodium, processed meats, and sugar-sweetened beverages and low intake of nuts/seeds, seafood omega-3 fats, fruits, and vegetables have been implicated in diet-related cardiometabolic deaths in the USA. [8] Intervention studies in healthy or high-risk populations have confirmed that dietary manipulations can improve modifiable CVD risk factors [20, 21] and reduce the incidence of cardiovascular events [22].
Physical activity (PA) is defined as any movement of the body by skeletal muscle that causes energy to be utilized past resting expenditure [23]. Many studies have shown that routine PA can reduce complications and mortality from cardiovascular diseases [9, 10]. PA has been shown to improve many cardiovascular risk factors, including insulin resistance [24], hypertension [25], blood lipids [26], and coronary artery calcium [26]. Because of the strength of evidence for PA for cardiovascular risk reduction, the Centers for Disease Control and Prevention, the American College of Sports Medicine, and the American Heart Association all recommend that all adults engage in a minimum of 30 min of moderate to intense exercise 5 days a week. [27, 28] Physical activity is also beneficial for most individuals with established CVD. A meta-analysis of 44 randomized controlled trials (RCTs) demonstrated that aerobic exercise reduces blood pressure in normotensive and hypertensive individuals, with a stronger effect in the latter group [29, 30]. Furthermore, evidence suggests that exercise-based cardiac rehabilitation effectively reduces cardiovascular mortality and hospital admission in CVD patients [31••].
In 2004, 10% of cardiovascular deaths worldwide in adults aged 30 and older were attributable to tobacco; among individuals between the ages 30–44, this figure was 35% [32]. Tobacco smoke contains more than 7000 compounds [33], at least 98 of which are considered hazardous for human inhalation [34]. The mechanisms by which smoking causes harmful effects on the cardiovascular system are complex, but likely include vascular endothelial cell dysfunction initiated by reduced nitric oxide bioavailability, increased lipid oxidation, and activation of proinflammatory and pro-coagulant states [11]. Smoking is associated with increased risk of heart failure (HF) and death. [26, 35] For past smokers, there is a dose-response relationship between pack-years of exposure and HF risk. [35] Current smoking and, to a lesser extent, former smoking have also been associated with subclinical atherosclerosis in multiple populations [36,37,38].
Psychological stress may also increase risk of CVD [13, 39]. Chronic stress, whether in childhood or adulthood, has been associated with a 40–60% increased risk of CHD [39]. Job-related stress and social isolation have been associated with 1.3-fold and 1.5-fold increased risk of CHD, respectively [13]. Furthermore, the acute stress associated with receiving bad news, like being diagnosed with cancer or the loss of a loved one [40], may increase the risk of myocardial infarction or cardiovascular death. Outbursts of intense anger may also trigger cardiovascular events in the short term [40].
The pivotal role of lifestyle in the prevention of CVD is well documented, and some lifestyle interventions for CVD patients have demonstrated effectiveness [30, 31••, 41]. The purpose of this review is to provide an update to the existing literature on the role of lifestyle medicine in managing CVD. Therefore, we describe relevant randomized controlled trials published between 2012 and 2017 that tested a lifestyle intervention in individuals with any of the following diagnoses: hypertension, coronary heart/artery disease (CHD/CAD) stroke, congestive heart failure (CHF), atrial fibrillation (AF), or peripheral vascular disease. We included interventions that targeted diet, physical activity, smoking, stress, or a combination of these. Because of low availability of interventions targeting sleep or social connectedness, we did not include studies on these topics. We also excluded studies of dietary supplements or programs to improve disease-specific management behaviors (e.g., medication adherence, self-monitoring). Details of included trials are presented in Appendix Tables 1 and 2.
Diet Interventions
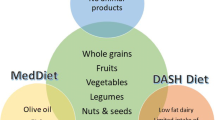
Among the most-studied dietary patterns for primary and secondary prevention of cardiovascular disease are the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet [42]. The DASH diet is generally high in fruits, vegetables, whole grains, low-fat dairy, lean meats, and fish; and low in total fat, saturated fat, and cholesterol [43, 44]. Traditional Mediterranean diets are high in fruits, vegetables, and whole grains, but also emphasize fatty fish, oils, and nuts [45, 46]. The 2013 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines endorse the DASH diet, in addition to the USDA Food Pattern and the AHA Diet as a heart-healthy dietary pattern that can help lower LDL cholesterol (LDL-C) and blood pressure [45]. Plant-based diets such as the portfolio diet [47, 48] and the Ornish Program [41] have produced significant improvements in cardiovascular risk. Recent observational studies also lend support to plant-based diets. Such studies have found increased risk of cardiovascular mortality associated with higher intake of processed meat [8] and animal protein. [49] On the other hand, greater intake of fruits, vegetables, nuts/seeds [8], and plant protein [49] were protective.
There is an abundance of literature on the cardiovascular risk-reducing effects of the Mediterranean diet, most notably from the Lyon Diet Heart Study in patients recovering from myocardial infarction (MI), which reported a 70% reduction in CV events and mortality, [50] and the PREDIMED trial in Spanish adults at high risk for CVD, which reported an approximately 30% reduction in major cardiovascular events [22, 51, 52]. However, we did not locate any recent (2012 or later) papers reporting effects of the Mediterranean diet on relevant clinical outcomes in individuals with CVD. The CORDIOPREV trial currently underway in Spain promises to shed light on the comparative effects of a Mediterranean or low-fat diet recommended by the National Cholesterol Education Program and the American Heart Association on cardiovascular events in CHD patients [53]. While both diets limit saturated fat and cholesterol and emphasize a variety of healthful foods (e.g., fruits, vegetables, whole grains, legumes, and dairy products), the Mediterranean diet also includes advice to reduce consumption of meat, particularly red meat, and to consume a higher percentage of calories from monounsaturated fats. Olive oil is provided to assist participants in achieving the latter goal. Thus far, this study has produced reports on baseline data and gene-diet interactions [54, 55].
Recent intervention trials of the DASH diet have demonstrated beneficial effects on blood pressure and other cardiovascular risk markers in individuals with hypertension [56, 57]. Paula and colleagues [57] found that a DASH diet was more effective than one based on American Diabetes Association (ADA) recommendations in reducing blood pressure in diabetic patients with hypertension. After 4 weeks, the intervention significantly reduced 24-h ambulatory SBP (− 12.5 vs − 1.5 mmHg, P < 0.001) and DBP (− 7.0 vs − 2.0 mmHg, P = 0.013) relative to control. Increases in daily number of steps and changes in urinary sodium and potassium suggest that intervention participants adhered reasonably well to the protocol. The groups experienced similar reductions in weight. Therefore, treatment effects on blood pressure were likely attributable to the DASH diet and/or increased PA rather than weight loss.
On the other hand, Miller et al. [58] did not observe significant effects of a “DASH-Plus” intervention on blood pressure or lipids in African American adults with controlled hypertension. DASH-Plus included in-person coaching, weekly phone calls, and a $30/week allowance for purchasing selected foods high in potassium (specific fruit, vegetable, nut, and bean products). It is possible that, because participants had well-controlled baseline blood pressure, the ability to further reduce blood pressure was limited. Unexpectedly, the DASH-Plus intervention increased fasting blood glucose relative to control (mean difference between groups 10.9 mg/dL, P = 0.002), though this effect was not evident in participants without diabetes (mean difference 3.9 mg/dL, P = 0.20). In contrast with the findings of the study by Miller et al., Paula et al. [57] did not observe detrimental effects of the DASH diet on glucose metabolism.
Although we did not identify any studies published in the last 5 years on plant-based diets such as Ornish, portfolio, or others in individuals with CVD, we are compelled to acknowledge the robust existing literature on these dietary patterns. Plant-based diets have been shown to consistently reduce plasma lipids in individuals with and without hyperlipidemia [59]. In the 1990s, the Lifestyle Heart Study demonstrated that a comprehensive lifestyle change program including a low-fat vegetarian diet (the Ornish Program) could induce regression of coronary atherosclerosis [60], with continued improvement and reduction in cardiac events over 5 years [41]. The Ornish Program has since been approved for reimbursement by Medicare as an intensive cardiac rehabilitation program [61]. The addition of plant sterols, viscous fibers (e.g., psyllium, β-glucan), soy protein, and nuts to a vegetarian diet low in saturated fat produces even greater improvements in the lipid profile [62]. This “portfolio” diet has been shown to reduce LDL-C and C-reactive protein (CRP) as effectively as statin treatment in hyperlipidemic adults [48], and the diet’s lipid-lowering effects have been demonstrated consistently in subsequent studies [63]. A 2015 trial comparing a portfolio diet to a DASH-type diet in individuals with hyperlipidemia reported better reduction in blood pressure, total cholesterol, LDL-C, CRP, and measures of 10-year CHD and CVD risk with the portfolio diet [64].
Some trials have tested the effects of specific foods in the diet on markers of cardiovascular health [65,66,67,68]. Nuts are especially prominent in the literature on cardiovascular effects of foods [67,68,69]. A 2017 review of 14 meta-analyses determined that observational studies and intervention trials consistently report significant improvements in total cholesterol and reduced risk of CVD and hypertension with regular nut intake [69]. Of two recent trials of almonds in CAD patients [67, 68], one found that daily consumption improved serum lipid concentrations after 12 weeks relative to usual diet [67], whereas the other did not find that the addition of almonds to an overall healthful diet (National Cholesterol Education Program Step 1 diet) had any additional effect on blood lipids, inflammation, or vascular function [68].
Chocolate and cocoa have been also been well-studied for their antihypertensive effects. A 2017 systematic review concluded that flavanol-rich chocolate (FRC) and cocoa products can reduce blood pressure slightly (2 mmHg) in healthy adults [70]. However, the studies in this review varied greatly in duration and dose of flavanols and most were in healthy populations. Flammer and colleagues recently demonstrated beneficial effects of FRC in patients with CHF [65]. These effects were evident both acutely and after long-term consumption. In the FRC group, endothelial function measured by flow-mediated dilation of the brachial artery (FMD) improved significantly 2 h post-ingestion (P = 0.02 for between-group difference) and after 5 weeks of daily chocolate consumption (2 bars/day) (P = 0.002 for between-group difference). This sustained effect on FMD was observed despite a 12-h abstinence from chocolate consumption. No effects were observed on blood pressure, heart rate, or blood lipids.
Physical Activity Interventions
Physical activity is associated with reduced risk of cardiovascular events in individuals with CAD [71] or heart failure [72]. A 2016 Cochrane review and meta-analysis of exercise-based cardiac rehabilitation programs concluded that these programs reduce the risk of CV mortality (RR 0.74, 95% CI 0.64 to 0.86) and hospitalization (RR 0.82, 95% CI 0.70 to 0.96) but not all-cause mortality, MI, or revascularization [31••]. Overall, recent studies confirm the benefits of PA for managing CVD [73,74,75,76].
A 2014 trial in elderly CAD patients found that exercise training significantly improved aerobic capacity measured by peak metabolic equivalent and metabolic equivalents at the anaerobic threshold (P < 0.0001) and some measures of physical function relative to usual care [73]. In their 2015 study, Karjalainen et al. [71] found that a 2-year exercise training intervention improved exercise capacity in CAD patients with (P = 0.03) and without type 2 diabetes mellitus (T2DM) (P = 0.002) and waist circumference in those with T2DM only (P = 0.027), but did not influence other measures of cardiometabolic risk, including blood pressure and lipids.
Whether aerobic interval training (AIT) or moderate continuous training (MCT) is best for CVD patients remains uncertain because relatively few studies have compared these approaches, but a 2014 meta-analysis of nine studies (N = 206) concluded that AIT increased VO2peak significantly more than MCT (mean difference 1.60 ml/kg/min, P = 0.03) but MCT reduced body weight more than AIT (mean difference − 0.78 kg, P = 0.05). [74] Subsequent to that analysis, Conraads et al. [75] reported the findings of their trial in CAD patients, which demonstrated comparable improvement in VO2peak with either AIT or aerobic continuous training.
There have been, as well, several recent studies on the effects of routine PA on both AF and CHF. Despite observational studies suggesting an increased risk of AF with intense exercise in men [77], both observational studies and intervention trials have demonstrated clear benefit of moderate exercise for men and women with AF [76, 78]. Salient findings include reduced time in AF, improved symptom frequency and severity, and improvement in VO2peak [76, 78, 79]. Studies comparing low- and high-intensity exercise generally reported comparable benefits of both interventions [78, 79].
Stress Reduction Interventions
Stress reduction programs have been tested extensively in individuals with hypertension, with mixed results [80, 81]. A 2007 meta-analysis found that only transcendental meditation was effective in reducing blood pressure in individuals with hypertension or prehypertension [81]. In that review, transcendental meditation produced mean reductions in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of − 5.0 (P = 0.0002) and − 2.8 mmHg (P = 0.02), respectively. Interventions using simple biofeedback, relaxation-assisted biofeedback, progressive muscle relaxation, and stress management did not significantly affect blood pressure [81]. A more recent review of stress reduction trials in hypertensive patients did not report pooled effect sizes because of high heterogeneity, but most studies reported reductions in SBP and DBP [80].
Duraimani et al. [82] compared a stress reduction intervention using transcendental meditation with an extensive health education program in African American adults with stage I hypertension. Both interventions produced significant reductions in SBP, but only the health education group experienced a reduction in DBP (− 5.3 ± 6.4 mmHg, P = 0.04 for between-group difference). Schneider and colleagues also investigated the effects of transcendental meditation (TM) for stress reduction in African Americans, but targeting CHD patients [83]. The primary outcome was a composite measure including all-cause mortality, myocardial infarction, or stroke. Over a mean follow-up period of 5.4 years, risk of this outcome was 48% lower in the TM group (HR 0.52, 95% CI 0.29 to − 0.92, P = 0.025). There were no significant differences between groups in the composite of cardiovascular mortality, revascularizations, and cardiovascular hospitalizations (HR 0.76, 95% CI 0.51 to 1.13, P = 0.17). TM also reduced blood pressure and psychosocial stress factors.
In contrast, mindfulness-based stress reduction (MBSR) did not reduce daytime or 24-h ambulatory BP in adults with unmedicated stage 1 hypertension [84]. Because the intervention was intensive and the control group received no attention, the null findings in this study do not support MBSR for reducing BP in stage 1 hypertension. However, it is possible that MBSR would be effective in a higher-risk group. Another study in individuals aged ≥ 60 years with hypertension found little effect of Zen meditation on blood pressure [85].
Stress management and coping skills training [86••, 87] are more promising strategies for CVD patients. Blumenthal and colleagues [86••] compared standard cardiac rehabilitation (CR) and CR with stress management training (CR + SMT) in CHD patients. SMT topics included time management, progressive muscle relaxation, visual imagery, effective communication, and anger management. After 12 weeks, CR + SMT significantly reduced perceived stress compared with CR alone (P = 0.022). Although there were no significant differences between groups in serum lipids, aerobic fitness, CHD biomarkers, the CR + SMT group had lower risk of clinical events over a median follow-up of 3.2 years (HR = 0.49, 95% CI 0.25 to 0.95, P = 0.035). Both intervention groups fared better than a non-randomized comparison group that received no CR.
In a subsequent study by the same group of researchers [87], a 16-week coping skills training (CST) intervention, similar to SMT described above, but delivered by phone, improved quality of life (QOL) to a greater extent than a heart failure education program (P < 0.01) and reduced risk of HF-related hospitalization or death (HR 0.65, 95% CI 0.44 to 0.98, P = 0.040). However, CST was not superior in effects on HF disease biomarkers or risk of all-cause hospitalization or death (HR 0.84, 95% CI 0.59 to 1.21).
Heart rate variability has emerged as important cardiac risk indicator [88]. Studies of various stress mitigation approaches and mind-body techniques, including yoga [89] and mindfulness-based interventions [90] suggest salutary effects in healthy individuals, but additional research on potential benefits of mind-body approaches for CVD patients is needed.
Comparisons of Lifestyle Interventions
Pedersen et al. compared a low-energy diet (LED) to aerobic interval training (AIT) in individuals with CAD [91]. AIT and the LED similarly reduced total cholesterol, non-HDL cholesterol, and triglycerides. However, LED improved body composition to a significantly greater extent. For example, the LED group lost 10.6% of their body weight and 26.6% of their body fat mass, whereas the AIT group experienced reductions of 1.6 and 5.5%, respectively, (P < 0.001 for both between-group differences). On the other hand, AIT improved peak VO2 by a mean of 12.9 mL/kg fat-free mass/min, whereas LED did not significantly affect this outcome (P < 0.001 for between-group difference). While both LED and AID reduced total lipoprotein and LDL concentrations, LED was significantly better for reducing overall lipoprotein atherogenicity [92].
In a 2016 study, Kitzman et al. [93•] used a 2 × 2 factorial design to assess the effects of caloric restriction, aerobic exercise training, or both on exercise capacity, and QOL in obese adults aged ≥ 60 years with stable heart failure (HF) with preserved ejection fraction. Both interventions increased peak VO2 (P < 0.001 for both) and the combined effects of diet and exercise were additive [93•]. Diet and exercise improved HF symptoms similarly (P < 0.002). Diet improved CRP (− 2.8 μg/dL, P = 0.023), total cholesterol (− 14 mg/dL, P = 0.008), and LDL-C (− 13 mg/dL, P = 0.008), whereas exercise had no effect on these measures. Diet also improved some, but not all, measures of QOL.
Multifaceted Interventions
Because there are many lifestyle behaviors that influence cardiovascular disease progression, the most effective interventions are likely those that target more than one behavior. Diet and exercise are commonly combined in lifestyle interventions [94, 95], sometimes including tobacco cessation [96•, 97]. The most recent trials, described below, have had mixed results.
Hua et al. [98] found no effect of a relatively low-intensity lifestyle intervention for hypertensive patients on risk of composite cardiovascular events (including non-fatal stroke, myocardial infarction, and cardiovascular death). Over a median follow-up period of 3.5 years, the rates of cardiovascular events were similar in intervention and control groups (2.2 vs 2.4%, respectively, P = 0.86). Conversely, risk was reduced for participants who improved in at least two out of seven lifestyle behaviors (adjusted HR 0.45, 95% CI 0.32 to 0.63), suggesting a substantial protective effect of achieving lifestyle change. However, confounding by other variables linked to motivation to change cannot be ruled out.
Saffi and colleagues [99] demonstrated a beneficial effect of a nurse-led lifestyle counseling intervention targeting weight, PA, smoking, diet, and medication adherence on overall cardiovascular risk in CAD patients. Relative to general lifestyle advice, the intervention significantly reduced Framingham Risk Score (13.6% reduction vs 11% increase, P = 0.011), body weight (− 2 kg, P = 0.04), SBP (− 15 mmHg, P = 0.005), and DBP (− 6 mmHg, P = 0.02). There were no between-group differences in blood lipids, fasting glucose, or HbA1c.
Gallagher and colleagues tested a healthy eating and exercise program in overweight and obese adults with CHD and/or T2DM [94]. Compared with control, the intervention produced greater reductions in body weight (mean difference between groups − 2.06 kg, P < 0.001) and waist circumference (− 2.54 cm, P < 0.001) and greater increases in frequency of exercise (mean difference between groups 1.13 days/week, P = 0.02) and duration of exercise (102.31 min/week, P = 0.003). No differences in BP were observed. After 12 months, participants maintained significant reductions in body weight, BMI, and waist circumference (P < 0.001 for all), but not exercise duration [100].
Chow et al. [96•] assessed the impact of a text message-based intervention targeting diet, PA, smoking, and general cardiovascular health in patients with CHD. Compared with usual care, the intervention group experienced significant reductions in LDL-C (mean difference − 5 mg/dL, P = 0.04), SBP (mean difference − 7.6 mmHg, P < 0.001), DBP (mean difference − 3 mmHg, P < 0.001), and BMI (mean difference − 1.3 kg/m2, P < 0.001).
Another text message-based intervention, Text4Heart, was tested by Pfaeffli et al. [101] in CHD patients. In addition to usual care, the intervention group received daily text messages related to smoking, alcohol consumption, fruit and vegetable consumption, salt and saturated fat intake, and PA. After 3 months, intervention participants were more likely to adhere to at least three of four lifestyle recommendations (AOR 2.55, 95% CI 1.12 to 5.84, P = 0.03), but this effect was not maintained at 6 months (AOR 1.93, 95% CI 0.83–4.53, P = 0.13). Other secondary outcomes, assessed at 6 months only, included BMI, serum lipids, and blood pressure. Aside from a trend toward lower LDL in the intervention group (P = 0.053), there were no significant differences between groups.
Weight loss with calorie reduction and exercise may also be beneficial for AF patients [102]. After a median follow-up of 15 months, such an intervention produced greater reductions in body weight (− 14.3 vs − 3.6 kg, P < 0.001), Atrial Fibrillation Severity Scale (AFSS) symptom burden scores (− 11.8 vs − 2.6, P < 0.001), AFSS symptom severity scores (− 8.4 vs − 1.7 points, P < 0.001), number of AF episodes (− 2.5 vs no change, P = 0.01), and cumulative duration of AF episodes (− 692 min vs 419 min, P = 0.002) compared with control. Beneficial changes were also observed in SBP (− 3 vs − 1 mmHg, P < 0.001), DBP (− 2 vs − 1 mmHg, P = 0.02), and high-sensitivity CRP (− 1.2 vs − 0.4, P < 0.001), but not blood lipid concentrations.
Conclusions
Studies and reviews spanning decades [2, 5, 103,104,105] suggest the powerful influence of lifestyle factors on the overall risk for cardiovascular disease and mortality. In the aggregate, such work suggests that lifestyle could account for roughly 80% or more of all coronary disease in particular, and thus, at least this fraction of the disease prevalence is preventable. Recent assessments of dietary contributions to all-cause mortality [8, 106••] and to the potential for lifestyle factors to overcome even high genetic risk, [107] fortify this proposition. Realizing such promise in populations of patients, however, has proven challenging. Studies of lifestyle interventions and recommendations predicated on them, [108] are prone to report lackluster results at odds with the promise of epidemiologic impact. This literature, however, tends to be more about absence of evidences than evidence of absence. In other words, changing lifestyle tends to be hard, particularly in a cultural context that may conspire actively against such goals [109]. If lifestyle is the best of “medicine,” it may be that culture is the requisite spoon needed to help such medicine go down. Clinical interventions opposed by the forces of prevailing culture may be destined to failure, or at best, very limited success.
Another key consideration is that while studies of lifestyle interventions predominantly focus on component parts and attribution to specific, targeted interventions, such reductionism may directly conspire against the effectiveness of lifestyle intervention. There is a case to be made that lifestyle is intrinsically “holistic” [110]. Sleep disorders or poor sleep quality may attenuate stress tolerance [111], foster endocrine and psychological changes that promote overconsumption of calories, reduce energy expenditure at rest, and deplete the physical energy and motivation needed for exercise [112]. Lack of exercise, in turn, may increase stress, which may further erode appetite regulation. Increased energy intake and reduced exercise may contribute to weight gain, which may further impair sleep by several mechanisms [112]. A toxic cascade is readily set in motion, and only a comprehensive and holistic set of countermeasures may suffice to remediate and reverse it.
Despite such challenges, evidence is clear that lifestyle interventions can exert powerfully salutary effects at both the individual [63, 93•] and population level [113]. Cardiac risk factors can be modified, MIs prevented, and atherosclerotic plaque regressed. Though arguably harder to apply, dietary change can reduce LDL as effectively as statins [63]. In direct comparison to state-of-the-art pharmacotherapy, a lifestyle intervention was nearly twice as effective in the DPP at preventing progression to T2DM, a potent risk for cardiovascular disease, in at-risk adults [114].
The key components of a salutary lifestyle are for the most part well established and buoyed by global consensus [115]. They include sleep of adequate quantity and quality, robust social connections and support, stress mitigation by whatever means, routine physical activity, the avoidance of toxins such as tobacco, and a health-promoting dietary pattern. While the diet variable is complex, the basic theme of healthful eating is clear [116] and also a matter of prevailing consensus [115, 117•].
In the aggregate, the evidence for the potential of lifestyle as medicine to prevent, treat, and even reverse prevailing varieties of cardiovascular disease is very strong. The evidence that we can translate this knowledge into routine action in the context of modern culture is rather less clear. We have, in other words, found the best of medicine. We are still seeking the spoon required to help get that medicine to go down.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
National Center for Health Statistics. Health US, 2015. With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD; 2016.
Keys A, Menotti A, Aravanis C, Blackburn H, Djordevic BS, Buzina R, et al. The seven countries study: 2,289 deaths in 15 years. Prev Med. 1984;13:141–54.
Menotti A, Keys A, Kromhout D, Blackburn H, Aravanis C, Bloemberg B, et al. Inter-cohort differences in coronary heart disease mortality in the 25-year follow-up of the seven countries study. Eur J Epidemiol. 1993;9(5):527–36.
Verschuren WM, Jacobs DR, Bloemberg BP, Kromhout D, Menotti A, Aravanis C, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274(2):131–6.
Benfante R. Studies of cardiovascular disease and cause-specific mortality trends in Japanese-American men living in Hawaii and risk factor comparisons with other Japanese populations in the Pacific region: a review. Hum Biol. 1992;64(6):791–805.
Robertson TL, Kato H, Rhoads GG, Kagan A, Marmot M, Syme SL, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California. Incidence of myocardial infarction and death from coronary heart disease. Am J Cardiol. 1977;39(2):239–43.
Worth RM, Kato H, Rhoads GG, Kagan K, Syme SL. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: mortality. Am J Epidemiol. 1975;102(6):481–90.
Micha RPJ, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–24. https://doi.org/10.1001/jama.2017.0947.
Lee DC, Pate R, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64(5):472–81. https://doi.org/10.1016/j.jacc.2014.04.058.
Katzmarzyk PT. Standing and mortality in a prospective cohort of Canadian adults. Med Sci Sports Exerc. 2014;46(5):940–6. https://doi.org/10.1249/MSS.0000000000000198.
Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15. https://doi.org/10.1161/atvbaha.113.300156.
O'Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ. Alcohol and cardiovascular health: the dose makes the poison...or the remedy. Mayo Clin Proc. 2014;89(3):382–93. https://doi.org/10.1016/j.mayocp.2013.11.005.
Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–54. https://doi.org/10.1146/annurev-publhealth-031912-114452.
Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11(1):81–9. https://doi.org/10.1016/j.jsmc.2015.10.007.
Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102(13):1009–16. https://doi.org/10.1136/heartjnl-2015-308790.
Anthony D. Diagnosis and screening of coronary artery disease. Prim Care. 2005;32(4):931–46.
Poredos P. Endothelial dysfunction and cardiovascular disease. Pathophysiol Haemost Thromb. 2002;32(5–6):274–7.
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–43.
Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Corella D, Fito M, Ros E. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis. 2015;58(1):50–60. https://doi.org/10.1016/j.pcad.2015.04.003.
Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M et al. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(8):Cd009825. https://doi.org/10.1002/14651858.
Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of dietary approaches to stop hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24(12):1253–61. https://doi.org/10.1016/j.numecd.2014.06.008.
Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90.
Hills AP, Mokhtar N, Byrne NM. Assessment of physical activity and energy expenditure: an overview of objective measures. Front Nutr. 2014;1:5. https://doi.org/10.3389/fnut.2014.00005.
Roberts CK, Little JP, Thyfault JP. Modification of insulin sensitivity and glycemic control by activity and exercise. Med Sci Sports Exerc. 2013;45(10):1868–77. https://doi.org/10.1249/MSS.0b013e318295cdbb.
Cornelissen VASN. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(1):e004473. https://doi.org/10.1161/JAHA.112.004473.
Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109(2):288–95. https://doi.org/10.1016/j.amjcard.2011.08.042.
Pate R, Pratt M, Blair S, Haskell W, Macera C, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7.
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34.
Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33(6 Suppl):S484–92. discussion S93-4
Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity). Circulation. 2003;107(24):3109–16. https://doi.org/10.1161/01.cir.0000075572.40158.77.
•• Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1–12. https://doi.org/10.1016/j.jacc.2015.10.044. Recent meta-analysis of exercise-baed cardiac rehabilitation.
World Health Organization. WHO global report: mortality attributable to tobacco. 2012. 2012. http://apps.who.int/iris/bitstream/10665/44815/1/9789241564434_eng.pdf. Accessed 19 July 2017.
U.S. Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. 2010. https://www.ncbi.nlm.nih.gov/books/NBK53017/. Accessed 19 July 2017.
Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8(2):613–28. https://doi.org/10.3390/ijerph8020613.
Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, et al. Cigarette smoking exposure and heart failure risk in older adults: the health, aging, and body composition study. Am Heart J. 2012;164(2):236–42. https://doi.org/10.1016/j.ahj.2012.05.013.
McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):1002–10. https://doi.org/10.1161/atvbaha.114.304960.
Hisamatsu T, Miura K, Arima H, Kadota A, Kadowaki S, Torii S, et al. Smoking, smoking cessation, and measures of subclinical atherosclerosis in multiple vascular beds in Japanese men. J Am Heart Assoc. 2016;5(9) https://doi.org/10.1161/jaha.116.003738.
Kianoush S, Yakoob MY, Al-Rifai M, DeFilippis AP, Bittencourt MS, Duncan BB, et al. Associations of cigarette smoking with subclinical inflammation and atherosclerosis: ELSA-Brasil (The Brazilian Longitudinal Study of Adult Health). J Am Heart Assoc. 2017;6(6) https://doi.org/10.1161/jaha.116.005088.
Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–70. https://doi.org/10.1038/nrcardio.2012.45.
Mostofsky E, Maclure M, Sherwood JB, Tofler GH, Muller JE, Mittleman MA. Risk of acute myocardial infarction after the death of a significant person in one’s life: the determinants of myocardial infarction onset study. Circulation. 2012;125(3):491–6. https://doi.org/10.1161/circulationaha.111.061770.
Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–7.
Ravera A, Carubelli V, Sciatti E, Bonadei I, Gorga E, Cani D et al. Nutrition and cardiovascular disease: finding the perfect recipe for cardiovascular health. Nutrients. 2016;8(6). https://doi.org/10.3390/nu8060363.
Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, et al. Rationale and design of the dietary approaches to stop hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5(2):108–18.
Steinberg D, Bennett GG, Svetkey L. The DASH diet, 20 years later. JAMA. 2017;317(15):1529–30. https://doi.org/10.1001/jama.2017.1628.
Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. https://doi.org/10.1161/01.cir.0000437740.48606.d1.
Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009;338:b2337.
Kendall CW, Jenkins DJ. A dietary portfolio: maximal reduction of low-density lipoprotein cholesterol with diet. Curr Atheroscler Rep. 2004;6(6):492–8.
Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–10.
Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453–63. https://doi.org/10.1001/jamainternmed.2016.4182.
de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–85.
Martinez-Gonzalez MA, Toledo E, Aros F, Fiol M, Corella D, Salas-Salvado J, et al. Extravirgin olive oil consumption reduces risk of atrial fibrillation: the PREDIMED (Prevencion con Dieta Mediterranea) trial. Circulation. 2014;130(1):18–26. https://doi.org/10.1161/circulationaha.113.006921.
Toledo E, Hu FB, Estruch R, Buil-Cosiales P, Corella D, Salas-Salvado J, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med. 2013;11:207. https://doi.org/10.1186/1741-7015-11-207.
Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Perez-Caballero AI, Gomez-Delgado F, et al. CORonary diet intervention with olive oil and cardiovascular PREVention study (the CORDIOPREV study): rationale, methods, and baseline characteristics: a clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on cardiovascular disease in coronary patients. Am Heart J. 2016;177:42–50. https://doi.org/10.1016/j.ahj.2016.04.011.
Gomez-Delgado F, Garcia-Rios A, Alcala-Diaz JF, Rangel-Zuniga O, Delgado-Lista J, Yubero-Serrano EM, et al. Chronic consumption of a low-fat diet improves cardiometabolic risk factors according to the CLOCK gene in patients with coronary heart disease. Mol Nutr Food Res. 2015;59(12):2556–64. https://doi.org/10.1002/mnfr.201500375.
Gomez-Delgado F, Alcala-Diaz JF, Garcia-Rios A, Delgado-Lista J, Ortiz-Morales A, Rangel-Zuniga O, et al. Polymorphism at the TNF-alpha gene interacts with Mediterranean diet to influence triglyceride metabolism and inflammation status in metabolic syndrome patients: from the CORDIOPREV clinical trial. Mol Nutr Food Res. 2014;58(7):1519–27. https://doi.org/10.1002/mnfr.201300723.
Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170(2):126–35.
Paula TP, Viana LV, Neto AT, Leitao CB, Gross JL, Azevedo MJ. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: a randomized controlled trial. J Clin Hyperten (Greenwich). 2015;17(11):895–901. https://doi.org/10.1111/jch.12597.
Miller GFCE, Leroy Z, Wallin R. Prevalence and costs of five chronic conditions in children. J Sch Nurs. 2016;32(5):357–64. https://doi.org/10.1177/1059840516641190.
Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104(7):947–56. https://doi.org/10.1016/j.amjcard.2009.05.032.
Ornish D, Brown S, Scherwitz L. Can lifestyle changes reverse coronary heart disease? The lifestyle heart trial. Lancet. 1990;336:129–33.
Horrigan BJ. Ornish and Pritikin programs approved by CMS. Explore (New York, NY). 2010;6(6):346–8. https://doi.org/10.1016/j.explore.2010.08.007.
Jenkins DJ, Kendall CW, Marchie A, Faulkner D, Vidgen E, Lapsley KG, et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism. 2003;52(11):1478–83.
Jenkins DJ, Josse AR, Wong JM, Nguyen TH, Kendall CW. The portfolio diet for cardiovascular risk reduction. Curr Atheroscler Rep. 2007;9(6):501–7.
Jenkins DJ, Jones PJ, Frohlich J, Lamarche B, Ireland C, Nishi SK, et al. The effect of a dietary portfolio compared to a DASH-type diet on blood pressure. Nutr Metab Cardiovasc Dis. 2015;25(12):1132–9. https://doi.org/10.1016/j.numecd.2015.08.006.
Flammer AJ, Sudano I, Wolfrum M, Thomas R, Enseleit F, Periat D, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. 2012;33(17):2172–80. https://doi.org/10.1093/eurheartj/ehr448.
Rodriguez-Leyva D, Weighell W, Edel AL, LaVallee R, Dibrov E, Pinneker R, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62(6):1081–9. https://doi.org/10.1161/HYPERTENSIONAHA.113.02094.
Jamshed H, Sultan FA, Iqbal R, Gilani AH. Dietary almonds increase serum HDL cholesterol in coronary artery disease patients in a randomized controlled trial. J Nutr. 2015;145(10):2287–92. https://doi.org/10.3945/jn.114.207944.
Chen CY, Holbrook M, Duess MA, Dohadwala MM, Hamburg NM, Asztalos BF, et al. Effect of almond consumption on vascular function in patients with coronary artery disease: a randomized, controlled, cross-over trial. Nutr J. 2015;14:61. https://doi.org/10.1186/s12937-015-0049-5.
Schwingshackl L, Hoffmann G, Missbach B, Stelmach-Mardas M, Boeing H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Des. 2017;23(7):1016–27. https://doi.org/10.2174/1381612822666161010121356.
Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;4:CD008893. https://doi.org/10.1002/14651858.CD008893.pub3.
Karjalainen JJ, Kiviniemi AM, Hautala AJ, Piira OP, Lepojarvi ES, Perkiomaki JS, et al. Effects of physical activity and exercise training on cardiovascular risk in coronary artery disease patients with and without type 2 diabetes. Diabetes Care. 2015;38(4):706–15. https://doi.org/10.2337/dc14-2216.
Miura Y, Fukumoto Y, Miura T, Shimada K, Asakura M, Kadokami T, et al. Impact of physical activity on cardiovascular events in patients with chronic heart failure. A multicenter prospective cohort study. Circ J. 2013;77(12):2963–72.
Chen CH, Chen YJ, Tu HP, Huang MH, Jhong JH, Lin KL. Benefits of exercise training and the correlation between aerobic capacity and functional outcomes and quality of life in elderly patients with coronary artery disease. Kaohsjung J Med Sci. 2014;30(10):521–30. https://doi.org/10.1016/j.kjms.2014.08.004.
Pattyn N, Coeckelberghs E, Buys R, Cornelissen VA, Vanhees L. Aerobic interval training vs. moderate continuous training in coronary artery disease patients: a systematic review and meta-analysis. Sports Med. 2014;44(5):687–700. https://doi.org/10.1007/s40279-014-0158-x.
Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203–10. https://doi.org/10.1016/j.ijcard.2014.10.155.
Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133(5):466–73. https://doi.org/10.1161/CIRCULATIONAHA.115.018220.
Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol. 2016;27(9):1021–9. https://doi.org/10.1111/jce.13023.
Skielboe AK, Bandholm TQ, Hakmann S, Mourier M, Kallemose T, Dixen U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation—a randomized controlled trial. PLoS One. 2017;12(2):e0170060. https://doi.org/10.1371/journal.pone.0170060.
Ellingsen O, Halle M, Conraads V, Stoylen A, Dalen H, Delagardelle C, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017;135(9):839–49. https://doi.org/10.1161/CIRCULATIONAHA.116.022924.
Nagele E, Jeitler K, Horvath K, Semlitsch T, Posch N, Herrmann KH, et al. Clinical effectiveness of stress-reduction techniques in patients with hypertension: systematic review and meta-analysis. J Hypertens. 2014;32(10):1936–1944; discussion 44. https://doi.org/10.1097/HJH.0000000000000298.
Rainforth MV, Schneider RH, Nidich SI, Gaylord-King C, Salerno JW, Anderson JW. Stress reduction programs in patients with elevated blood pressure: a systematic review and meta-analysis. Curr Hypertens Rep. 2007;9(6):520–8.
Duraimani S, Schneider RH, Randall OS, Nidich SI, Xu S, Ketete M, et al. Effects of lifestyle modification on telomerase gene expression in hypertensive patients: a pilot trial of stress reduction and health education programs in African Americans. PLoS One. 2015;10(11):e0142689. https://doi.org/10.1371/journal.pone.0142689.
Schneider RH, Grim CE, Rainforth MV, Kotchen T, Nidich SI, Gaylord-King C, et al. Stress reduction in the secondary prevention of cardiovascular disease: randomized, controlled trial of transcendental meditation and health education in blacks. Circ Cardiovasc Qual Outcomes. 2012;5(6):750–8. https://doi.org/10.1161/CIRCOUTCOMES.112.967406.
Blom K, Baker B, How M, Dai M, Irvine J, Abbey S, et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: results from the HARMONY randomized controlled trial. Am J Hypertens. 2014;27(1):122–9. https://doi.org/10.1093/ajh/hpt134.
de Fatima Rosas Marchiori M, Kozasa EH, Miranda RD, Monezi Andrade AL, Perrotti TC, Leite JR. Decrease in blood pressure and improved psychological aspects through meditation training in hypertensive older adults: a randomized control study. Geriatr Gerontol Int. 2015;15(10):1158–64. https://doi.org/10.1111/ggi.12414.
• Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, et al. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation. 2016;133(14):1341–50. https://doi.org/10.1161/CIRCULATIONAHA.115.018926. Trial of stress management reporting long-term clinical outcomes.
Sherwood A, Blumenthal JA, Koch GG, Hoffman BM, Watkins LL, Smith PJ, et al. Effects of coping skills training on quality of life, disease biomarkers, and clinical outcomes in patients with heart failure: a randomized clinical trial. Circ Heart Fail. 2017;10(1) https://doi.org/10.1161/CIRCHEARTFAILURE.116.003410.
Huikuri HV, Stein PK. Heart rate variability in risk stratification of cardiac patients. Prog Cardiovasc Dis. 2013;56(2):153–9. https://doi.org/10.1016/j.pcad.2013.07.003.
Tyagi A, Cohen M. Yoga and heart rate variability: a comprehensive review of the literature. Int J Yoga. 2016;9(2):97–113. https://doi.org/10.4103/0973-6131.183712.
Wolever RQ, Bobinet KJ, McCabe K, Mackenzie ER, Fekete E, Kusnick CA, et al. Effective and viable mind-body stress reduction in the workplace: a randomized controlled trial. J Occup Health Psychol. 2012;17(2):246–58. https://doi.org/10.1037/a0027278.
Pedersen LR, Olsen RH, Jurs A, Astrup A, Chabanova E, Simonsen L, et al. A randomised trial comparing weight loss with aerobic exercise in overweight individuals with coronary artery disease: the CUT-IT trial. Eur J Prev Cardiol. 2015;22(8):1009–17. https://doi.org/10.1177/2047487314545280.
Pedersen LR, Olsen RH, Anholm C, Walzem RL, Fenger M, Eugen-Olsen J, et al. Weight loss is superior to exercise in improving the atherogenic lipid profile in a sedentary, overweight population with stable coronary artery disease: a randomized trial. Atherosclerosis. 2016;246:221–8. https://doi.org/10.1016/j.atherosclerosis.2016.01.001.
• Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315(1):36–46. https://doi.org/10.1001/jama.2015.17346. Assessed independent and combined effects of caloric restriction and exercise in heart failure.
Gallagher R, Kirkness A, Zelestis E, Hollams D, Kneale C, Armari E, et al. A randomised trial of a weight loss intervention for overweight and obese people diagnosed with coronary heart disease and/or type 2 diabetes. Ann Behav Med. 2012;44(1):119–28. https://doi.org/10.1007/s12160-012-9369-2.
Miller ER 3rd, Cooper LA, Carson KA, Wang NY, Appel LJ, Gayles D, et al. A dietary intervention in urban African Americans: results of the “five plus nuts and beans” randomized trial. Am J Prev Med. 2016;50(1):87–95. https://doi.org/10.1016/j.amepre.2015.06.010.
• Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314(12):1255–63. https://doi.org/10.1001/jama.2015.10945. Evaluation of a low-cost text-message-based intervention.
Ijzelenberg W, Hellemans IM, van Tulder MW, Heymans MW, Rauwerda JA, van Rossum AC, et al. The effect of a comprehensive lifestyle intervention on cardiovascular risk factors in pharmacologically treated patients with stable cardiovascular disease compared to usual care: a randomised controlled trial. BMC Cardiovasc Disord. 2012;12:71. https://doi.org/10.1186/1471-2261-12-71.
• Hua K, Hao G, Li W. Cardiovascular outcomes of lifestyle intervention in hypertensive patients with antihypertensive agents. Int J Cardiol. 2017;227:751–6. https://doi.org/10.1016/j.ijcard.2016.10.062. Large RCT reporting long-term clinical outcomes.
Saffi MA, Polanczyk CA, Rabelo-Silva ER. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: a randomized clinical trial. Eur J Cardiovasc Nurs. 2014;13(5):436–43. https://doi.org/10.1177/1474515113505396.
Alharbi M, Gallagher R, Kirkness A, Sibbritt D, Tofler G. Long-term outcomes from healthy eating and exercise lifestyle program for overweight people with heart disease and diabetes. Eur J Cardiovasc Nurs. 2016;15(1):91–9. https://doi.org/10.1177/1474515114557222.
Pfaeffli Dale L, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text message and internet support for coronary heart disease self-management: results from the Text4Heart randomized controlled trial. J Med Internet Res. 2015;17(10):e237. https://doi.org/10.2196/jmir.4944.
Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–60. https://doi.org/10.1001/jama.2013.280521.
Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. https://doi.org/10.1016/S0140-6736(13)61752-3.
Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45.
Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European prospective investigation into cancer and nutrition-Potsdam study. Arch Intern Med. 2009;169(15):1355–62.
•• Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, et al. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. https://doi.org/10.3945/ajcn.117.153148. Latest meta-analysis of associations between dietary factors and mortality.
Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349–58. https://doi.org/10.1056/NEJMoa1605086.
Jorgensen T, Jacobsen RK, Toft U, Aadahl M, Glumer C, Pisinger C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ. 2014;348:g3617. https://doi.org/10.1136/bmj.g3617.
Katz DL. Lifestyle is the medicine, culture is the spoon. AJLM. 2014;8(5):301–5.
Katz DL, Colino S. Disease proof. New York: Hudson Street Press; 2013.
Massar SAA, Liu JCJ, Mohammad NB, Chee MWL. Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men. Psychoneuroendocrinology. 2017;81:151–6. https://doi.org/10.1016/j.psyneuen.2017.04.013.
Hargens TA, Kaleth AS, Edwards ES, Butner KL. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep. 2013;5:27–35. https://doi.org/10.2147/NSS.S34838.
Jousilahti P, Laatikainen T, Salomaa V, Pietila A, Vartiainen E, Puska P. 40-year CHD mortality trends and the role of risk factors in mortality decline: the North Karelia project experience. Glob Heart. 2016;11(2):207–12. https://doi.org/10.1016/j.gheart.2016.04.004.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. https://doi.org/10.1056/NEJMoa012512.
Katz DL, Frates EP, Bonnet JP, Gupta SK, Vartiainen E, Carmona RH. Lifestyle as medicine: the case for a true health initiative. Am J Health Promot. 2017;890117117705949 https://doi.org/10.1177/0890117117705949.
Katz DL, Meller S. Can we say what diet is best for health? Annu Rev Public Health. 2014;35:83–103. https://doi.org/10.1146/annurev-publhealth-032013-182351.
• Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017; https://doi.org/10.1161/CIR.0000000000000510. Recent AHA scientific statement on dietary fats and cardiovascular risk.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kimberly N. Doughty, Nelson X. Del Pilar, Amanda Audette, and David L. Katz declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Public Health Policy
Rights and permissions
About this article
Cite this article
Doughty, K.N., Del Pilar, N.X., Audette, A. et al. Lifestyle Medicine and the Management of Cardiovascular Disease. Curr Cardiol Rep 19, 116 (2017). https://doi.org/10.1007/s11886-017-0925-z
Published:
DOI: https://doi.org/10.1007/s11886-017-0925-z