Abstract
Purpose of Review
This review will discuss the application of various imaging modalities including their advantages and disadvantages in the evaluation of the most common pericardial masses with a focus on pericardial cysts, tumors, and hematomas.
Recent Findings
Accurate identification of pericardial masses and assessment of potential hemodynamic compromise is imperative for management. Cardiac imaging plays a central role in tissue characterization as well as evaluation of extension into neighboring structures. Currently, echocardiography is the preferred modality for the initial evaluation due to its low cost and widespread availability. However, due to potential limitations with echocardiography, computed tomography (CT), and cardiac magnetic resonance (CMR) imaging have become robust complementary imaging tests. CT provides superior spatial resolution and is the ideal test for evaluation of calcified masses while CMR provides excellent tissue characterization through various CMR sequences. Finally, positron emission tomography (PET) imaging can provide additional unique information in the assessment of potentially malignant tumors.
Summary
An integrated, multi-modality imaging approach is helpful to evaluate the pericardium and diagnose pericardial masses. Advancements in imaging technology have provided improved diagnostic accuracy, with CT and CMR currently serving as complementary imaging techniques to traditional echocardiography imaging. Because each imaging modality has its unique sets of advantages and disadvantages, the choice of modality must be individualized to each patient. Through careful consideration, an integrated imaging approach is crucial in noninvasively providing information on cardiac structure, morphology, function, and associated complications that are important to the diagnosis and management of a variety of pericardial masses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pericardium consists of the visceral pericardium, composed of mesothelial cells along with collagen and elastin fibers, and the parietal pericardium, a 2-mm structure which is mostly acellular but composed of collagen and elastin fibers [1]. Although the pericardium has a variety of functions, including maintaining the heart in a constant position, preventing transmission of infections, and secreting prostaglandins, the lack of a pericardium does not appear to have negative repercussions [1]. Masses of the heart and pericardium are classified as neoplastic (both primary and secondary), or non-neoplastic. Primary cardiac tumors are rare, with a 0.001 to 0.03% incidence found in autopsies while secondary tumors are more common with an incidence of 1.7 to 14% [1]. Secondary tumors can occur either through direct extension into the pericardium, or through hematologic or lymphatic pathways [2]. Pericardial tumors are even less common than other cardiac tumors and account for only 6.7–12.8% of all primary cardiac tumors [3, 4]. A classification scheme of the most common pericardial tumors encountered is shown in Table 1 with mesothelioma being the most common and lymphomas, melanomas, and lung and breast carcinomas as the most common secondary pericardial tumors [5].
Imaging Modalities
Echocardiography
Echocardiography is a type of ultrasound imaging in which high-frequency sound waves are emitted from piezoelectric crystals, reflected off internal structures, and then back to the transducer from which microprocessors generate an image [1]. Echocardiography is considered the first-line imaging modality of choice for the assessment of pericardial masses as well as potential hemodynamic consequences, which can be followed through serial studies. Echocardiography often readily displays the mass in question (Fig. 1, Videos 1 and 2) is safe, readily available, and low cost. [6••] Currently, 2D echocardiography techniques are most often used to assess tumor characteristics such as shape, size, tumor attachment, and location as related to adjacent structures [7]. While transthoracic echocardiography (TTE) is a useful starting point, it has a reported sensitivity of 55–93% in the detection of intracardiac masses [8]. Thus, transesophageal echocardiography (TEE) can be a complementary diagnostic test that is especially helpful in characterizing masses in the posterior structures of the heart, particularly in the left atrium, left atrial appendage, right heart, and descending thoracic aorta [8]. Further use of 3D echocardiography can be helpful by allowing for measurement of the entire volume of a mass, which may be underestimated by 2D echocardiography [9]. Additionally, 3D echo provides incremental diagnostic yield by allowing for more accurate assessment of the size, location, mobility of the mass, and feasibility for surgical resection [7]. Further information on tumor identification can be obtained through using ultrasound contrast agents. Highly vascular or malignant tumors result in contrast hyper-enhancement of the tumor compared to the adjacent myocardium while stromal tumors which have poor blood supply show hypo-enhancement and thrombi show no enhancement [10]. Although echocardiography is considered the first-line test, image quality is operator dependent, and may be limited depending on the acoustic windows available to visualize the heart along with limited tissue characterization [6••].
A fifty-eight-year old woman with myxoid sarcoma. TTE images in the apical 4 chamber (a) and oblique short axis (b) views readily demonstrate a lobulated heterogeneous pericardial mass with associated pericardial effusion that encompasses the left ventricle. Contrast enhanced CT images (c, d) further delineate the full extent of the pericardial mass with evidence of compression of the left ventricle (arrowhead) and pulmonary veins (arrow). Please refer to Videos 1 and 2 for associated movie files
Cardiovascular Magnetic Resonance
Cardiac magnetic resonance (CMR) creates images of the heart by applying magnet forces to align the intrinsic weak magnetic forces generated by hydrogen protons present in the human body, then turning off the magnet to allow the protons to return back to their normal states [1]. CMR is currently used for assessment of the pericardium and pericardial masses by delineating tissue characteristics through various CMR sequences and determining mobility through cine images and potential mass effects on ventricular and valvular functions [11]. Further, since it does not require iodizing radiation, CMR is the ideal imaging modality in conjunction with echocardiography for pediatric patients or those with renal impairment [12•]. However, because of its relatively higher cost, longer acquisition time, and the need for patient cooperation, CMR may not be feasible on every patient [13]. CMR has been shown to improve detection of cardiac masses, particularly paracardiac masses, which may not be well visualized on routine TTE [14, 15]. Studies have demonstrated that 10–20% of cardiac tumors detected by CMR are missed on routine TTE [14, 15], with most of them located in the pericardium or adjacent mediastinum [14]. CMR provides multiplanar imaging with a wide field of view, high spatial and temporal resolutions, and high intrinsic soft tissue contrast without the need for ionizing radiation or iodinated contrast. Specific sequences allow for different tissue weightings and intravenous contrast can be applied for further insight into the internal composition of the mass [16] (Fig. 2). Both still and cine images are often acquired to further characterize pericardial masses and evaluate for pericardial effusion, myocardial invasion, myocardial infarction, involvement of coronary arteries, and secondary functional and hemodynamic effects on the heart such as compression, diastolic dysfunction, and/or constrictive physiology. CMR also provides additional information on resectability of masses as well as associated complications such as invasion of mediastinal structures, regional or distant metastases, and encasement of vital structures.
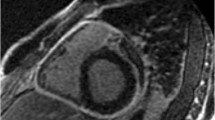
A fifty-two-year-old woman with paraganglioma. TEE image (a) show an echodensity between the aortic root and pulmonary artery (asterisk). Contrast CT in an axial plane (b) show patchy contrast enhancement of the mass (asterisk). PET image in the axial plane (c) demonstrates mild increased FDG uptake. CMR images in an oblique sagittal view with various sequences to assess the tissue characteristics. The paraganglioma demonstrates intermediate signal intensity on pre-contrast T1-weighted turbo spin echo sequences (d) and high signal intensity on pre-contrast T2-weighted inversion recovery sequences (e). There is minimal contrast enhancement noted on post-contrast T1-weighted turbo spin echo sequence (f). Please refer to Video 3 for associated movie file. Ao aorta, PA pulmonary artery, LA left atrium
Cardiac Computed Tomography
Cardiac computed tomography (CT) uses ionizing radiation captured by a detector array synchronized to the cardiac cycle to convert raw data into computer-reconstructed cross-sectional images [1]. It is a useful imaging modality for further assessment of pericardial masses after an initial echocardiogram evaluation, especially in situations in which CMR is contraindicated or not available. These pericardial masses may be incidental findings on CT; in one series, 40–50% of multidetector CTs displayed incidental findings with 5–10% of these requiring follow-up [1]. Not only does CT yield high-quality images with excellent spatial resolution, it is the modality of choice for imaging calcified masses as well as other non-cardiac intrathoracic structures [11, 12•]. Additionally, due to its high-contrast resolution, CT allows for specific evaluation of pericardial fluid as well as the pericardium itself and delineation of pericardial masses (Fig. 1) [6••]. Specific CT protocols such as variation in timing of contrast administration with multiphasic contrast administration can be further applied to optimize visualization of the right heart as routine CT contrast studies yield limited visualization of the right heart chambers due to intermixing of opacified and non-opacified blood. Delayed imaging can also be used to evaluate for presence of intracardiac thrombus, which is especially important in patients with atrial fibrillation [17]. Cardiac CT angiography may be helpful in delineating presence of coronary artery disease and also degree of neovascular growth of a tumor, both of which would guide surgical planning if needed [11]. Some advantages of CT include fast acquisition time, high spatial and temporal resolution, ability to create multiplanar image reconstructions, and evaluation of thoracic, extracardiac structures. Although CT can add valuable information to the assessment of pericardial masses, risk of ionizing radiation and contrast-induced nephropathy must be also be considered [6••].
Positron Emission Tomography
Positron emission tomography (PET) is a nuclear medicine imaging technique that uses radiotracers labeled with positron-emitting isotopes with properties of naturally occurring elements to evaluate myocardial perfusion or metabolism [1]. 18F-FDG PET is infrequently used to assess cardiac tumors although there have been small studies demonstrating the use of 18F-FDG for differentiation between benign and malignant cardiac tumors [12•, 18]. Fusion imaging of CT with 18F-FDG positron emission tomography (18F-FDG PET) can be helpful in distinguishing between benign versus malignant tumors as increased 18F-FDG uptake indicates either primary malignant or metastatic tumors rather than benign tumors (Fig. 2) [12•]. However, care must be made to differentiate masses that have increased 18F-FDG uptake due to high content of brown fat in the mitochondria as seen within benign lipomatous hypertrophy of the interatrial septum [11]. Although there are no reports of using PET specifically for assessment of pericardial tumors, this imaging technique may be a useful tool for assessing the malignant nature of a pericardial mass in question.
Diagnostic Evaluation of Pericardial Masses
Clinical diagnosis of pericardial masses can be difficult since patients may be asymptomatic or may present with diverse, nonspecific symptoms depending on whether there is associated pericardial effusion with or without tamponade physiology, pericarditis, or invasion of adjacent structures with resultant hemodynamic effects. Thus, imaging plays an important role in the evaluation of such patients. Pericardial masses may also be incidentally detected on imaging tests that are performed for other clinical indications. The diagnostic workup often starts with chest roentgenography and/or transthoracic echocardiography, which usually identifies the mass. Further evaluation using TEE may be indicated to complement the transthoracic echocardiographic assessment. Often, investigation with other imaging modalities such as CT, CMR, or PET is warranted for more detailed assessment by visualization of the entire pericardium, tissue characterization, and evaluation of surrounding structures [6••, 13]. Although imaging may help in identification and characterization of the mass, biopsy is often needed for definitive tissue diagnosis [1, 6••].
Based on the clinical question and each patient’s unique characteristics, judicious use of additional imaging should be targeted to avoid inappropriate or unnecessary testing and also incomplete or non-diagnostic testing [6••, 19••]. Information on whether a mass is malignant or benign is important to direct surgical versus medical management. In addition, the extent of cardiac and extracardiac involvement is also important to determine potential for complications such as pericardial effusion with or without cardiac tamponade, constrictive pericarditis, superior vena cava syndrome, and valvular disease [1], and also help track progression of disease. Large masses deemed to be benign based on imaging can often be completely surgically excised to not only provide tissue for pathologic analysis, which remains the gold standard for definitive identification, but also to provide symptom relief. [2, 6••] In contrast, primary malignant tumors are often difficult to resect at the time of diagnosis, with little chemotherapy and radiotherapy treatment options. Small masses may be best assessed using CT given its high spatial resolution while larger masses may be better assessed using CMR due its superior tissue characterization abilities [12•]. Progression of disease can be assessed using TTE if there are optimal acoustic windows or using CT or CMR if not easily visualized by TTE while metabolic response to treatment can be evaluated using PET. A comparison of advantages and limitations of these 4 imaging modalities are outlined in Table 2.
Pericardial Masses
Pericardial Cysts
The most common primary pericardial masses are benign pericardial cysts. These fluid-containing structures are thought to be formed from sections of the pericardium that pinch off during embryonic development [16]. Although they can occur anywhere along the pericardium, they are most commonly seen in the right cardiophrenic angle [16, 20, 21•]. Pericardial cysts are often visualized on chest roentgenography or echocardiography (Fig. 3). On TTE, cysts are visualized as echolucent structures that may abut the right atrium (Fig. 3), with color-flow and pulsed-wave Doppler used to demonstrate lack of flow into the structure [13]. Although may provide further details [22], CT or CMR is often required for further characterization [19••]. On CT, these thin-walled, homogenous structures are non-enhancing with iodinated contrast and have an attenuation between −10 to 20 HU (Fig. 3) [12•] (Fig. 1b). On CMR, cysts display simple fluid characteristics with low signal intensity on T1 and high signal intensity on T2 images without contrast enhancement [20, 21•, 23]. (Fig. 1c). Pericardial cysts are benign and do not usually have clinical significance except for rare cases of rupture with resultant cardiac tamponade. As such, asymptomatic patients are monitored through a conservative, serial imaging monitoring approach using CT or CMR every 1–2 years while symptomatic patients with large cysts require percutaneous aspiration or surgical excision [6••].
42 year old man with pericardial cyst. Chest X-ray (a) demonstrates abnormality at the right heart border (arrow). TTE (b) shows echolucent structure noted inferior to the right atrium/right ventricle on short axis view (arrow). Contrast enhanced CT (c) demonstrates low attenuation round mass attached to the pericardium along the right heart border (arrow). CMR (d) image with T2-weighted IR sequence demonstrates high signal intensity (arrow) which is characteristic for fluid filled simple cyst
Pericardial Tumors
Primary pericardial tumors are rare and occur much less frequently than secondary pericardial metastasis [21•]. Primary tumors can be benign, including lipoma, hemangioma, teratoma or malignant, including mesothelioma, sarcoma, and lymphoma [24]. Metastatic tumors are usually of breast, lung, and bone marrow origin [24]. While TTE usually provides initial identification of pericardial tumors, CT and CMR can provide further information on tumor location and site of insertion as well as relationship to adjacent structures, allowing for evaluation of feasibility for surgical resection [12•, 24], and assess for additional masses including extracardiac lesions (Figs. 1 and 2). CMR can provide crucial information on the histopathology of cardiac masses (Fig. 2) [14, 21•, 25], although tissue biopsy is often still required for diagnosis. CMR can accurately detect the high fatty content of lipoma and liposarcomas noted by homogenous high T1- and T2-weighted signal intensity [14, 25]. For most other tumors, there is medium signal intensity on T1-weighted images but high signal intensity on T2-weighted images with the exception of melanoma, which displays high signal intensity on T1-weighted images due to high content of paramagnetic melanin [13, 23]. Specific CMR sequences, most notably the T1-weighted dynamic phase fast gradient-echo (SSFP) sequence, can be used to assess vascularity of the mass in question [11]. Cardiac CT can also be used for tissue characterization. Because CT is sensitive in detecting calcification, it can be used to detect pericardial thickening and calcification as well as characterize chronic thrombi which often contains spotty calcification [12•, 19••]. Further, CT detects fat attenuation and vascularity of tumors, which help to determine tumor type [12•]. Compared to benign tumors, malignant tumors often demonstrate additional features of nonmobility, associated pericardial effusion, and myocardial invasion [14]. On both CT and CMR, presence of disrupted pericardium, hemorrhagic effusion, invasion into the epicardial fat, myocardium, or cardiac chambers and associated mediastinal or pericardial lymphadenopathy are additional signs of aggressive disease [12•, 19••]. Despite the limited use of PET in the evaluation of cardiac tumors in clinical practice, it does provide serial quantitation of physiologic and biochemical processes of the tumor tissue (Fig. 2). Specifically, PET can help track tumor response to therapy for prognostication as well as earlier detection of potential non-responding tumors [26].
Pericardial Hematomas
Pericardial hematomas are a common mimic of tumors, making differentiation between the two entities essential. Hematomas usually form after surgery or trauma [27], and although can be initially diagnosed by echocardiography, CT or CMR is often performed for confirmation and delineation of the extent of involvement. In an acute hematoma, CT will detect the high attenuation of initial blood, which decreases as time progresses. As the hematoma matures, CT will detect fibrosis and calcification with high sensitivity. Further, hematomas do not enhance with iodinated contrast. [28] CMR has been shown to have excellent accuracy in the differentiation of cardiac thrombi or hematoma from tumors [14, 21•, 25]. In general, thrombi tend to be smaller, more homogeneous, and less mobile compared to tumors [21•] while tumors often demonstrate increased hyperintensity on T2-weighted images and contrast enhancement [14]. Acute phase hematomas display high intensity on T1- and T2-weighted sequences [23]; subacute-phase hematomas appear as a fluid collection with heterogeneous intermediate to high signal on T1- and T2-weighted sequences; and chronic-phase hematomas display low signal with a dark rim due to its lower water content [23, 27].
Conclusion
An integrated, multi-modality imaging approach is helpful to evaluate the pericardium and diagnose pericardial masses. Advancements in imaging technology have provided improved diagnostic accuracy, with CT and CMR currently serving as complementary imaging techniques to traditional echocardiography imaging. Because each imaging modality has its unique sets of advantages and disadvantages, the choice of modality must be individualized to each patient. Through careful consideration, an integrated imaging approach is crucial in noninvasively providing information on cardiac structure, morphology, function, and associated complications that are important to the diagnosis and management of a variety of pericardial masses.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s heart disease. 10th ed. Philadelphia: Elsevier Saunders; 2015.
Basso C, Rizzo S, Valente M, Thiene G. Prevalence and pathology of primary cardiac tumours. Int J Cardiol. 2012;15(1):18–29.
Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol [Internet] Elsevier. 2016;84(1):69–75. Available from: doi:10.1016/S0167-5273(02)00136-5.
Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK centre: surgical and autopsy series. Cardiovasc Pathol [Internet] Elsevier. 2016;19(6):343–52. Available from: doi:10.1016/j.carpath.2009.07.005.
Klatt EC, Heitz DR. Cardiac metastases. Cancer United States. 1990;65(6):1456–9.
•• Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography. J Am Soc Echocardiogr [Internet] Elsevier Inc. 2013;26(9):965–1012.e15. doi:10.1016/j.echo.2013.06.023. These guidelines provide an overview of pericardial disease pathology with emphasis on using different imaging modalities for diagnosis and management.
Lang RM, Goldstein SA, Kronzon I, Khandheria B, Mor-Avi V, American Society of Echocardiography. ASE’s comprehensive echocardiography [Internet]. 2015. Available from: https://www.clinicalkey.com/dura/browse/bookChapter/3-s2.0-C20120012501.
Mügge A, Daniel WG, Haverich A, Lichtlen PR. Diagnosis of noninfective cardiac mass lesions by two-dimensional echocardiography. Comparison of the transthoracic and transesophageal approaches. Circulation [Internet]. 1991;83(1):70–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1984900.
Asch FM, Bieganski SP, Panza JA, Weissman NJ. Real-time 3-dimensional echocardiography evaluation of intracardiac masses. Echocardiography [Internet] Blackwell Publishing Inc. 2006;23(3):218–24. doi:10.1111/j.1540-8175.2006.00196.x.
Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, et al. American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J Am Soc Echocardiogr. 2008;21(11):1179–201.
Buckley O, Madan R, Kwong R, Rybicki FJ, Hunsaker A. Cardiac masses, part 1: imaging strategies and technical considerations. Am J Roentgenol. 2011;197(5):837–41.
• Kassop D, Donovan MS, Cheezum MK, Nguyen BT, Gambill NB, Blankstein R, et al. Cardiac masses on cardiac CT: a review. Curr Cardiovasc Imaging Rep. 2014;7(8):1–13. Provides overview of using cardiac CT to diagnose cardiac masses.
Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J. Multimodality imaging of pericardial diseases. JACC Cardiovasc Imaging Elsevier Inc. 2010;3(6):650–60.
Patel R, Lim RP, Saric M, Nayar A, Babb J, Ettel M, et al. Diagnostic performance of cardiac magnetic resonance imaging and echocardiography in evaluation of cardiac and paracardiac masses. Am J Cardiol Elsevier Inc. 2016;117(1):135–40.
Staab W, Bergau L, Schuster A, Hinojar R, Dorenkamp M, Obenauer S, et al. Detection of intracardiac masses in patients with coronary artery disease using cardiac magnetic resonance imaging: a comparison with transthoracic echocardiography. Int J Cardiovasc Imaging [Internet]. 2014;647–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24384859
Grizzard JD. Magnetic resonance imaging of pericardial disease and intracardiac thrombus. Heart Fail Clin. 2009;5(3):401–19.
Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging [Internet]. 2013;6(2):185–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23406625.
Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T, et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med [Internet]. 2012;53(6):856–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22577239.
•• Verhaert D, Gabrie RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3(3):333–43. Comparison of strengths and limitations of different imaging modalities in evaluation of pericardial masses.
Rajiah P. Cardiac MRI: part 2, pericardial diseases. Am J Roentgenol. 2011;197(4):W621–34.
• Pazos-López P, Pozo E, Siqueira ME, García-Lunar I, Cham M, Jacobi A, et al. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc Imaging. 2014;7(9):896–905. This study uses CMR to differentiate cardiac thrombi from tumors and benign versus maligant neoplasms.
Patel J, Park C, Michaels J, Rosen S, Kort S. Pericardial cyst: case reports and a literature review. Echocardiography [Internet] Blackwell Science Inc. 2004;21(3):269–72. doi:10.1111/j.0742-2822.2004.03097.x.
Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics [Internet]. 2005;25(5):1255–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16160110.
Bogaert J, Francone M. Cardiovascular magnetic resonance in pericardial diseases. J Cardiovasc Magn Reson [Internet]. 2009;11:14. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2685792&tool=pmcentrez&rendertype=abstract.
Hong YJ, Hur J, Kim YJ, Lee HJ, Nam JE, Kim HY, et al. The usefulness of delayed contrast-enhanced cardiovascular magnetic resonance imaging in differentiating cardiac tumors from thrombi in stroke patients. Int J Cardiovasc Imaging. 2011;1:89–95.
Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol [Internet] American Society of Clinical Oncology. 2006;24(20):3282–92. doi:10.1200/JCO.2006.06.6068.
Axel L. Assessment of pericardial disease by magnetic resonance and computed tomography. J Magn Reson Imaging. 2004;19(6):816–26.
O’Leary SM, Williams PL, Williams MP, Edwards AJ, Roobottom CA, Morganhughes GJ, et al. Imaging the pericardium: appearances on ECG-gated 64-detector row cardiac computed tomography. Br J Radiol. 2010;83(987):194–205.
Zhou W, Srichai-Parsia MB. CMR and Pericardial Masses. Accessed January 31, 2017. http://www.acc.org/latest-in-cardiology/articles/2016/07/12/13/06/cmr-and-pericardial-masses?w_nav=TI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Wunan Zhou and Monvadi Barbara Srichai declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pericardial Disease
Rights and permissions
About this article
Cite this article
Zhou, W., Srichai, M.B. Multi-modality Imaging Assessment of Pericardial Masses. Curr Cardiol Rep 19, 32 (2017). https://doi.org/10.1007/s11886-017-0845-y
Published:
DOI: https://doi.org/10.1007/s11886-017-0845-y