Abstract
Functional mitral regurgitation (FMR) occurs when normal or nearly normal mitral leaflets are prevented from proper coaptation by underlying left ventricular (LV) dysfunction, mitral annular dilation, or both. FMR is associated with an adverse prognosis in nonischemic or ischemic LV dysfunction. Multiple studies have confirmed that even mild FMR portends a worse prognosis, and that the risk of FMR is independent of LV volumes and other clinical risk factors. FMR can be difficult to quantitate echocardiographically because it is load dependent and can vary considerably from exam to exam. There is a systematic tendency to underestimate FMR severity by echocardiography because the regurgitant orifice in FMR is typically elliptical, but the formula for calculating regurgitant orifice area assumes circular geometry. Treatment of FMR begins with guideline-directed medical therapy (GDMT) for LV dysfunction and heart failure, including cardiac resynchronization, if indicated. Revascularization should be considered for ischemic FMR, when indicated. Finally, mitral valve surgery should be considered in patients undergoing CABG in whom moderate or greater FMR is present, and also when severe symptomatic FMR persists despite optimal GDMT and revascularization. Percutaneous options for treatment of FMR are in development but are not currently approved in the US.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mitral valve regurgitation (MR) represents nearly one-third of acquired left-sided valve pathology in developed countries [1]. In broad terms, MR is classified as either primary or secondary. Primary MR is due to pathologic abnormalities of the leaflets such as myxomatous degeneration, rheumatic heart disease, endocarditis, autoimmune diseases, radiation injury, drugs, etc. Severe primary MR causes a pure volume overload on the left ventricle (LV), which can cause heart failure (HF) symptoms, pulmonary venous hypertension, and atrial fibrillation even when LV ejection fraction (LVEF) is preserved by favorable loading conditions. Correction of primary MR in a timely fashion reverses these consequences. The gold standard for correction of primary MR is surgical mitral valve repair, which has been shown to restore normal age-adjusted longevity in multiple studies [2-4]. Secondary MR, also known as functional MR, occurs when the mitral leaflets are structurally normal or nearly normal, but leaflet coaptation is restricted by abnormal structure and function of the left ventricle (LV). In functional MR (FMR), there is controversy whether surgical correction of MR is beneficial since the underlying problem is LV dysfunction. This review will focus on the role of FMR in ischemic and non-ischemic LV dysfunction, specifically focusing on natural history, mechanism, quantification of MR severity, and treatment options.
Prognosis of Functional MR
MR is detected in 45 %–75 % of patients with systolic dysfunction [5-7]. Moderate/severe MR occurs in about 12 % and severe MR in 4 % of patients with systolic HF [8]. Several observational studies have demonstrated that FMR confers a poor prognosis relative to patients with LV dysfunction who do not have FMR. This is true for patients with recent acute myocardial infarction (MI) and for patients with chronic ischemic or nonischemic cardiomyopathy. In the Survival and Ventricular Enlargement (SAVE) trial [9], patients with FMR within 16 days following acute MI were more likely to experience cardiovascular mortality (29 % vs 12 %; P < .001), and severe heart failure (24 % vs 16 %; P = .0153) than those without MR. The presence of MR was an independent predictor of cardiovascular mortality (relative risk, 2.00; 95 % CI, 1.28–3.04). Data from trials of thrombolysis for acute MI also showed poor prognosis for ischemic MR [10]. Post-MI patients had a 1-year mortality rate of 52 % if they had severe MR, 22 % if they had mild-moderate MR, and 11 % if they had no MR. Grigioni et al [11] studied 303 patients presenting at least 16 days following acute MI. The adjusted relative risks of total and cardiac mortality associated with the presence of ischemic MR (n = 194) was 1.88 (P = 0.003) and 1.83 (P = 0.014), respectively, compared with patients without ischemic MR (n = 104). Trichon et al [5] reported 2057 patients with ischemic MR and chronic LV systolic dysfunction and showed MR to be an independent predictor of mortality and also found significantly lower survival rates at 1, 3, and 5 years in HF patients with moderate to severe MR vs those without MR or with mild MR. Outcomes in HF patients with FMR are similar whether the cause of HF is ischemic or nonischemic cardiomyopathy. In the BEST trial [12], vena contracta width >0.4 cm (moderate or greater MR) was an independent predictor of mortality along with LV end-diastolic volume and a restrictive diastolic filling pattern. Cioffi et al [13] studied 170 patients with EF ≤ 40 % (ischemic and nonischemic) and varying degrees of MR. MR severity was the strongest predictor of 1-year mortality, independent of the presence of diabetes mellitus, older age, and larger LV end-diastolic volume. Rossi et al [14] studied 1256 patients with FMR because of ischemic and nonischemic cardiomyopathy. Using quantitative measures of FMR severity, FMR was an independent predictor of poor survival, even when effective regurgitant orifice area (EROA) was 0.2 cm2, a finding typically considered to be the threshold for distinguishing mild from moderate MR. Results from an MR substudy of the STICH (Surgical Treatment for Ischemic Heart Failure) trial confirmed the previous observational studies [15]. Of 1212 randomized patients with EF ≤ 35 % and CAD amenable to CABG, 554 (46 %) had mild MR, 181 (15 %) had moderate MR, and 39 (3 %) had severe MR. There was a graded worsening of prognosis with increasing MR severity. This study clearly demonstrated the adverse prognosis of untreated ischemic FMR in a cohort of patients with ischemic cardiomyopathy treated with current guideline-directed medical therapy with rigorous long-term follow-up in a randomized controlled trial setting. Furthermore, the consistent finding in multiple studies that FMR predicts mortality independently of LV volume or LVEF suggests that although FMR is caused by LV dysfunction, it may be a reasonable target for therapy.
Mechanism(s) of Functional MR
Carpentier [16] described 3 different types of MR according to leaflet motion. Carpentier class I MR is associated with normal leaflet motion and can be due to either structural abnormalities of the leaflets themselves, such as endocarditis or cleft leaflet, or may be due to pure annular dilation, such as chronic atrial fibrillation or restrictive cardiomyopathy. Class II MR is due to excessive motion of the leaflets into the left atrium because of prolapse or flail. This is the most common form of primary MR. Carpentier class IIIa is restriction of leaflet motion in both systole and diastole, classically seen in rheumatic heart disease or other inflammatory conditions. In Class IIIa, the mitral leaflets are typically thickened, calcified and relatively immobile. Class IIIb is leaflet restriction in systole only, which is the most common leaflet motion abnormality in FMR. Leaflet thickening and calcification is usually absent or age-related, and the abnormal systolic motion of the leaflets is due to underlying LV dysfunction.
The mechanism of ischemic FMR has been reviewed in detail by Levine and Schwammenthal [17]. Normally, as the LV shortens, papillary muscle contraction maintains the distance between the papillary muscle tips and mitral annulus to prevent prolapse of the leaflets into the left atrium. The papillary muscles, normally parallel to the LV long axis and perpendicular to the leaflets, efficiently balance forces generated by ventricular pressure on the leaflet surface. Ischemia or HF displaces the myocardial segments underlying the papillary muscles in an outward and/or apical direction, restricting leaflet closure in systole. This is known as tethering of the mitral leaflets and is often most on the posterior leaflet [15] and particularly on the posteromedial scallop [18]. The annulus is often dilated, and when present, annular dilation is associated with assumption of circular geometry rather than its typical “D” shape. Annular dilation may also be asymmetric with predominance of dilation next to the posteromedial scallop of the posterior leaflet [19]. In addition, there is often reduced closing force on the leaflets from LV systolic dysfunction. Contrary to earlier beliefs, pure papillary muscle dysfunction is not usually a major cause of FMR. Kaul et al [20] showed that reducing papillary muscle perfusion did not cause MR. In contrast, global hypoperfusion with LV dilatation caused FMR with incomplete leaflet closure correlating directly with the degree of LV dysfunction.
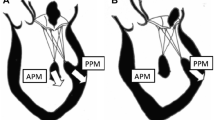
In the VALIANT trial [21] of 610 patients following acute MI, tenting area, coaptation depth, annular dilatation, and left atrial size were all associated with the degree of baseline MR. Tenting area, defined as the area enclosed between the annular plane and the mitral leaflets in mid-systole, was the only significant and independent predictor of worsening MR. Interestingly, not all patients with a dilated left ventricle develop MR [22]. This is, in part, because of adaptive increases in mitral leaflet surface area that occur in some patients with LV dysfunction. A recent study by Chaput et al [23] elegantly showed that patients with ischemic MR had lower leaflet area to closure area than those without MR. Inadequate leaflet expansion to compensate for LV dilatation appear to play a role in the mechanism of ischemic MR [24]. Finally, atrial functional MR is a subcategory of secondary MR, which occurs secondary to atrial fibrillation or restrictive cardiomyopathy [25]. As noted earlier, this form of FMR is Carpentier class I leaflet motion, in which pure annular dilation secondary to atrial dilation is the underlying mechanism of FMR. An interesting subgroup of patients with FMR have isolated inferobasal myocardial infarction with marked posterior leaflet tethering but preserved EF. In such patients, HF symptoms are clearly due to the FMR itself and not to LV dysfunction. This contrasts with the more typical variant of FMR wherein it can be difficult to determine whether HF symptoms are due to the underlying LV dysfunction, FMR, or both. Figure 1 illustrates different categories of FMR, highlighting the heterogeneity of this condition. Ischemic FMR is further complicated by CAD, ongoing ischemia, the need for and adequacy of coronary revascularization, and myocardial viability.
Heterogeneity of FMR. The classic cause of FMR is LV dysfunction, which can be nonischemic or ischemic cardiomyopathy. Treatment options for both start with GDMT for LV dysfunction/heart failure. If patient is not a transplant/LVAD candidate, mitral valve surgery can be considered. For ischemic FMR, in addition to GDMT, revascularization and viability may be considered. A subgroup of ischemic FMR patients have isolated inferobasal MI with preserved LV function. These patients clinically resemble primary MR in that their symptoms are due to the FMR, not the underlying LV dysfunction. Mitral valve surgery should be considered early. Finally, there is a group of FMR patients for whom the cause is pure mitral annular dilation secondary to atrial dilation, not LV dysfunction. These include chronic atrial fibrillation and restrictive cardiomyopathy. In addition to medical therapy or arrhythmia control, these patients may benefit from mitral valve annuloplasty
Assessing Severity of FMR
Echocardiography is the most widely used method for assessing the presence, mechanism and severity of MR. Evaluation of the severity of MR by echocardiography is complex, and all methods have inherent strengths and weaknesses. Simple “eyeball” grading of MR color flow jets is prone to error and should be discouraged. Both the American Society of Echocardiography [26] and European Association of Echocardiography guidelines [27•] state that the size of the color Doppler MR jet in the left atrium should not be used as the sole measure of MR severity. Instead, an integrative approach that incorporates several quantitative and qualitative measures should be used. The integrative approach combines quantitative measures of MR severity, such as EROA and regurgitant volume (RgV) with qualitative parameters including mitral filling pattern, pulmonary vein flow pattern, density of the MR signal on continuous wave Doppler, left atrial size, and pulmonary artery pressure. Some of these qualitative methods, though subjective, are highly specific. For example, systolic flow reversal in the pulmonary veins is highly specific for severe MR [26, 27•]. Conversely, an A-wave dominant mitral inflow pattern virtually excludes severe MR [26, 27•]. These patterns can help mitigate errors that are inherent in quantitative methods. Unfortunately, most of the studies evaluating prognosis of FMR or response of FMR to treatment did not use either quantitation of MR severity or the integrative method, as currently recommended.
The most accepted and well-studied method of quantitating MR severity is calculation of EROA. This is usually done by the proximal isovelocity surface area (PISA) method which assumes that flow approaches a circular orifice in a flat surface in an infinite series of hemispheric shells, each of which has the same velocity [26, 27•]. The assumption of a circular orifice is a major limitation of the PISA method in FMR because the elliptical orifice is usually elliptical [28•]. As a result, PISA tends to underestimate EROA in FMR (Fig. 2). This may be largely responsible for the fact that prior studies have shown an adverse prognosis even with EROA >0.2 cm2 [14, 29]. Recent studies have shown that 3D echocardiographic measurement of EROA directly (without assumption of circular geometry) results in larger and more accurate values in FMR. Regurgitant volume can be calculated by the formula EROA × VTI, where VTI is the velocity-time integral of the MR jet measured by continuous wave Doppler. Regurgitant volume and fraction can also be determined accurately from cardiac magnetic resonance (CMR) imaging, which may be very helpful when echocardiography is of poor quality or conflicts with the clinical picture or catheter-based data. A detailed review of quantitation of MR severity, including the integrative approach combining multiple variables was recently published [28•].
Transesophageal echocardiographic images from a typical patient with FMR because of dilated ischemic cardiomyopathy. The top left panel shows a PISA radius (yellow arrow) of 0.8 cm with an aliasing velocity of 29.3 cm/s, giving a peak MR flow rate of 118 mL/s. EROA is calculated as 0.25 cm2 by dividing peak MR flow rate by peak MR velocity of 476 cm/s from the continuous wave Doppler spectrum (Lower left panel). This is a classic underestimation of the true EROA because the orifice is not circular, but highly elliptical, as shown in the top right panel, which is a direct 3D TEE image of the EROA, which measures 0.61 cm2. The RgVol is 36 mL by 2D PISA method, but 87 mL by 3D EROA. The bottom right panel shows systolic flow reversal (yellow arrows) in the left upper pulmonary vein, which is highly specific for severe MR
In patients with functional MR (FMR) caused by LV dysfunction, EROA tends to decrease during mid-systole [30] and can vary significantly with loading conditions. This can result in FMR severity varying considerably on sequential echocardiograms. It is best to optimize medical therapy and diuretic doses prior to evaluating FMR severity, but it should be recognized that FMR severity can change over time in response to therapy, HF exacerbations, underlying ischemia, hypertensive episodes, and/or day to day variations in loading conditions.
There is some disparity among the guidelines as to what constitutes severe MR. All the guidelines agree that EROA ≥ 0.4 cm2, RgV ≥ 60 mL, and RgF ≥ 50 % constitute severe primary MR [26, 27•, 31••, 32], although these values are derived from a single-center observational study [33]. The Cardiothoracic Surgery Network trials originally used EROA ≥ 0.4 cm2 for the severe MR trial and 0.2 to 0.39 cm2 for the moderate MR trial [34]. These entry criteria were subsequently modified to allow lower values for EROA if accompanied by other echocardiographic signs that indicated that MR severity was worse, such as RgV, VCW, elevated E-wave velocity, or pulmonary vein flow reversal. This takes into account the tendency of PISA to underestimate EROA in FMR. The recent ACC/AHA guidelines on valvular heart disease propose a new definition for severe FMR at EROA >0.2 cm2 [31••]. This lower threshold accounts for the possibility that the actual EROA is larger than calculated by PISA in FMR, as well as the fact that MR in the setting of a cardiomyopathy has been associated with an adverse prognosis. However, it is not clear what to do with a patient with a EROA of 0.2 cm2 measured accurately by 3D TEE or CMR techniques, nor are there convincing data showing that correction of FMR at the lower threshold improves patient outcomes in FMR. Therefore, this new recommendation is likely to be controversial. While it is clear than EROA >0.2 cm2 confers an adverse prognosis in FMR, it is neither clear nor likely that EROA of 0.2 cm2 constitutes severe MR in terms of actual regurgitant volume.
EAE guidelines emphasize the potential value of exercise echocardiography in the evaluation of patients with ischemic FMR [27•]. Exercise echocardiography is useful in the evaluation of exercise-related changes in LV systolic function, ROA, and pulmonary artery pressure. Changes in EROA during exercise are not related to resting MR severity [30]; however, increases in EROA (≥13 mm2) during exercise in FMR have been shown to be associated with symptom status and a worsened prognosis [35, 36]. Finally, increased pulmonary artery pressure during exercise (≥60 mmHg) is considered a Class IIa indication for surgery in asymptomatic severe MR [31••, 32].
CMR offers an excellent method for quantifying MR severity [37-39]. CMR offers the potential to directly quantify RgV, RgF, and EROA in patients with MR. Because of its accuracy, reproducibility, and precision in measuring LV volumes, CMR is useful for evaluation of LV remodeling in MR [40]. Finally, CMR can identify the presence of scar or fibrosis using delayed hyperenhancement of gadolinium contrast agents. Limitations of CMR include cardiac arrhythmias that preclude adequate gating, pacemakers or implantable cardioverter-defibrillators, and claustrophobia. CMR should be used when echocardiography is technically difficult or equivocal.
Management
Medical Therapy
The first line of therapy for FMR is to address the underlying LV dysfunction with guideline-directed medical therapy (GDMT) [41••, 42], which is indicated (Class I, level of evidence A) in the most recent guidelines. The goal of such medical therapy is to optimize cardiac performance, reduce symptoms, and enhance survival by unloading the LV and maintaining euvolemia. This includes carefully titrated diuretic therapy, ACE inhibitors or angiotensin receptor antagonists and beta-blockers, especially carvedilol. The use of carvedilol has been shown to decrease severity of FMR, and is also associated with an increase in the forward aortic stroke volume [43]. Neurohormonal antagonists should be titrated to the targeted dose or the highest tolerated dose. It has been shown that in patients who respond to such therapy with improved LV function, there is a reduction (at least on qualitative grading) of MR severity.
Cardiac Resynchronization Therapy
Like GDMT, cardiac resynchronization therapy (CRT) is indicated (Class I, level of evidence A) for FMR patients who meet the indications for CRT. Dyssynchrony is presumed to exacerbate the underlying causes of FMR. Uncoordinated LV contraction worsens papillary muscle displacement and leaflet tethering, and inefficient LV systolic function results in decreased closing forces. CRT has been shown to cause a reduction in FMR through decreased leaflet tenting and presumably increased closing forces. Improvement in MR severity might be dependent on the improvement in LV dyssynchrony [44-46]. Interruption of CRT can result in immediate recurrence of MR [47, 48]. MR reduction could also be related to CRT-related reverse remodeling that requires weeks/months to occur [49, 50].
Results from the MIRACLE trial [51] showed an improvement in MR severity index following CRT institution in HF patients (EF ≤ 35 %) with a prolonged QRS (≥130 ms) and LVEDD ≥ 55 mm. Similar results were seen in the multicenter trial by Biase et al [52] (n = 794, median follow-up = 26 months). In this study, nonischemic patients (n = 379), compared with the ischemic group (n = 379), had a significantly higher rate of CRT responders ie, LV reverse remodeling (P >0.001). Severity of MR at baseline (regression coefficient = 0.76, R-square = 0.44, P <0.001) and MR change at 3 months (regression coefficient = 0.69, R-square = 0.41, P <0.001) were found to have strong correlation with MR (improvement/worsening) at 12 months. Also, marked (≥1 grade) improvement of MR at 3 months was associated with a better response to CRT. In the study by Onishi et al [53] (n = 274), there were 114 (48 %) patients with significant MR (≥moderate) at baseline; of whom 48 (42 %) patients had MR improvement at 6 month follow-up, and 24 (19 %) patients had MR worsening after CRT. Three echocardiographic features were independently associated with improved MR after CRT on multivariable analysis: anteroseptal to posterior wall radial strain dyssynchrony >200 ms, lack of severe LV dilatation (end-systolic dimension index <29 mm/m(2)), and lack of echocardiographic scar at papillary muscle insertion sites (all P <0.05). In this study, significant MR after CRT was strongly associated with less favorable long-term survival. There were a total of 66 events (47 deaths, 10 transplants, and 9 LV assist devices). Events were strongly associated with significant MR after CRT (hazard ratio, 3.58; 95 % confidence interval (CI), 2.18–5.87; P <0.0001). Similar findings with longer follow-up were shown by Van Bommel et al [54] (n = 85, median follow-up = 32 months). However, most of these studies used inaccurate measures of MR severity, such as jet area to LA area, which have several limitations as discussed previously.
Revascularization
Patients with MR because of ischemic cardiomyopathy should undergo appropriate revascularization as indicated for documented ischemia. In the STICH trial, which randomized 1212 patients with HF because of ischemic cardiomyopathy to GDMT vs GDMT and CABG, there was a trend for improved all-cause mortality with CABG (hazard ratio 0.86, 95 % CI 0.72–10.4, P = 0.12) [55•]. The secondary endpoint of cardiac mortality (hazard ratio 0.81, 95 % CI 0.66–1.00, P = 0.05) was improved significantly by CABG, as was the combined endpoint of all-cause mortality and hospitalization for cardiac causes (hazard ratio 0.74, 95 % CI 0.64–0.85, P < .001). Revascularization of ischemic cardiomyopathy can result in improved LV size and systolic function, improved HF symptoms and reduction of FMR. Kang et al [56] reported a nonrandomized study of 185 undergoing coronary revascularization by PCI (n = 66) or CABG (n = 119). In a propensity-matched analysis, cardiac events were lower with CABG compared with PCI (hazard ratio 0.499, 95 % CI 0.25–0.99, P = 0.043). There are no prospective randomized trials comparing CABG to PCI for FMR patients, but this study suggests that CABG may be superior. Because reverse LV remodeling after revascularization is not immediate, it is usually appropriate to wait 3 months to determine if significant reverse remodeling and improvement in FMR have occurred.
Surgical Therapy for MR
In patients who have significant symptomatic FMR despite optimal GDMT and revascularization (if indicated), surgical correction of FMR can be considered. The current trend amongst most clinicians is to repair MR greater than mild severity when the patient is already scheduled for CABG [57, 58]. This is supported by the recent ACC/AHA guidelines, which state that mitral valve surgery is reasonable for patients with symptomatic severe FMR who are undergoing CABG or AVR (Class IIa, level of evidence C) [31••]. Mitral surgery can also be considered for 2 other situations: (1) severely symptomatic patients (NYHA Class III or IV) with chronic severe FMR (Class IIb, level of evidence B), or (2) chronic moderate FMR who are undergoing other cardiac surgery (Class IIb, level of evidence C) [31••].
When considering mitral valve surgery for FMR, it is important to distinguish between ischemic and nonischemic etiology. For moderate ischemic MR, some have argued that revascularization by CABG reverses LV remodeling, resulting in normalization of ventricular geometry and therefore improvement of elimination of FMR [59]. However, several studies showed that CABG alone is often insufficient and leaves many patients (40 %) with significant residual FMR [58, 60]. Randomized clinical trials have been performed in this area, but are not definitive. Fattouch [61] reported no significant benefit to adding mitral valve annuloplasty to CABG in a small single-center randomized trial of 102 patients. This study was underpowered and does not exclude the possibility of a benefit. In a substudy of the STICH trial [15], there was a strong trend for benefit to adding mitral valve repair to CABG vs medical therapy alone. Patients who received CABG plus mitral valve repair had a lower point estimate for mortality, but this was not statistically significant (hazard ratio 0.62; 95 % CI, 0.35–1.08). After adjustment for baseline prognostic variables, CABG with mitral surgery was superior to CABG alone (hazard ratio 0.41, 95 % CI, 0.22–0.77; P = 0.006). Unfortunately, although STICH was a randomized trial, the decision to treat the mitral valve during CABG was not randomized, but left to the discretion of the surgeon. In the RIME trial [62], 73 patients with moderate FMR were randomized to CABG alone vs CABG plus mitral valve repair. The trial was stopped early for a benefit in the primary endpoint of peak oxygen consumption. LV remodeling, MR severity and functional class were better with mitral valve repair, but the trial was not powered for mortality.
In nonischemic cardiomyopathy, a retrospective analysis by Wu et al [63] failed to show a benefit of mitral valve annuloplasty over medical therapy alone with a primary end point of death, LV assist device implantation, or heart transplantation. However, in the ACORN trial [64], mitral valve repair, with or without an external cardiac restraint device in patients with heart failure (NYHA 3-4), LVEF ≤ 35 % and LV dilation, was associated with progressive reduction in LV mass, increased EF, and sphericity index, all consistent with reverse remodeling. In the study by Braun et al [65], LV end-diastolic dimension was the best predictor of the extent of reverse remodeling where patients with LV end-diastolic dimension ≤ 65 mm showed the most benefit. This suggests that earlier intervention may be more beneficial than waiting until severe LV dilation has taken place.
MV Repair vs MV Replacement
Currently, the decision between repair and replacement is left to the surgeon’s discretion. Mitral repair is generally preferred whenever possible based on valve pathology and patient stability, because it avoids long-term anticoagulation, decreases infective endocarditis risk, and provides greater leaflet durability. Also, retrospective studies have shown lower peri-operative mortality and, in 1 propensity-matched comparison, improved 5-year survival for repair vs replacement (58 vs 36 %) [66]. Among repair techniques, ring annuloplasty is considered the gold standard. Partial-ring annuloplasty is believed to be inadequate for preventing progressive annular dilation [67]. Similarly, flexible rings also permit annular distortion and have been found to have a 4-fold greater recurrence of MR over rigid rings [68]. Full ring annuloplasty undersized by 1 to 2 sizes restores annular geometry, ensuring proper leaflet coaptation and providing proper annular reduction and minimal risk for stenosis [69, 70]. Failure to downsize can result in a recurrence rate of 30 % to 40 % [69]. Saddle-shaped complete rings have also reported to improve restoration of annular geometry, decrease leaflet strain, and increase durability [71]. However, even with ring-induced annular reduction, concomitant prolapse or tethering of the valve leaflets may persist, requiring additional procedures. Tethered or elongated chordae of the anterior leaflet can be relocated by transposition of chordae or replacement with suture. Papillary muscles may also be relocated to a more favorable position on the ventricular wall [72].
MVR is usually reserved for situations in which the valve cannot be reasonably repaired, or repair is unlikely to be tolerated clinically. MVR is more appropriate for complex valve disease with both structural and functional MR, involving multiple or eccentric regurgitant jets. MVR is also usually faster than repair with a shorter CPB time, and, therefore, may be more appropriate for high-risk surgical candidates. Biological prostheses are generally used for older patients, those with expected survival less than 10 years, and in patients unable to tolerate or maintain compliance with anticoagulation. MVR without preservation of the mitral apparatus and chordal structures is not recommended because preservation of the mitral apparatus has shown to preserve LV geometry and systolic function, and improve survival [73-76]. In FMR, operative mortality for MV surgery is approximately 3 %–4 % [77-80]. Five-year survival has been traditionally low, at approximately 30 %–40 %, because of the significance of this disease and the underlying cardiomyopathy.
The Cardiothoracic Surgical Trials Network (CTSN) [81•] enrolled 251 patients with moderate to severe ischemic MR and coronary artery disease between 2009 and 2011 and randomized them to ether receive mitral valve repair (126 patients) or chord-sparing replacement (125 patients). The primary endpoint was LV reverse remodeling assessed by LV end-systolic volume index (LVESVI) measured at 12 months. At 12 months, LVESVI was 54.6 mL/m2 in the repair group vs 60.7 mL/m2 in the replacement group, for a mean change from baseline of -6.6 and -6.8 mL/m2, respectively. There was no significant between-group difference in LVESVI after adjustment for death. The 1-year mortality rate was 14.3 % in the repair group vs 17.6 % in the replacement group. However, the repair group showed a significantly higher rate of recurrence of moderate or severe MR at 12 months (32.6 % vs 2.3 %). There were no significant differences between the groups in the rate of a composite of major adverse cardiac or cerebrovascular events, functional status or quality of life at 12 months. The investigators concluded that although replacement provided a more durable correction of MR, there was no significant between-group difference in clinical outcomes. Long-term follow-up is ongoing. The high recurrence rate of significant MR, despite the use of complete rigid rings in the hands of excellent surgeons, may turn out to change the current practice of favoring annuloplasty over chord-sparing MVR. It may also dampen enthusiasm for percutaneous annuloplasty devices in current development.
Percutaneous Options
A variety of percutaneous approaches have been devised for the minimally invasive treatment of MR, with the goal of serving the large demand for restoration of mitral function without the increased perioperative risk of traditional surgery. Feldman and Young [82] recently published an excellent review of such devices, which generally attempt to mimic a surgical technique but in a beating heart without cardiopulmonary bypass or aortic cross-clamping. Each percutaneous system attempts to address a component of the pathophysiology associated with the mechanism of MR. These devices enable leaflet repair, chordal replacement, annular shape change, LV geometry change, or even complete valve replacement. Although the use of percutaneous approaches is often limited to patients who present as poor surgical candidates, their effectiveness and usefulness should continue to increase as engineering improvements occur. The most widely used of these is the MitraClip, which is a cloth-covered cobalt-chromium clip that pins the anterior and posterior leaflets together in a manner analogous to the Alfieri stitch. MitraClip has been used in over 13,500 patients worldwide and is considered Class IIb for treatment of FMR in the ACC/AHA HF guidelines [41••] and the European valve guidelines [32]. Although MitraClip has been shown to improve MR severity, symptoms, and cause reverse remodeling in both primary MR and FMR [83-88], it is currently only approved in the US for use in patients with primary degenerative MR who are at prohibitive risk for surgical repair. Approval for FMR will not be forthcoming until the results of the COAPT trial are available. COAPT is a randomized controlled trial of FMR patients treated with GDMT including CRT, if indicated) vs GDMT plus MitraClip. The primary endpoint is rate of HF hospitalization. Results are not expected until 2016.
Several annuloplasty devices have been developed using a variety of approaches. Indirect annuloplasty via the coronary sinus has shown good preliminary results [89] but is limited because the coronary sinus overlies the left circumflex coronary artery in a substantial number of patients, is often >1 cm from the mitral annulus, which is especially true as the LV dilates, and does not allow for a complete ring repair. Direct annuloplasty devices can be performed via the left atrium through direct surgical approaches or trans-septal approaches. Such devices have anchoring systems to connect to the mitral annulus and can be tightened under TEE guidance to achieve maximal reduction of MR in a beating heart. An alternative approach is to place the annuloplasty ring in the LV behind the subvalvular apparatus. A criticism of these devices is that they are not complete rings. Given the 32 % recurrence rate of significant MR in the CTSN trial with direct surgical implantation of complete rigid rings, these percutaneous annuloplasty rings will be required to demonstrate durability. Chordal repair techniques are being developed, but these are more appropriate for primary degenerative MR than for FMR. Finally, there are several devices being developed for mitral valve replacement, either via transapical or trans-septal approaches. Like TAVR, TMVR offers the potential to provide beating heart valve replacement to high risk patients and may be particularly suitable for FMR. In contrast to TAVR, TMVR will be harder to develop because of the noncircular shape of the mitral annulus, the potential for pushing the anterior mitral leaflet into the LV outflow tract, and difficulty anchoring the device to the anterior portion of the mitral annulus (the aortomitral curtain). The usefulness and effectiveness of these devices will need to be compared with the gold standard of ring annuloplasty.
Conclusions
FMR is a complex disorder in which MR occurs because of disordered LV geometry and contractile function or pure annular dilation because of atrial enlargement. Treatment of FMR should first be targeted to the underlying LV dysfunction using guideline-directed medical therapy, as well as CRT and/or coronary revascularization, when indicated. Mitral valve surgery can be considered when patients are already scheduled for CABG or other cardiac surgery, or when symptoms persist and are attributable to FMR, despite optimal guideline-directed medical therapy. Advances in percutaneous devices for treatment of FMR are promising and will continue to evolve.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–43.
Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. 1995;91:1022–8.
Jokinen JJ, Hippeläinen MJ, Pitkänen OA, Hartikainen JE. Mitral valve replacement vs repair: propensity-adjusted survival and quality-of-life analysis. Ann Thorac Surg. 2007;84:451–8.
David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127:1485–92.
Trichon BH, Felker GM, Shaw LK, et al. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–43.
Blondheim DS, Jacobs LE, Kotler MN, Costacurta GA, Parry WR. Dilated cardiomyopathy with mitral regurgitation: decreased survival despite a low frequency of left ventricular thrombus. Am Heart J. 1991;122:763–71.
Robbins JD, Maniar PB, Cotts W, Parker MA, Bonow RO, Gheorghiade M. Prevalence and severity of functional mitral regurgitation in chronic systolic heart failure. Am J Cardiol. 2003;91:360–2.
Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–91.
Lamas GA, Mitchell GF, Flaker GC, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and ventricular enlargement investigators. Circulation. 1997;96:827–33.
Tcheng JE, Jackman Jr JD, Nelson CL, et al. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117:18–24.
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64.
Grayburn PA, Appleton CP, De Maria AN, Greenberg B, Lowes B, Oh J, et al. BEST Trial Echocardiographic Substudy Investigators. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST). J Am Coll Cardiol. 2005;45:1064–71.
Cioffi G, Tarantini L, De Feo S, et al. Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure. Eur J Heart Fail. 2005;7:1112–7.
Rossi A, Dini FL, Faggiano P, Agricola E, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–80.
Deja M, Grayburn P, Sun B, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–48.
Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–37.
Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–58.
Nakai H, Kaku K, Takeuchi M, et al. Different influences of left ventricular remodeling on anterior and posterior mitral leaflet tethering. Circ J. 2012;76:2481–7.
Kwan J, Shiota T, Agler DA, Popovic ZB, Qin JX, Gillinov MA, et al. Geometric differences of the mitral apparatus between ischemic and dilated cardiomyopathy with significant mitral regurgitation. Real-time three-dimensional echocardiography study. Circulation. 2003;107:1135–40.
Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA. Mechanism of ischemic mitral regurgitation: an experimental evaluation. Circulation. 1991;84:2167–80.
Meris A, Amigoni M, Verma A, et al. Mechanisms and predictors of mitral regurgitation after high-risk myocardial infarction. J Am Soc Echocardiogr. 2012;25:535–42.
Beaudoin J, Handschumacher MD, Zeng X, Hung J, Morris EL, Levine RA, et al. Mitral valve enlargement in chronic aortic regurgitation as a compensatory mechanism to prevent functional mitral regurgitation in the dilated left ventricle. J Am Coll Cardiol. 2013;61:1809–16.
Chaput M, Handschumacher MD, Tournoux F, et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–52.
Grayburn PA. New concepts in functional mitral regurgitation: it is not just a disease of the left ventricle. J Am Coll Cardiol. 2013;61:1817–9.
Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474–81.
Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler Echocardiography. J Am Soc Echocardiogr. 2003;16:777–802.
Lancellottti P, Moura L, Pierard L, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation, part 2: mitral and tricuspid regurgitation (native disease). Eur J Echocardiogr. 2010;11:307–32. European Association of Echocardiography Guidelines for assessment of MR.
Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation. 2012;126:2005–17. Review of imaging methods for quantitation of MR, including 3D echocardiography.
Lancelloti P, Lebrun F, Pierard LA. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2003;42:1921–8.
Mittal AK, Langston Jr M, Cohn KE, Selzer A, Kerth WJ. Combined papillary muscle and left ventricular wall dysfunction as a cause of mitral regurgitation: an experimental study. Circulation. 1971;44:174–80.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC Guidelines for the Management of Patients With Valvular Heart Disease, J Am Coll Cardiol. 2014;[Epub ahead of print]. Updated AHA/ACC guidelines for valvular heart disease.
Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–68.
Dujardin KS, Enriquez-Sarano M, Bailey KR, Nishimura RA, Seward JB, Tajik AJ. Grading of mitral regurgitation by quantitative Doppler echocardiography: calibration by left ventricular angiography in routine clinical practice. Circulation. 1997;96:3409–15.
Smith PK, Michler RE, Woo YJ, et al. Design, rationale, and initiation of the Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial: a report from the Cardiothoracic Surgical Network. J Thorac Cardiovasc Surg. 2012;143:111–7.
Pierard LA, Lancelloti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med. 2004;35:1627–34.
Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation. 2003;108:875–83.
Hundley WG, Li HF, Willard JE, et al. Magnetic resonance imaging assessment of the severity of mitral regurgitation: comparison with invasive techniques. Circulation. 1995;92:1151–8.
Gelfand EV, Hughes S, Hauser TH, et al. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson. 2006;8:503–7.
Van de Heyning CM, Magne J, Vrints CJ, Piérard L, Lancellotti P. The role of multi-imaging modality in primary mitral regurgitation. Eur Heart J Cardiovasc Imaging. 2012;13:139–51.
Schiros CG, Dell'italia LJ, Gladden JD, et al. Magnetic resonance imaging with 3-dimensional analysis of left ventricular remodeling in isolated mitral regurgitation: implications beyond dimensions. Circulation. 2012;125:2334–42.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. Updated AHA/ACC guidelines for heart failure.
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69.
Comin-Colet J, Sánchez-Corral MA, Manito N, et al. Effect of carvedilol therapy on functional mitral regurgitation, ventricular remodeling, and contractility in patients with heart failure due to left ventricular systolic dysfunction. Transplant Proc. 2002;34:177–8.
Kanzaki H, Bazaz R, Schwartzman D, et al. A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1619–25.
Porciani MC, Macioce R, Demarchi G, et al. Effects of cardiac resynchronization therapy on the mechanisms underlying functional mitral regurgitation in congestive heart failure. Eur J Echocardiogr. 2006;7:31–9.
Goland S, Rafique AM, Mirocha J, Siegel RJ, Naqvi TZ. Reduction in mitral regurgitation in patients undergoing cardiac resynchronization treatment: assessment of predictors by two-dimensional radial strain echocardiography. Echocardiography. 2009;26:420–30.
Brandt RR, Reiner C, Arnold R, Sperzel J, Pitschner HF, Hamm CW. Contractile response and mitral regurgitation after temporary interruption of long-term cardiac resynchronization therapy. Eur Heart J. 2006;27:187–92.
Kuppahally SS, Fowler MB, Vagelos R, Wang P, Al-Ahmad A, Paloma A, et al. Worsening of left ventricular end-systolic volume and mitral regurgitation without increase in left ventricular dyssynchrony on acute interruption of cardiac resynchronization therapy. Echocardiography. 2009;26:759–65.
Cazeau S, Leclercq C, Lavergne T, et al. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effect of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80.
Abraham WT, Fisher WG, Smith AL, et al. Multicenter InSync randomized clinical evaluation. Cardiac resynchronization therapy in chronic heart failure. N Engl J Med. 2002;346:1845–53.
Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation. 2006;113:266–72.
Di Biase L, Auricchio A, Mohanty P, et al. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace. 2011;13:829–38.
Onishi T, Onishi T, Marek JJ, et al. Mechanistic features associated with improvement in mitral regurgitation after cardiac resynchronization therapy and their relation to long-term patient outcome. Circ Heart Fail. 2013;6:685–93.
Van Bommel RJ, Marsan NA, Delgado V, et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation. 2011;124:912–9.
Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. Randomized trial of GDMT vs CABG + GDMT in ischemic heart failure.
Kang DH, Sun BJ, Kim DH, et al. Percutaneous vs surgical revascularization in patients with ischemic mitral regurgitation. Circulation. 2011;124(11 Suppl):S156–62.
Prifti E, Bonacchi M, Frati G, et al. Should mild-to-moderate and moderate ischemic mitral regurgitation be corrected in patients with impaired left ventricular function undergoing simultaneous coronary revascularization? J Card Surg. 2001;16:473–83.
Harris KM, Sundt III TM, Aeppli D, Sharma R, Barzilai B. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thorac Surg. 2002;74:1468–75.
Balu V, Hershowitz S, Zaki Masud A, et al. Mitral regurgitation in coronary artery disease. Chest. 1982;81:550–5.
Grossi EA, Zakow PK, Sussman M, et al. Late results of mitral valve reconstruction in the elderly. Ann Thorac Surg. 2000;70:1224–6.
Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E, et al. Point: efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278–85.
Chan KM, Punjabi PP, Flather M, Wage R, Symmonds K, Roussin I, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502–10.
Wu A, Aaronson K, Bolling S, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005;45:381–7.
Acker MA, Bolling S, Shemin R, et al. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg. 2006;132:568–77.
Braun J, Bax JJ, Versteegh MI, Voigt PG, Holman ER, Klautz RJ, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2005;27:847–53.
Gillinov A, Wierup P, Blackstone E, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–41.
Mihaljevic T, Lam B, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–201.
Ryan L, Jackson B, Hamamoto H, et al. The influence of annuloplasty ring geometry on mitral leaflet curvature. Ann Thorac Surg. 2008;86:749–60.
Acker M. Should moderate or greater mitral regurgitation be repaired in all patients with LVEF <30 %? Mitral valve repair in patients with advanced heart failure and severe functional mitral insufficiency reverses left ventricular remodeling and improves symptoms. Circ Heart Fail. 2008;1:281–4.
Braun J, van de Veire NR, Klautz RJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85:430–6.
Jimenez J, Liou S, Padala M, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J Thorac Cardiovasc Surg. 2007;134:1562–8.
Kron I, Green G, Cope J. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74:600–1.
David TE, Uden DE, Strauss HD. The importance of the mitral apparatus in left ventricular function after correction of mitral regurgitation. Circulation. 1983;68:1176–82.
David TE, Burns RJ, Bacchus CM, Druck MN. Mitral valve replacement for mitral regurgitation with and without preservation of chordae tendineae. J Thorac Cardiovasc Surg. 1984;88:718–25.
Rozich JD, Carabello BA, Usher BW, Kratz JM, Bell AE, Zile MR. Mitral valve replacement with and without chordal preservation in patients with chronic mitral regurgitation. Mechanisms for differences in postoperative ejection performance. Circulation. 1992;86:1718–26.
Horskotte D, Schulte HD, Bircks W, Strauer BE. The effect of chordal preservation on late outcome after mitral valve replacement: a randomized study. J Heart Valve Dis. 1993;2:150–8.
Khabbaz KR, Mahmood F, Shakil O, et al. Dynamic 3-dimensional echocardiographic assessment of mitral annular geometry in patients with functional mitral regurgitation. Ann Thorac Surg. 2013;95:105–10.
Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–241.
Filsoufi F, Salzberg S, Adams D. Current management of ischemic mitral regurgitation. Mt Sinai J Med. 2005;72:105–15.
Adams D, Filsoufi F, Aklog L. Surgical treatment of the ischemic mitral valve. J Heart Valve Dis. 2002;11:S21–5.
Acker MA, Parides MK, Perrault LP, Moskowitz AJ, et al. Mitral-valve repair vs replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. Randomized trial of mitral valve replacement vs repair in ischemic FMR.
Feldman T, Young A. Percutaneous approaches to valve repair in mitral regurgitation. J Am Coll Cardiol. 2014; [Epub ahead of print].
Feldman T, Foster E, Glower D, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406.
Mauri L, Foster E, Glower DD, Apruzzese P, Massaro JM, Herrmann HC, et al. Four-year results of a randomized controlled trial of percutaneous repair vs surgery for mitral regurgitation. J Am Coll Cardiol. 2013;62:317–28.
Foster E, Kwan D, Feldman T, Weissman N, Grayburn P, Schwartz A, et al. Percutaneous mitral valve repair in the initial EVEREST cohort: evidence of reverse left ventricular remodeling. Circ Cardiovasc Interv. 2013;6:522–30.
Grayburn PA, Sangli C, Massaro J, Mauri L, Weissman NJ, Glower DG, et al. The relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after MitraClip therapy. Circulation. 2013;128:1667–74.
Auricchio A, Schillinger W, Meyer S, Maisano F, Hoffmann R, Ussia GP, et al. PERMIT-CARE Investigators. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol. 2011;58:2183–9.
Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, et al. Percutaneous MitraClip® device therapy for mitral regurgitation in 351 patients: high risk subset of the EVEREST II Study. J Am Coll Cardiol. 2013; [In press].
Siminiak T, Wu JC, Haude M, Hoppe UC, Sadowski J, Lipiecki J, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail. 2012;14:931–8.
Compliance with Ethics Guidelines
Conflict of Interest
Mina M. Benjamin declares that he has no conflict of interest. Robert L. Smith declares that he has no conflict of interest. Paul A. Grayburn reports grants and personal fees from Abbott Vascular, grants from Medtronic, grants from Edwards, grants from Aastrom, grants from Guided Delivery Systems, and grants from Valtech Cardio.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Congestive Heart Failure
Rights and permissions
About this article
Cite this article
Benjamin, M.M., Smith, R.L. & Grayburn, P.A. Ischemic and Functional Mitral Regurgitation in Heart Failure: Natural History and Treatment. Curr Cardiol Rep 16, 517 (2014). https://doi.org/10.1007/s11886-014-0517-0
Published:
DOI: https://doi.org/10.1007/s11886-014-0517-0