Abstract
Functional ischemic mitral regurgitation may occur as part of the complex process of left ventricular remodeling and affects the prognosis unfavourably. Chronic ischemic MR occurs in approximately 30 % of patients followed up after a myocardial infarction and in 50 % of those with post-infarct congestive heart failure. The treatment of ischemic mitral regurgitation is still debated. Medical management alleviates symptoms but does not alter the progression of the disease. The matter of surgery for ischemic mitral regurgitation, in terms of whether, when and how it should be corrected is still considerably controversial. Surgery is recommended for moderate-to-severe or severe mitral regurgitation in patients with symptoms or evidence of left ventricular dysfunction. Myocardial revascularization paired to valve surgery can be performed to treat the underlying coronary artery disease. Surgical Ventricular Reconstruction offers either the possibility to repair the mitral valve through the left ventricular opening or the potential of improving mitral functioning by reducing left ventricular volumes and rebuilding a more normal geometry.
This chapter will discuss the principles of treatment for ischemic mitral regurgitation according to the different phenotypes of left ventricular remodelling, the surgical techniques and the results, focusing on which patients may have greater benefit to the best of our knowledge.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Myocardial infarction
- Left ventricular remodeling
- Ischemic mitral regurgitation
- Surgical ventricular reconstruction
Introduction
Functional mitral regurgitation (MR) broadly indicates abnormal function of normal mitral leaflets in the context of impaired left ventricular function; it typically occurs in globally dilated and hypokinetic ventricles or with segmental damage that affects valve closure. Functional ischemic MR is caused by changes in ventricular structure and function related ultimately to ischemia [1]; it is predominantly postinfarction MR and occurs as part of the complex process of left ventricular (LV) remodeling (Fig. 13.1). Despite advances in coronary reperfusion, MR remains common following an acute MI, occurring in up to 30 % of patients [2, 3] and in 50 % of those with congestive heart failure [4], adversely affecting the prognosis, even when mild [5].
Although the knowledge of the mechanisms underlying ischemic mitral regurgitation has improved over half a century, many aspects remain controversial, leaving the therapeutic strategies perplexing in their diversity and not fully effective. Indeed, chronic ischemic MR is a “complex and dynamic disease”, involving coronary arteries, mitral annulus, subvalvular apparatus and ventricle, in which the large number of geometric and hemodynamic variables and the complex interaction between each other carries the risk of a suboptimal result when treated.
From Ischemic LV Remodeling to Mitral Regurgitation: In Search of the Target Lesion
LV remodeling is a complex, dynamic and time-dependent process, which may occur in different clinical conditions, including MI, leading to chamber dilatation, altered configuration and increased wall stress [6]. It begins within the first few hours after an MI and results from fibrotic repair of the necrotic area with scar formation, elongation, and thinning of the infarcted zone. LV volumes increase, a response that is sometimes considered adaptive, associated with stroke volume augmentation in an effort to maintain a normal cardiac output as the ejection fraction declines. However, beyond this early stage, the remodeling process is driven predominantly by eccentric hypertrophy of the non-infarcted remote regions, resulting in increased wall stress, chamber enlargement and geometric distortion [7]. These changes, along with a decline in performance of hypertrophied myocyte, increased neurohormonal activation, collagen synthesis, fibrosis and remodeling of the extracellular matrix within the non-infarcted zone, result in a progressive decline in ventricular performance [8]. At the same time, the papillary muscle (PM) displacement, which may occur as a consequence of the LV dilatation, results in leaflet tethering of the mitral valve at closure with lack of a proper coaptation, in turn leading to secondary MR (Fig. 13.2). In addition, ventricular dilatation results in annular enlargement, which further increases valve incompetence. Indeed, after the first description by Burch et al. [9] in 1963 supporting the central role of PM dysfunction as the basic mechanism of ischemic MR, a large number of experimental and clinical studies have reported contrary results. Large-animal models have shown that both left ventricular dilation and posterior PM infarction were necessary for the development of MR [10]. A retrospective study by Okayama et al. [11] in patients with single-vessel coronary disease using cardiac magnetic resonance (CMR) to quantify PM infarction and MR found an association between the presence of delayed enhancement (DE) in PM and MR, specifically in patients with large infarctions and bilateral PM enhancement. Other studies, however, have underlined a weaker role for PMs in ischemic MR. Dog models of ischemic MR showed that when PM was selectively infarcted, it did not produce MR, whereas larger infarctions encompassing the PM and adjacent myocardium did produce MR [12]. In the same study by Okayama et al. [11], patients with single vessel right coronary artery disease as well as PM infarction had less MR than patients who had no PM infarction.
One of the latest studies seems to have elegantly reconciled this old debate to better clarify the role of papillary muscles compared to that of the adjacent remodeled LV wall. In a large prospective cohort of 153 patients with first ST-segment elevation MI without intrinsic mitral valve disease, Chinitz et al. [13] evaluated the incidence and severity of ischemic MR as well as coronary and ventricular anatomy using a multimodality imaging (echocardiography to quantify MR, angiography to identify the culprit coronary lesions, and a high resolution DE-CMR sequence to define the extent of PM infarction – partial vs. complete – and ventricular infarction). The results showed that neither complete nor partial PM infarction necessarily led to the development of MR. However, the amount of infarcted myocardium was significantly associated with the development of ischemic MR, even after a multivariate analysis, confirming the role of the underlying ventricular infarction and adverse remodeling as the primary culprit for the development and progression of chronic ischemic MR. Furthermore, once established, ischemic MR, can itself worsen the remodeling process, altering LV loading conditions, increasing diastolic wall stress (which can worsen eccentric hypertrophy with further LV dilatation and dysfunction) and end-systolic wall stress in patients with chronic MR because of induced LV remodeling, with decreased contractility and increased end-systolic volume, driving a vicious circle in which MR begets more MR [14]. Having said that, it is still unclear if the volume overload created by MR adds a greater pathologic burden to an already adverse condition or, simply, the worse prognosis is related to a poorer LV function and functional MR is merely an indicator of this bad condition.
Ischemic Mitral Regurgitation According Different Phenotypes of LV Remodeling
Usually, ischemic MR occurs in nearly 50–60 % of patients with previous inferior MI due to a bulging of the inferior and posterior LV basal and midventricular segments underlying the PMs [3, 15, 16]. However, clinical studies, including one of the most recent from Levine and co-workers, outlined the importance of anteroapical MI causing MR despite the absence of inferobasal wall motion abnormalities [17]. In this case, mitral regurgitation grade correlated most strongly with tethering length due to the displacement of the papillary muscles.
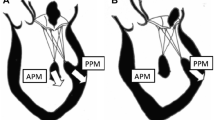
Recently, our group addressed the differences between anterior and posterior remodeling in patients with previous MI undergoing surgical ventricular reconstruction (SVR) [18]. From a morphological point of view, post-infarction remodeling occurred at different LV levels in the two study groups (A, anterior versus P, posterior). The LV apex is primarily involved after an anterior MI (Fig. 13.3, left panel), as we previously reported [19]. As a consequence, the conicity index (obtained from the apical to short axis ratio) was significantly greater in the anterior remodeling group (A) compared to posterior (P). Conversely, a previous inferior MI determined a regional remodeling of the basal and mid segments of the inferopostero-lateral wall (Fig. 13.3, right panel), with a significant increase in LV transverse diameters and consequently in the sphericity index (obtained from the short to long axis ratio). LV basal involvement in posterior dilatation causes lateral displacement of the posteromedial PM leading to a significant increase in the interpapillary distance in Group P compared to Group A. As a consequence, patients in group P presented with a higher incidence of severe MR (55 % vs 25 %, respectively, p = 0.01), which determined higher LV mass, larger left atrium dimensions, higher pulmonary artery pressure and higher rate of right ventricular dysfunction. After analyzing the data according to the presence or not of moderate to severe MR in the two different patterns of LV remodeling, we observed that in posterior remodeling the main geometrical change associated with severe MR was an increase in the interpapillary distance, without significant difference in the tenting area. On the contrary in anterior remodeling, MR occurs mainly in the setting of global LV dilatation, with tethering of both mitral valve leaflets due to apical displacement of PMs; hence, increased mitral tenting area was the major determinant of severe MR, without a concomitant significant increase in interpapillary distance. Furthermore, when Cox Regression analysis was applied separately to the two study groups, severe preoperative MR remained a significant independent predictor of long-term mortality in Group A but not in Group P. We speculated that, behind the above mentioned geometrical assumptions, in patients with previous anterior MI, MR occurs mainly in the setting of global LV dilatation and severe dysfunction, reflecting a more advanced stage of disease.
(a) Left panel: the LV apex is primarily involved after an anterior MI. As a consequence, the conicity index (CI, obtained from the apical – c – to short axis ratio – b) is significantly greater in the anterior remodeling compared to posterior remodeling group. (b) Right panel: a previous inferior MI induces a regional remodeling of the basal and mid segments of the inferopostero-lateral wall, with a significant increase in LV transverse diameters and consequently in the sphericity index (SI, obtained from the short – b -to long axis ratio – a)
This is also consistent with the fact that, in the group A, patients with severe MR showed worsened right ventricular function compared to patients with mild MR. This phenomenon was not observed in the group P even though in both groups, patients with severe MR showed a similar increase in systolic pulmonary artery pressure.
Surgical Operative techniques
The Rationale to Perform Surgical Ventricular Reconstruction to Reverse LV Remodeling
Surgical Ventricular Reconstruction (SVR) of the LV chamber has been introduced as an optional therapeutic strategy aiming to reduce LV volumes through the exclusion of the scar tissue from the LV cavity, thereby reducing the ventricle size to a more physiological volume, reshaping the distorted chamber and improving cardiac function through a reduction of LV wall stress in accordance with the principle of Laplace’s law. Since LV wall stress is directly proportional to LV internal radius and pressure, and inversely proportional to wall thickness, any intervention to optimize this relationship would be beneficial either in terms of improving wall compliance and reducing filling pressure, or as wall stress is a crucial determinant of afterload, in terms of enhancing contractile performance of the LV by increasing the extent and velocity of systolic fiber shortening [20]. Furthermore, SVR of failing ventricles is usually combined with myocardial revascularization with the aim of treating the underlying coronary artery disease, although in recent years the percentage of patients with ischemic LV dysfunction without significant coronary disease has increased due previous percutaneous coronary interventions (PCI). Lastly, SVR offers either the possibility to repair the mitral valve through the LV opening or the potential of improving mitral functioning by improving the LV [21, 22].
SVR Technique
The operation is performed under cardiac arrest, with antegrade cold crystalloid cardioplegia. A complete myocardial revascularization is performed first, when indicated, with particular attention to revascularize the proximal left anterior descending segment, to preserve the upper part of the septum. For this purpose, a left internal mammary artery is almost always placed on the left anterior descending artery. Revascularization is completed, when indicated, through sequential saphenous vein coronary bypass grafting on other diseased coronary arteries.
After completion of coronary grafting, the ventricle is opened with an incision parallel to the left anterior descending artery, starting at the middle scarred region and ending at the apex. The cavity is carefully inspected and any thrombus is removed if present. The surgeon identifies the transitional zone between scarred and non-scarred tissue, driven by cardiac magnetic resonance with late gadolinium enhancement (LGE), when previously performed, or alternatively by echocardiographic analysis. After that, a pre-shaped mannequin is inserted into the LV chamber and inflated with saline (Fig. 13.4, upper panel on the left). The mannequin is useful to optimize the size and shape of the new LV, particularly when the ventricle is not very enlarged (to reduce the risk of a too small residual cavity), or when the transitional zone between scarred and non-scarred tissue is not clearly demarcated, as occurs in akinetic remodeling. Furthermore, the mannequin is useful in giving the surgeon the correct position of the apex and in maintaining the long axis of the ventricle in a physiologic range (7.5/8.5), reducing thereby the risk of sphericalization of the new ventricle. The size of the device is defined by multiplying the body surface area of the patient by 50 ml. The exclusion of dyskinetic or akinetic LV free wall is performed through an endoventricular circular suture passed in the tissue of the transitional zone (Fig. 13.4, upper panel on the right). The conical shape of the mannequin guides the orientation of the plane of the endoventricular circular suture at the transitional zone, obliquely towards the aortic flow tract, mainly in rebuilding the new apex. The reconstruction of the apex may be difficult when the apical and inferior regions are severely dilated and scarred; in this case, a plication of the distal inferior wall is performed before patch suturing, thus placing the apex in a more superior position leaving a small portion of scar. The mannequin is removed before the closure of the ventricle and the opening is closed with a direct suture (simple stiches) if it is less than 3 cm large or with an elliptical, synthetic patch if greater than 3 cm to avoid distortion of the cavity (Fig. 13.4, lower panel on the left). In the first case, a second stratum with the excluded tissue is sutured on the first suture to avoid bleeding. If the closure is performed by using a patch (usually a Dacron patch), a few millimeters of border is left when sewing the patch in an everting way (Fig. 13.4, lower panel on the right). This technique assures a good hemostasis and makes it easier to put some additional stiches, if needed. The position of the patch is crucial in determining the residual shape of the new ventricle. To this aim, the surgeon pays attention to positioning the patch with an oblique orientation, toward the aortic outflow tract. In this way we avoid obtaining a box-like shape of the ventricle that may occur when the orientation of the patch is almost parallel to the mitral valve. More recently, the growing number of PCI has changed the pattern of LV remodeling in that the classical, dyskinetic aneurysm has almost disappeared and there is an increased incidence of a more diffuse akinetic remodeling. In the latter case, the use of the mannequin is crucial in determining the final result. The LV cavity is restored using the mannequin as a cast and the wall is closed with a running suture over the mannequin without a suture on the transitional zone. The final shape is more elliptical because the surgeon starts the suture in a higher position close to the aortic valve.
SVR procedure for anterior remodeling (schematic). Upper panel: The mannequin is inside the ventricle (on the left); the circular suture follows the curvature of the mannequin to re-shape the ventricle in an elliptical way (on the right). Lower panel: The patch is used to close the ventricular opening
Mitral Valve Repair
When indicated, the mitral valve is repaired through the ventricular opening with a double arm stitch running from one trigone to the other one, embedding the two arms in the posterior anulus of the mitral valve (Fig. 13.5). To avoid tears of the posterior left of the mitral valve (as has previously occurred), the suture is reinforced with a Teflon strip. After that, the suture is tied to undersize the mitral orifice. A Hegar sizer no. 26 is used to size the mitral annulus. Alternatively, a restrictive mitral annuloplasty with a ring implantation may be performed in selected patients, when the LV opening is not big enough to have a good exposition of the mitral valve.
Tailored Approaches
The surgical procedure as described above is usually performed to reverse LV remodeling after an anterior MI. However, the procedure may be tailored to approach different patterns of post-infarction LV remodeling, varying from the classic posterior aneurysm with a bulging of the inferior wall and a well-defined neck (Fig. 13.6), to a global LV dilatation with regional wall dysfunction at the inferior and posterior region, according to the site of coronary occlusion (Fig. 13.7). Surgery for the posterior aneurysm generally involves a patch to close the neck of the aneurysm. Otherwise, the treatment of global dilatation of the infero-posterior wall is more complex and varies according to the relationship between the site of the scar and the dilatation (with or without involvement of the posterior septum) with respect to the papillary muscles. After an inferior MI, there are two possibilities: a – the dilatation is mainly between the two papillary muscles (Fig. 13.8) or b – the dilatation is between the posteromedial papillary muscle and the septum, which is deeply involved (Fig. 13.9). We use two techniques for LV dilatation after an inferior MI. The first involves opening the scarred wall at the level of the scar or at the level of the collapsed area, parallel to the posterior descending artery (Fig. 13.10, on the left panel). A continuous 2/0 Prolene suture is performed to obtain the re-approximation of the two papillary muscles and the exclusion of the entire dilated zone. The suture is started at the beginning of the dilatation (sometimes just at the level of the mitral annulus) and continues toward the apex. According to the second technique, the wall is opened and the continuous suture is brought behind the posteromedial papillary muscle, bringing the posterior wall against the septum.
SVR procedure for posterior remodeling (schematic). The picture shows the two different techniques, from top to bottom on the left (which is applied when the dilatation occurs mainly between the two papillary muscles) and from top to bottom on the right (which is applied when the dilatation occurs between the posteromedial papillary muscle and the septum)
Results
The matter of functional chronic ischemic MR in terms of whether, when and how it should be corrected is one of the most common and controversial dilemmas faced by cardiac surgeons in the daily practice [23]. Certainly, the existing body of mostly retrospective and scarce literature has not contributed to general agreement. Authors advocating MV repair refer to the well-established negative impact that ischemic MR has on survival in patients undergoing CABG alone [24, 25], for whom the presence of even mild degrees of ischemic MR is thought to increase long-term mortality. Clinicians supporting the role of the LV ventricle in causing ischemic MR argue in favour of CABG alone which should theoretically improve regional wall motion abnormalities, papillary muscle function, and stimulate reverse LV remodeling avoiding the incremental perioperative morbidity and mortality with which adjunctive MV repair has been historically associated [26]. Not surprisingly, some retrospective studies show that CABG alone improves both ischemic MR and functional status in the short term [27, 28], whereas others show that MR is not reversed [29] and may progress further [30] with negative impact on survival [29]. Recently, Gelsomino and co-workers [31] showed that anterior mitral leaflet tethering is a powerful predictor of MR recurrence after undersized mitral ring annuloplasty, suggesting the need for concomitant or alternative surgery addressing the leaflet tethering (i.e., papillary muscle repositioning). In this regard, LV reconstruction has the advantage of addressing both the ventricle and mitral apparatus.
In 2002 we reported our initial experience on a small group of patients undergoing complete coronary revascularization, SVR and mitral valve repair for moderate or severe MR [32]. The mitral valve was repaired through the LV cavity without a prosthetic ring. Postoperative MR was absent or minimal in 84 % of patients; only one patient experienced severe MR a few days after surgery, requiring MV replacement. The follow-up analysis showed a cumulative survival of 63 % at 30-months.
Later, we addressed the effectiveness of SVR on unrepaired mild ischemic mitral regurgitation, showing that SVR improves mitral functioning by improving geometrical abnormalities [22]. Overall mid-term survival, including early mortality, was 93 % at 1 year and 88 % at 3 years, higher than it would be expected in patients with post-infarction remodelled ventricles and depressed LV function, suggesting that mitral repair in conjunction with SVR would be unnecessary in such patients.
Most recently, we re-analysed the data from our database, which comes from a prospective registry in which follow-up is carried out every 6 months. From January 2001 to October 2014, 175 patients out of 626 (28 %) heart failure patients undergoing SVR had associated MV repair. CABG was performed in 86 % of patients. The mean follow-up for death from any cause was 42 ± 37 months and was 100 % complete. In this latter population, the actuarial survival rate of the whole population, including operative mortality (25/175, 14,3 %), was 72 % ± 4 %, 65 % ± 4 %, and 45 % ± 6 % at 3, 5 and 8 years, respectively [33].
Although the comparison between populations of different studies is always difficult because of differences in baseline characteristics, our findings deserve some considerations. The operative mortality is relatively high but not disproportionally when compared to mortality associated with CABG plus MV surgery in previous reports (10,8 % by Schurr et al. [34]; 12 % by Kang et al. [35]). Surprisingly, possibly due to the large number of participating centers with different experience, Deja and colleagues, addressing the matter of MV surgery in the STICH hypothesis 1 population (among 104 patients assigned to CABG with moderate to severe MR, 91 underwent CABG and 49 received an adjunctive concomitant mitral valve procedure), showed a significantly higher operative mortality in patients treated with CABG only compared to patients treated with CABG plus an added MV repair (14,3 % vs 2 %, p = 0.046) [36]. Overall, it seems that it is not MV surgery per se which increase the operative risk rather the ischemic MV regurgitation in patients with LV dysfunction carries a higher risk regardless of treatment. In this regard, our results are not a cause of concern, especially if the relative “higher price to pay” early is compensated by a late benefit. Indeed, the observational data from the STICH trial indicate that adding MV repair to CABG in patients with LV dysfunction and moderate to severe MR may improve survival compared with CABG alone or MED alone (50 % of mortality risk at 5 years in the latter). Compared with these data, our results, coming from a larger population with a longer follow-up, show that combining MV repair with SVR added to CABG in the majority may further improve survival at 5 year (59 % in the STICH population vs 65 % in our series).
The Black Hole Beyond Repair: The Issue of Durability
LV adverse remodeling is a dynamic process as well as LV reverse remodeling, both evolving over time, the latter depending on the completeness of revascularization, the residual shape of the LV (in case of performing SVR), the decision to repair or not the mitral valve (left to the surgeon at the time of surgery), the technique to repair (with or without a prosthetic ring and type of ring) and the complex interaction between each other.
Accordingly, the rate of recurrence of MR after MV repair surgery is significantly high, extremely variable among different series, and poorly predictable. Recently, Kron and coworkers reported a particularly high rate of recurrence (ranging from 25.5 % at 6 months up to 39 % at 24 months) coming from the Cardiothoracic Surgical Trials Network [37]. The authors identified basal aneurysm/dyskinesis as one of the mechanisms of mitral valve annuloplasty failure, at least in this population, calling the need for different repair techniques. In 2002, the same Author described the direct relocation of the posterior papillary muscle tip in a small group of patients with previous inferior MI, not severely dilated ventricles (<6 cm in end-systolic diameter) and regional left ventricular (LV) geometric changes causing MR. In patients with dilated left ventricles (> 6 cm end-systolic diameter), the Dor procedure, relocating the papillary muscle base, was advocated [38]. In this regard, our surgical approach of the left ventricle, as described above, and tailored according to different phenotypes of LV remodeling, has the advantage to act on different mechanisms, although with different results. Preliminary data from the echocardiographic follow-up of 114 patients who underwent MV repair combined with SVR at our Institution (overall actuarial survival free from recurrence of 84 % ± 4 % and 63 % ± 7 % at 1 and 5 years, respectively) show that, both anterior remodeling (OR = 3.7, p = 0.05) and end-diastolic diameter (OR 1.08 per mm, p = 0.009) are independent predictors of recurrence, but not posterior remodeling (including basal aneurysm/dyskinesis) (unpublished data). The reason can probably be ascribed to the fact that the surgical technique reduces the short axis in the posterior remodeling, while acting mostly on the longitudinal axis in the anterior remodeling leading to an increase of the sphericity (Fig. 13.11) and a higher rate of recurrence. Recently, Oh and colleagues claimed that the significant increase in sphericity index observed after SVR in the STICH hypothesis 2 population was accompanied by a worsening of diastolic function and less improvement in mitral regurgitation [39]. The increase in sphericity index was ascribed to the amputation of the apex as result of SVR, which is not exactly the aim of this procedure. Rather, the apex should be reshaped (if distorted) while the surgeon must be aware to preserve the length of the ventricle (Fig. 13.12).
Changes in LV geometry Upper panel: LV anterior remodeling before SVR. The LV geometry is altered and the apex is widely dilated, without significant MR; SI is within the normal range, conversely the CI is increased SId = 0.53 CId = 1.10 SIs = 0.49 CIs = 1.22 Lower panel: After SVR, the apex has been rebuilt excluding the scar tissue and shortening inevitably the longitudinal diameter; SI increased although not disproportionally, while CI decreased, as expected after reshaping of the apex. The red arrow indicates the Dacron patch used to close the LV cavity SId = 0.67 CId = 0.87 SIs = 0.63 CIs = 0.86
Which Patient Should Have This Procedure: A Complex Surgery for Complex Patients
Ischemic MR is a complex disease which adversely affect the prognosis of patients with post-infarction LV remodeling. While the therapeutic strategy remains controversial, a greater focus must be placed on understanding the complex interaction between the ventricle, in terms of geometry and function, and valve functioning. According to our knowledge, the reconstruction of the left ventricle should be considered in selected HF patients with a baseline LVESVI ≥ 60 ml/m2 and a scar either in the antero-septal wall or in the infero-lateral wall. Mitral valve repair is indicated in presence of moderate to severe MR or even mild if the mitral annulus is greater than 40 mm. When the internal diameter is greater than 65 mm, it is probably reasonable to consider to replace the valve in patients with anterior remodeling. In the presence of posterior remodeling, MV replacement is not advised because the LV reconstruction, addressing mainly the internal diameter, produces a greater shortening of the short axis compared to the long one, fixing the mitral valve in a stable way.
References
Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;2(112):745–58.
Lamas GA, Mitchell GF, Flaker GC, Smith Jr SC, Gersh BJ, Basta L, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–33.
Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, et al. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg. 2003;125:135–43.
Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–43.
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64.
Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure. Current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108.
Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72.
Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;15(358):2148–59.
Burch GE, Depasquale NP, Phillips JH. Clinical manifestations of papillary muscle dysfunction. Arch Intern Med. 1963;112:112–7.
Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, et al. Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432–9.
Okayama S, Uemura S, Soeda T, Onoue K, Somekawa S, Ishigami K, et al. Clinical significance of papillary muscle late enhancement detected via cardiac magnetic resonance imaging in patients with single old myocardial infarction. Int J Cardiol. 2011;146:73–9.
Mittal AK, Langston Jr M, Cohn KE, Selzer A, Kerth WJ. Combined papillary muscle and left ventricular wall dysfunction as a cause of mitral regurgitation. An experimental study. Circulation. 1971;44:174–80.
Chinitz JS, Chen D, Goyal P, Wilson S, Islam F, Nguyen T, et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging. 2013;6:220–34.
Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M, et al. Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation. J Am Coll Cardiol. 2008;51:476–86.
Sabbah HN, Rosman H, Kono T, Alam M, Khaja F, Goldstein S. On the mechanism of functional mitral regurgitation. Am J Cardiol. 1993;72:1074–6.
Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;18(58):1733–40.
Yosefy C, Beeri R, Guerrero JL, Vaturi M, Scherrer-Crosbie M, Handschumacher MD, et al. Mitral regurgitation after anteroapical myocardial infarction: new mechanistic insights. Circulation. 2011;123:1529–36.
Garatti A, Castelvecchio S, Bandera F, Guazzi M, Menicanti L. Surgical ventricular restoration: is there any difference in outcome between anterior and posterior remodeling? Ann Thorac Surg. 2015;99:552–9.
Di Donato M, Dabic P, Castelvecchio S, Santambrogio C, Brankovic J, Collarini L, et al.; RESTORE Group. Left ventricular geometry in normal and post-anterior myocardial infarction patients: sphericity index and ‘new’ conicity index comparisons. Eur J Cardiothorac Surg. 2006;29 Suppl 1:S225–30.
Di Donato M, Sabatier M, Toso A, Barletta G, Baroni M, et al. Regional myocardial performance of non-ischaemic zones remote from anterior wall left ventricular aneurysm. Effects of aneurysmectomy. Eur Heart J. 1995;16:1285–92.
Menicanti L, Di Donato M, Frigiola A, Buckberg G, Santambrogio C, Ranucci M, et al. RESTORE Group. Ischemic mitral regurgitation: intraventricular papillary muscle imbrication without mitral ring during left ventricular restoration. J Thorac Cardiovasc Surg. 2002;123:1041–50.
Di Donato M, Castelvecchio S, Brankovic E, Santambrogio C, Montericcio V, Menicanti L. Effectiveness of surgical ventricular restoration in patients with dilated ischemic cardiomyopathy and unrepaired mild mitral regurgitation. J Thorac Cardiovasc Surg. 2007;134:1548–53.
Bouma W, van der Horst IC, Wijdh-den Hamer IJ, Erasmus ME, Zijlstra F, Mariani MA, et al. Chronic ischaemic mitral regurgitation. Current treatment results and new mechanism-based surgical approaches. Eur J Cardiothorac Surg. 2010;37:170–85.
Dion R. Ischemic mitral regurgitation: when and how should it be corrected? J Heart Valve Dis. 1993;2:536–43.
Chen FY, Adams DH, Aranki SF, Collins Jr JJ, Couper GS, Rizzo RJ. Mitral valve repair in cardiomyopathy. Circulation. 1998;98:II124–7.
Tolis Jr GA, Korkolis DP, Kopf GS, Elefteriades JA. Revascularization alone (without mitral valve repair) suffices in patients with advanced ischemic cardiomyopathy and mild-to-moderate mitral regurgitation. Ann Thorac Surg. 2002;74:1476–80.
Balu V, Hershowitz S, Zaki Masud AR, Bhayana JN, Dean DC. Mitral regurgitation in coronary artery disease. Chest. 1982;81:550–5.
Aronson D, Goldsher N, Zukermann R, Kapeliovich M, Lessick J, Mutlak D, et al. Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med. 2006;166:2362–8.
Lam BK, Gillinov AM, Blackstone EH, Rajeswaran J, Yuh B, Bhudia SK, et al. Importance of moderate ischemic mitral regurgitation. Ann Thorac Surg. 2005;79:462–70; discussion 470.
Campwala SZ, Bansal RC, Wang N, Razzouk A, Pai RG. Mitral regurgitation progression following isolated coronary artery bypass surgery: frequency, risk factors, and potential prevention strategies. Eur J Cardiothorac Surg. 2006;29:348–53.
Gelsomino S, van Garsse L, Lucà F, Lorusso R, Cheriex E, Rao CM, et al. Impact of preoperative anterior leaflet tethering on the recurrence of ischemic mitral regurgitation and the lack of left ventricular reverse remodeling after restrictive annuloplasty. J Am Soc Echocardiogr. 2011;24:1365–75.
Menicanti L, Di Donato M, Frigiola A, Buckberg G, Santambrogio C, Ranucci M, et al.; RESTORE Group. Ischemic mitral regurgitation: intraventricular papillary muscle imbrication without mitral ring during left ventricular restoration. J Thorac Cardiovasc Surg. 2002;123:1041–50.
Castelvecchio S, Parolari A, Garatti A, Gagliardotto P, Mossuto E, Canziani A, et al. Surgical ventricular restoration plus mitral valve repair in patients with ischemic heart failure. Risk factors for early and mid-term outcome. Oral presentation at 29th EACTS annual meeting, Amsterdam, 3–7 Oct 2015.
Schurr P, Boeken U, Limathe J, Akhyari P, Feindt P, Lichtenberg A. Impact of mitral valve repair in patients with mitral regurgitation undergoing coronary artery bypass grafting. Acta Cardiol. 2010;65:441–7.
Kang DH, Kim MJ, Kang SJ, Song JM, Song H, Hong MK, et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation. 2006;114(1 Suppl):I499–503.
Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–48.
Kron IL, Hung J, Overbey JR, Bouchard D, Gelijns AC, Moskowitz AJ, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149:752–61.
Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74:600–1.
Choi JO, Daly RC, Lin G, Lahr BD, Wiste HJ, Beaver TM, et al. Impact of surgical ventricular reconstruction on sphericity index in patients with ischemic cardiomyopathy: follow-up from the STICH trial. Eur J Heart Fail. 2015;17:453–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Castelvecchio, S., Garatti, A., Menicanti, L. (2017). Addressing the Left Ventricle in Functional Mitral Regurgitation. In: Chan, K. (eds) Functional Mitral and Tricuspid Regurgitation. Springer, Cham. https://doi.org/10.1007/978-3-319-43510-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-43510-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43508-4
Online ISBN: 978-3-319-43510-7
eBook Packages: MedicineMedicine (R0)