Abstract
Transesophageal echocardiography provides excellent visualization of the posteriorly located mitral valve. Over the last decade, 3-dimensional transesophageal echocardiography (3D TEE) has emerged as an exciting imaging modality, particularly of the mitral valve. The current generation matrix array technology allows the operator to perform 2D and 3D imaging with a single transducer. 3D TEE affords the unique ability to view the mitral valve and its surrounding structures “en face” in real time (RT), and provide contextual anatomical guidance during surgical and transcatheter interventions. Additionally, offline quantification has made significant contributions to our mechanistic understanding of the normal and diseased mitral valve, and alterations induced by therapeutic intervention such as surgical repair. This review will address recent advances in the incremental role of 3D TEE in mitral valve imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Echocardiography (Echo) is the primary imaging modality for the diagnosis, therapeutic guidance, and follow-up of mitral valvular disease. Due to the proximity of the imaging probe to the left atrium, transesophageal echocardiography (TEE) is ideally suited to image the mitral valve (MV). Recently, 3-dimensional transesophageal echocardiography (3D TEE) has emerged as an indispensable tool for imaging of the MV, and current matrix array technology confers both 2-dimensional (2D) and 3-dimensional (3D) functionality within a single transducer. A distinct advantage of 3D TEE over conventional 2D TEE is the ability to visualize the entire MV and support apparatus from a single transducer position and in real time. 3D echocardiography (3DE) allows an unprecedented “enface view” of the MV superiorly from the left atrium (LA), which corresponds to the surgeon’s view, or alternatively, inferiorly from the left ventricle (LV), which corresponds to the fluoroscopic view in the cardiac catheterization laboratory. The current review will explore the incremental role of 3D TEE in the assessment of mitral regurgitation and stenosis, and mitral percutaneous and surgical interventions, with emphasis on recent advances and research.
Normal Mitral Valve
The MV is a complex structure consisting of the mitral annulus, anterior and posterior leaflets, chordae tendinea, and the papillary muscles. A comprehensive understanding of normal mitral anatomy and its alteration in diseased states is fundamental to the appreciation of 3DE.
The mitral annulus is the D-shaped circumferential junction of the LA, LV, and mitral leaflets attachment, with a straight anterior and curved posterior portion. The anterior annulus is interposed between the right and left fibrous trigones and is in direct fibrous continuity with the aortic valve, referred to as the aortomitral curtain or more commonly, the mitral-aortic inter valvular fibrosa (MAIVF). This results in coupling of the mitral and aortic valve dynamics. The mitral annulus has a unique saddle shape, with anterior and posterior peaks and medial and lateral commissural valleys. This shape is best suited to minimize leaflet stress, and maintain optimal leaflet coaptation in systole. The entire annulus has a characteristic dynamic motion during the cardiac cycle, which is distinctly abnormal in various mitral pathologies [1].
The thicker anterior leaflet attaches to the anterior one-third or the straight portion of the D whereas the long and thin posterior leaflet attaches to the remaining two thirds of the annular circumference or the posterior curved portion of the D. When viewed from the left atrium, minor commissures that are cleft like indentations not associated with underlying papillary muscles, divide the posterior leaflet into 3 distinct scallops (segments). They are classified by Carpentier as P1 (anterolateral), P2 (middle), and P3 (posteromedial). Rarely, there are accessory scallops. The anterior leaflet is correspondingly divided into 3 segments A1, A2, and A3.
The chordae insert the inferior surface of the leaflets to the anterolateral and posteromedial papillary muscles and LV wall.
This intricate architecture of the MV apparatus results in optimal function by reducing leaflet stress and maintaining coaptation in systole, and leaflet excursion in diastole. It is difficult to visualize these anatomical details and dynamics with 2DE. With 3DE, not only can the entire apparatus be visualized in real time, but various aspects such as annular circumference, motion and nonplanar geometry, leaflet surface area, integrity of segments, and length of chordae, in normal and diseased mitral valves can be studied.
3D Acquisition, Display, and Orientation
3D TEE is in real-time (RT3D TEE) when the image in the form of a volume data set is acquired in a single heartbeat. A major limitation of RT3D TEE is the low frame rate or more accurately the volume rate when there is need for a large volume data set particularly if imaging includes 3D color flow Doppler. This limitation may be overcome by multi-beat acquisition, but the volume data set generated is postprocessed, hence, not in real time, and is subject to “stitch” artifacts. Conventionally, when viewed from the left atrium, the MV is displayed with the aorta on top (12 o’clock), the medial commissure to the right (3 o’clock) and the lateral commissure and left atrial appendage, an anterolateral structure, to the left (9 o’clock). The leaflet scallops are named from the left to the right as A1/P1, A2/P2, A3/P3. EAE/ASE recommendations for 3D echo image acquisition and display [2••], and ASE framework for 3D imaging of the MV [3] have been published.
Mitral Regurgitation
3DE has played a central role in understanding the functional anatomy and dynamics of the MV complex in the normal state and various etiologies of mitral regurgitation (MR) including degenerative and functional. Degenerative mitral valve disease (DMVD), commonly known as MV prolapse is due to an intrinsic abnormality of the MV leaflets and subvalvular apparatus. It results in excess leaflet tissue, chordal thinning, elongation or rupture and single or multi-segmental prolapse or flail. DMVD is the most common cause of MR requiring surgical intervention. The ability to view the entire surface of the leaflets allows easy identification and classification of prolapsing or flail segments. Several authors have demonstrated that 3D and 2D TEE are comparable (98 % vs 90 %) in accurately identifying degenerative and functional etiologies of severe MR in patients undergoing surgical MV repair [4–6]. But, 3D TEE has superior diagnostic accuracy in identifying scallop specific pathology [5–7], and in more complex disease with anterior leaflet, bileaflet, or commissural involvement. A recent study showed that physicians across a gamut of echocardiographic experience were better able to localize prolapsed and flail segments with 3D TEE. Both experienced and inexperienced echocardiographers (residents and fellows) achieved higher diagnostic scores with 3D TEE compared with 2D TEE [8•].
Mitral Annular Dynamics and Morphologic Changes in Degenerative Mitral Valve Disease
Early studies using 3DE elegantly demonstrated that the mitral annulus is not planar but saddle shaped with a unique dynamic motion throughout the cardiac cycle [9]. This nonplanar geometry and motion cannot be appreciated by 2D imaging. The saddle shape minimizes leaflet stress and maintains competency during systole. Due to various architectural changes, this motion is distinctly abnormal in DMVD. Compared with normals, the myxomatous mitral annulus is larger in surface area, wider in diameter, and flatter in early systole, and therefore less capable of maintaining leaflet coaptation. One study [10] demonstrated significant differences in mitral annular dynamics between normal controls, degenerative MR, and ischemic MR using 3D TEE. The normal mitral annulus remains dynamic throughout the cardiac cycle with a systolic decrease in the anteroposterior (AP) diameter and annular area, no change in inter commissural diameter (IC), and early-systolic accentuation of the saddle shape. In DMVD, the annulus demonstrates a systolic increase in all dimensions‒—AP and IC diameters, circumference and area‒—and a less prominent systolic accentuation of the saddle shape. In ischemic MR, the annulus is adynamic, with an increased AP diameter but unchanged IC diameter. Following standard MV repair with a flexible annuloplasty ring, the degenerative mitral annulus did not regain systolic accentuation of its saddle shape underscoring the fact that current surgical techniques do not completely restore normal mitral annular dynamics in DMVD.
DMVD covers a wide pathomorphologic spectrum with fibroelastic deficiency (FED) at one end, and Barlow’s Disease (BD) at the other. The hallmark features of FED are thin, translucent leaflets with abnormal single segmental thickening, and chordal elongation with prolapse or flail, typically involving the P2 scallop of the posterior leaflet. In BD, there is excessive annular dilatation, billowing of several thickened segments, with thickened and elongated chords (Fig. 1, Video 1). Chandra et al [11••] used RT3D TEE to demonstrate differences between FED and BD and identify predictors of surgical complexity. In both FED and BD, there was an increase in AP and IC diameters, leaflet surface area, leaflet billowing height and billowing volume. Not surprisingly, these changes were more exaggerated in BD. In FED, the P2 scallop was most frequently abnormal, followed by P1 and P3. Interestingly, the mitral annulus retained its normal contour in FED, but became circular in BD. They identified a billowing height of > 1.0 mm as the strongest predictor of differentiating normal from DMVD, and a billowing volume of > 1.15 ml as the strongest differentiator of FED from BD. Immediately postoperatively, the differences in dimensions between the 2 subgroups remained, but were significantly reduced. Maffessanti et al found similar results in a subset of patients with FED and BD undergoing mitral repair [12].
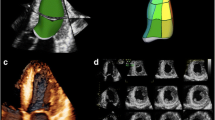
Three-dimensional transesophageal echocardiography ‘surgeons view’ from the left atrial perspective showing a mitral valve with P2 prolapse (asterisk) and a ruptured chord (yellow arrow). Demonstrates the aortic valve (AoV) at the 12 o’clock position, interatrial septum (black arrowheads), and ostium of the left atrial appendage (black arrow). © Mayo Foundation
In a more recent study, Lee and colleagues were the first to prospectively study mitral annular shape in DMVD with and without MR using RT3D TEE [13••, 14]. They identified flattening of the mitral annulus - quantified as the annular height to commissural width ratio - as an important prognostic parameter. As the annulus flattened, leaflet billowing volume, chordal rupture and MR severity increased. It suggests that flattening of the annulus maybe a marker for better risk stratifying patients with DMVD.
Prominent posterior leaflet inter scallop commissures referred to as “deep clefts” have been observed in MVP and are associated with immediate postoperative residual MR following mitral valve repair [15]. Accurate characterization of clefts with 2D echo is not possible. In a 3D TEE study, Ring and colleagues [16••] reported that deep clefts are significantly more prevalent in MVP at 84 %, compared with 16 % in functional MR, and 12 % in controls. Interestingly, they were only present around or within prolapsing or flail segments, and their numbers increased with the number of prolapsing segments. Regurgitation through the cleft was better detected by 3D rather than 2D TEE. They proposed that deep clefts represent a structural abnormality that could contribute to the burden of prolapse, mechanism of regurgitation, and results of surgical repair.
Such nuanced differentiation of morphology or dynamicity has previously not been possible with 2D TEE or direct surgical inspection of a flaccid heart during cardiopulmonary bypass.
Aortic Mitral Valve Coupling in Normal and Degenerative Mitral Valve
There is coupling of the aortic and mitral valvular dynamics because of their attachment through the MAIVF. Veronesi et al were the first to study aortic and MV coupling using 3D TEE [17•]. They demonstrated that the common fibrous anchoring renders a reciprocal dynamicity between the 2 valves. In early diastole, when the MV is open, the mitral annulus area (MAA) is maximal, and the aortic annular area (AoA) is minimal. Conversely, when the aortic valve opens in systole, the AoA is maximal and the MAA is minimal. The angle between the 2 valves decreases during ventricular ejection. In a separate study, the same authors noted changes in coupled dynamics before and after surgical repair for DMVD [18]. Prior to surgery, despite changes in mitral annular dimensions and geometry, the dynamic reciprocal coupled motion of the mitral and aortic valves is maintained. Following surgical repair and insertion of annuloplasty ring, there is a decrease in mitral annular dimensions, and an associated tightening of the mitral aortic complex. This results in a decreased intervalvular angle and attenuated reciprocal motion of the 2 valves. These findings support the concept of the aortic and MV as a single dynamic valvular unit rather than 2 truly independent structures.
Functional MR
In functional mitral regurgitation (FMR), the mitral leaflets are normal. FMR can occur in the setting of dilated cardiomyopathy or coronary artery disease. When the latter is the underlying etiology, FMR is referred to as ischemic mitral regurgitation (IMR). IMR is most commonly associated with prior inferior or inferolateral myocardial infarction, but may occur following an anterior myocardial infarction. The key pathophysiologic events leading to IMR are LV remodeling of the infarcted myocardial segment(s) underlying a papillar muscle with secondary lateral and apical papillary muscle displacement, which in turn results in asymmetric or symmetric tethering and mal coaptation of the mitral leaflets. Annular dilatation and left ventricular systolic dysfunction also contribute to mitral regurgitation. Similar mechanisms are in play in patients with nonischemic dilated cardiomyopathy. Papillary muscle displacement is not unique to FMR, however. Otani and colleagues [19••] demonstrated, that even in degenerative MR, mild to moderate LV dilatation and remodeling results in papillary muscle displacement and tenting of the nonprolapsed segments, which in turn, exacerbates the degree of mitral regurgitation.
Both 3D transthoracic [20, 21] and 3D TEE [10] studies have shown that in IMR the annulus dilates, flattens and becomes adynamic, with an increased tenting volume. 3D TEE has the advantage of quantifying indices of FMR severity such as increase in tenting length, tenting volume, papillary muscle displacement and LV volumes [22, 23].
Quantification of Mitral Regurgitation
The quantification of MR has important implications for severity classification and therapy. The complementary role of 3DE in this setting is being increasingly recognized [24•]. Two-D estimation of MR severity is frequently done by the proximal isovelocity surface area (PISA) method that assumes a circular mitral orifice. However, it has been shown that, the effective regurgitant orifice (ERO) or vena contracta (VC) is frequently asymmetrical or ellipsoid, particularly in functional and/or eccentric MR, leading to a consistent underestimation of ERO area (EROA) and regurgitant volume (RVol) by the 2D PISA method [25–27]. With 3D, the EROA and VC can be directly visualized and planimetered [28]. Shanks and colleagues compared MR quantification by 2D TEE, 3D TEE, and magnetic resonance imaging (MRI) [29]. EROA was obtained by direct planimetry in the en face view, and regurgitant volume (RVol) was calculated as the product of EROA and mitral velocity time integral (VTI). RVol-3D was comparable with that derived by MRI. There was a significant underestimation of both EROA-2D and RVol-2D when compared with 3D and MRI, particularly in eccentric jets. Hence, a significant proportion of patients were reclassified to a greater severity of MR by 3D TEE.
Current guidelines and expert opinion mention a potential role for 3D quantification of MR but do not make specific recommendations regarding the indications or normal reference values [2••, 30–32]. Studies addressing outcomes of patients undergoing 3D quantification of MR are lacking.
Intraoperative 3D TEE in Mitral Valve Repair
Accurate measurement of prognostic parameters such as leaflet and prolapse length, billowing height and volume, and inter-trigonal length can help plan surgical technique, and anticipate complications such as systolic anterior motion leading to postoperative MR [11••, 33]. Recent data suggest that the dynamicity of the mitral annulus is better preserved with surgical repair without leaflet resection compared with traditional leaflet resection techniques [34].
Up to 30 % of patients who undergo surgical band annuloplasty repair for ischemic MR develop recurrent MR. Traditional surgical repair with an undersized ring annuloplasty addresses the dilated annulus, but not the sub valvar abnormalities. Langer et al [35] identified a leaflet tenting height ≥10 mm as an echocardiographic marker of significant leaflet tethering and repair failure. When these patients underwent a modified technique of annuloplasty ring and relocation of the posterior papillary muscle (RING + STRING technique), a significant freedom from recurrent MR at 2 years was achieved.
Similarly, semi rigid band annuloplasty for functional MR has been shown to reestablish coaptation, and support regional LV remodeling [36].
In 1study, experienced operators could acquire a single focused RT-3D TEE MV image from the left atrial perspective in 60 ± 18 seconds, providing all the necessary information required for presurgical planning in patients undergoing surgical repair for MR [5]. Hence, intraoperative 3D TEE can be performed expeditiously and has a promising role in recognizing important variables to aid customized modifications in surgical technique, which would improve hemodynamic results and long-term durability of repair [11••, 22, 37].
Mitral Stenosis
In rheumatic mitral stenosis, there is extensive post inflammatory fibrosis, asymmetric scarring, and calcification of the MV leaflets and subvalvar apparatus. The anatomy is significantly distorted rendering 2D transthoracic imaging challenging. The orifice area should be planimetered at the leaflet tips, during their maximal separation in early to mid-diastole. Planimetry measurements below the leaflet tips will overestimate the area. 2D planimetry is prone to erroneous overestimation of valve area due to the technical difficulty in accurately establishing the plane of the leaflet tips in the parasternal short axis view. 3D echo overcomes this limitation by the ability to identify the plane of the leaflet tips. Studies with head to head comparison of transthoracic 2D and 3D have shown that 3D planimetry correlates more closely with invasively derived area, both before and after commissurotomy [38–40]. Schlosshan and colleagues [41] compared the mitral valve area (MVA) by 3D TEE with traditional 2D TTE methods. They concluded that 3D TEE is feasible and provides reproducible, superior imaging of the rheumatic MV and commissural fusion in nearly all patients. MVA-3D planimetry was significantly lower than MVA-2D planimetry and pressure half time, but agreed closely with the continuity method. Min and colleagues [42] confirmed that 2D planimetry significantly overestimated MVA by >0.2 cm2 in nearly half the patients. Two factors predicted area overestimation - left atrial diameter > 49 mm and an angle of deviation of the ultrasound plane from the leaflet tips ≥ 9.5°. 3D TEE may offer incremental value in patients with discrepant clinical and 2D echocardiographic severity of mitral stenosis.
However, the primary role for 3D TEE in mitral stenosis is in percutaneous mitral balloon valvuloplasty (PMBV). This is described in a subsequent section below.
Valvular Vegetations and Perforation
Complications of endocarditis include vegetations and perforations of both native and prosthetic mitral valves. Definitive therapy relies on echocardiographic diagnosis. Mitral valvular perforations are commonly caused by bacterial endocarditis. 2D TEE is established as the diagnostic modality of choice with a sensitivity of 94 % and specificity of 100 %. However, 2D imaging is unable to visualize the entire valve surface in a single view. A retrospective study comparing 2D- and 3D TEE reported that 3D TEE was superior in diagnosing and anatomically localizing perforations, particularly of the MV [43]. 3D TEE also provides incremental information such as quantification of vegetation volumes, area of valve destruction, site and extension of abscesses, and identification of chordal rupture, as confirmed by surgical inspection [44].
Prosthetic Mitral Valves
Krim et al were the first to report that 3D TEE diastolic orifice area correlated closely with the manufacture defined geometric area of St Jude mechanical mitral prostheses, but the standard Doppler derived effective orifice area was consistently smaller than both [45••]. Sugeng and colleagues [46] demonstrated good correlation between RT3D TEE of bioprosthetic and mechanical mitral prosthetic leaflets, struts and sewing rings, and subvalvar apparatus, and surgical findings. Mechanical MV images were superior from the LA perspective, whereas bioprosthetic valves were equally well visualized from the LA or LV. Due to the high spatial resolution, accurate anatomic delineation of prosthetic dehiscence, perivalvular regurgitation, and masses can be easily achieved. This is a huge advantage over 2D imaging. In a study of 2D vs 3D TEE assessment of postoperative MR secondary to mitral ring or prosthetic valve dehiscence, Kronzon et al [47] concluded that 3D TEE provided additional anatomical details of the dehisced segment and regurgitant jet that would help better plan intervention, either surgical or percutaneous.
3D TEE in Transcatheter Mitral Interventions
Conceivably the most clinically applicable, and incremental role for 3D TEE is in the patient selection, planning and procedural guidance of percutaneous or transcatheter mitral interventions [48, 49, 50•, 51]. A recent EAE/ASE guideline document concisely outlines the role of 2D and 3D echocardiography in various transcatheter valvular interventions [52]. The main mitral procedures are:
-
(1)
Closure of mitral paravalvular regurgitation.
-
(2)
Edge-to-edge repair of mitral regurgitation.
-
(3)
Valve in valve implantation.
-
(4)
Balloon valvuloplasty in mitral stenosis.
Transcatheter Closure of Paravalvular Regurgitation
Paravalvular regurgitation (PVR) or paravalvular leak is a regurgitant jet occurring through a defect between a prosthetic valve and surrounding tissue. Clinically significant PVR occurs in 3 %–6 % of all patients, most commonly in mitral prostheses [53]. Redo surgery is recommended in symptomatic patients. Over the last decade, percutaneous closure of PVR has emerged as a feasible alternative for poor surgical candidates [54–56]. Approximately 80 % of transcatheter closures are done for paramitral defects [55]. Technical success and safety depend on careful patient selection. Active endocarditis and presence of significant transvalvular regurgitation preclude PVR closure. Currently available closure plugs are predominantly circular in shape and therefore sub-optimally suited to most defects that are irregular or crescent shaped. In addition, large defects that involve greater than 20 % of the sewing ring limit successful closure [56]. Prior to offering transcatheter closure as a therapeutic option, anatomical details of location, number, size and shape of the defects have to be meticulously characterized.
Due to the length and complexity of the procedure, transcatheter closure of mitral PVR is done under general anesthesia with TEE as the preferred imaging modality. 3D TEE offers distinct incremental value over 2D imaging for patient selection, intraprocedural guidance, and post-procedure assessment. Due to the high spatial resolution, 3D TEE can separate paravalvular from transvalvular regurgitation, identify single vs multiple defects, describe their respective shapes and sizes, and elucidate spatial relationships to the prosthetic valve and surrounding structures. Only 3D echo permits an en face view of the defect(s), and simultaneous visualization of the catheter, guide wire, and closure device. Following deployment, technical success and potential complications such as device impingement on prosthetic leaflets, device migration or embolization, residual regurgitation, and iatrogenic atrial septal defect from the transeptal puncture can be rapidly assessed. As experience with transcatheter closure increases, several high volume centers have adopted 3D TEE for patient selection and procedural navigation [53–55].
Catheter Based Edge-to-Edge Repair of Mitral Regurgitation
Percutaneous mitral valve repair (PMVR) using the edge-to-edge technique has emerged as a feasible option for eligible patients with significant chronic mitral regurgitation and high surgical risk. Eligibility is determined by clinical variables and extensive echocardiographic criteria based on mitral anatomy, MR severity and LV function [57]. In the Phase I [58] and phase II [59] EVEREST (Endovascular Valve Edge-to-Edge REpair STudy) trials, all patients underwent TEE prior to selection, during and postprocedure. PMVR emulates the double orifice surgical technique or Alfieri stitch. A clip is used to approximate the free edges of A2/P2 leaflet scallops, resulting in a double orifice that diminishes the degree of MR.
The procedure is performed under general anesthesia, with fluoroscopic and TEE imaging. The MitraClip system consists of a steerable catheter delivery system with the clip attached distally. The clip has 2 arms that can be opened to grip mitral leaflet tissue, then closed shut and deployed. After transeptal puncture, the delivery system is navigated into the left atrium. Precise positioning of the clip is crucial to success of the procedure, and relies largely on TEE guidance. The clip has to be positioned perpendicular to the long axis of the leaflets, and in the center of the mitral orifice and regurgitant jet. This critical step can be optimally visualized using RT3D TEE. The clip is then carefully advanced into the LV and then pulled back to grip the leaflets in the mid portion. After TEE confirms a double orifice and maximal reduction of MR, the clip is deployed. Additional clips maybe used as indicated. Postprocedure, TEE evaluates clip stability, residual MR and mitral orifice area calculated by pressure half time and planimetry (sum of the 2 orifices at the level of the clip). Potential complications include pericardial effusion and tamponade, clip detachment or embolization, leaflet tear, injury to sub valvar apparatus and iatrogenic mitral stenosis.
RT3D TEE has been shown to be superior to 2D TEE in guiding most steps of PMVR. Altiok et al found that compared with 2D TEE, RT3D TEE offered incremental procedural guidance in 9 out of 11 steps [60]. Potentially prognostic data can also be obtained. A recent study [61] used 3D TEE to demonstrate a significant postprocedural reduction in RV, VC, mitral annular circumference, and diastolic MV area. Furthermore, at 6-month follow-up, the patients who had a >50 % reduction in VC post PMVR had smaller left atrial and left ventricular end diastolic volumes. Faletra et al have authored a side by side comparison of fluoroscopy, 2D and 3D TEE [62], as well as a detailed review of the role of RT3D TEE in percutaneous edge-to-edge mitral repair [63•]. A recent novel case report describes intraprocedural RT3D TEE with stereovision or depth vision capability provided by the use of red-blue/cyan glasses by the operator [64•]. The review by Delgado et al outlines the role of 3DE in the multimodality imaging of PMVR [65].
Valve in Valve Procedures
First reported in 2009 by Cheung and colleagues, the transcatheter mitral valve-in-valve implantation is an off-label option for high-risk patients with degenerated bioprosthetic surgical valves [66]. The procedure can be performed via an antegrade transfemoral approach, a retrograde transapical approach or by using both routes to create a rail. An appropriate valve is chosen based on at least 10 % oversizing of the inner diameter of the existing mitral bioprosthesis. The procedure is done under general anesthesia with fluoroscopic and TEE guidance. RT3D TEE may be superior in accurate sizing, and guiding precise positioning of the valve [67, 68]. Postdeployment, 2D TEE is used for assessing the gradient, degree of MR and other complications. RT3D TEE is complementary in ensuring appropriate seating and stability of the new valve, assessing for clots and differentiating new perivalvular from transvalvular MR by 3D color Doppler.
Percutaneous Mitral Balloon Valvuloplasty
PMBV is a long established treatment option for selected patients with symptomatic mitral stenosis in the absence of significant mitral regurgitation. Patient selection is based on clinical profile and favorable valve anatomy [31, 69]. An echocardiographic assessment of the extent of commissural calcification, fusion, and involvement of subvalvar apparatus is required. 3D TEE provides a superior description of commissural calcification [70] and fusion [41] from the LA and LV perspectives, and may identify the safest site for transeptal puncture with respect to surrounding structures. Special care is taken to avoid the aorta while allowing adequate room to maneuver the catheter and balloon tip coaxially towards the mitral orifice, thereby helping in appropriate sizing as well. The balloon is then positioned for inflation without damaging the sub valvar structures. 3D TEE is superior in post valvuloplasty detection of commissural splitting and leaflet tears [71].
Advantages
The well recognized advantages of traditional 2D TEE apply to 3D TEE as well – widely applicable, close proximity to the MV allowing excellent visualization, low complication rate, absence of radiation, and contrast exposure. Additionally, in contrast to the need for frequent transducer manipulation with 2D TEE to appreciate the full extent of the MV pathology, a panoramic view of the MV and surrounding anatomy including the left atrial appendage, pulmonary veins, atrial septum, and aortic valve is readily obtained by 3D TEE in real time from a single transducer position and with minimal or no probe manipulation. Dual capability transducers permit swapping between 2D and 3D via a touchscreen function. The “en face” views from the left atrial or left ventricular perspectives are unique to 3D echo. They facilitate a visually intuitive depiction of the MV in anatomical context to neighboring structures, catheters, and intracardiac devices that can be easily appreciated by surgeons, interventionalists, and imagers. 3D TEE can, therefore, enhance patient selection, procedural planning and safety. “Off line” quantitative analyses promote mechanistic understanding of the normal and diseased MV, and aid refinement of procedural and surgical techniques.
Limitations
Current 3DE limitations include low frame rates with color flow imaging and real time single beat acquisitions, and drop out artifacts. Multi-beat acquisition is frequently associated with stitch artifacts from respiratory motion and arrhythmias. Volumetric assessments require offline processing and analysis, which can be time consuming and laborious.
Conclusions
3DE is an imaging modality in evolution. Technological advances will, undoubtedly, address current limitations. Current literature supports that it has a sizable incremental advantage over 2DE in the assessment of MV disease. While 2D echo remains essential, 3D TEE is well on its way to becoming widely integrated into clinical practice as part of multimodality imaging of the MV. Echocardiographers, interventionalists, and cardiac surgeons stand to gain from familiarity with the technique.
Abbreviations
- 2D:
-
2-dimensional
- 3D:
-
3-dimensional
- DMVD:
-
Degenerative mitral valve disease
- Echo:
-
Echocardiography
- FMR:
-
Functional mitral regurgitation
- IMR:
-
Ischemic mitral regurgitation
- MR:
-
Mitral regurgitation
- MS:
-
Mitral stenosis
- MV:
-
Mitral valve
- MVP:
-
Mitral valve prolapse
- MAIVF:
-
Mitral-aortic inter valvular fibrosa
- PVR:
-
Paravalvular regurgitation
- TEE:
-
Transesophageal echocardiography
- RT3D TEE:
-
Real time 3-dimensional transesophageal
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. 2012;164:163–76.
•• Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46. This is the recent document for practicle principles of 3D echo and current role in echocardiography.
Salcedo EE, Quaife RA, Seres T, Carroll JD. A framework for systematic characterization of the mitral valve by real-time three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2009;22:1087–99.
Pepi M, Tamborini G, Maltagliati A, et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006;48:2524–30.
Grewal J, Mankad S, Freeman WK, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr. 2009;22:34–41.
Ben Zekry S, Nagueh SF, Little SH, et al. Comparative accuracy of two- and three-dimensional transthoracic and transesophageal echocardiography in identifying mitral valve pathology in patients undergoing mitral valve repair: initial observations. J Am Soc Echocardiogr. 2011;24:1079–85.
Hien MD, Rauch H, Lichtenberg A, et al. Real-time three-dimensional transesophageal echocardiography: improvements in intraoperative mitral valve imaging. Anesth Analg. 2013;116:287–95.
• Hien MD, Grossgasteiger M, Rauch H, Weymann A, Bekeredjian R, Rosendal C. Experts and beginners benefit from three-dimensional echocardiography: a multicenter study on the assessment of mitral valve prolapse. J Am Soc Echocardiogr. 2013;26(8):828–34. An interesting study case of interpretation in 3D TEE of the mitral valve.
Levine RA, Handschumacher MD, Sanfilippo AJ, et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–98.
Grewal J, Suri R, Mankad S, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121:1423–31.
•• Chandra S, Salgo IS, Sugeng L, et al. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images: objective insight into complexity and planning of mitral valve repair. Circ Cardiovasc Imaging. 2011;4:24–32. This study quantifies the association between morphological differences and surgical complexity in the spectrum of degenerative mitral valve disease.
Maffessanti F, Marsan NA, Tamborini G, et al. Quantitative analysis of mitral valve apparatus in mitral valve prolapse before and after annuloplasty: a three-dimensional intraoperative transesophageal study. J Am Soc Echocardiogr. 2011;24:405–13.
•• Lee AP, Hsiung MC, Salgo IS, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation. 2013;127:832–41. This article is the first to support that annulus flattening in mitral valve prolapse is a marker for severity of MR.
Jensen MO, Hagege AA, Otsuji Y, Levine RA. The unsaddled annulus: biomechanical culprit in mitral valve prolapse? Circulation. 2013;127:766–8.
Agricola E, Oppizzi M, Maisano F, et al. Detection of mechanisms of immediate failure by transesophageal echocardiography in quadrangular resection mitral valve repair technique for severe mitral regurgitation. Am J Cardiol. 2003;91:175–9.
•• Ring L, Rana BS, Ho SY, Wells FC. The prevalence and impact of deep clefts in the mitral leaflets in mitral valve prolapse. Eur Heart J Cardiovasc Imaging. 2013;14(6):595–602. This is the first comprehensive 3D study of deep clefts in mitral valve prolapse.
• Veronesi F, Corsi C, Sugeng L, et al. A study of functional anatomy of aortic-mitral valve coupling using 3D matrix transesophageal echocardiography. Circ Cardiovasc Imaging. 2009;2:24–31. The is the first study to describe the aortic and mitral valves as a coupled dynamic unit.
Veronesi F, Caiani EG, Sugeng L, et al. Effect of mitral valve repair on mitral-aortic coupling: a real-time three-dimensional transesophageal echocardiography study. J Am Soc Echocardiogr. 2012;25:524–31.
•• Otani K, Takeuchi M, Kaku K, et al. Evidence of a vicious cycle in mitral regurgitation with prolapse: secondary tethering attributed to primary prolapse demonstrated by three-dimensional echocardiography exacerbates regurgitation. Circulation. 2012;126:S214–21. First to describe secondary functional MR in primary degenrative MR with LV dilatation.
Watanabe N, Ogasawara Y, Yamaura Y, et al. Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. J Am Coll Cardiol. 2005;45:763–9.
Watanabe N, Ogasawara Y, Yamaura Y, et al. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation. 2005;112:I458–62.
Fattouch K, Castrovinci S, Murana G, et al. Multiplane two-dimensional vs real time three-dimensional transesophageal echocardiography in ischemic mitral regurgitation. Echocardiography. 2011;28:1125–32.
Saito K, Okura H, Watanabe N, et al. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc Imaging. 2012;5:337–45.
• Zamorano JL, Goncalves A. Three dimensional echocardiography for quantification of valvular heart disease. Heart. 2013;99:811–8. This is an up-to-date review of 3DE quantification of valvular disease.
Yosefy C, Levine RA, Solis J, Vaturi M, Handschumacher MD, Hung J. Proximal flow convergence region as assessed by real-time 3-dimensional echocardiography: challenging the hemispheric assumption. J Am Soc Echocardiogr. 2007;20:389–96.
Kahlert P, Plicht B, Schenk IM, Janosi RA, Erbel R, Buck T. Direct assessment of size and shape of noncircular vena contracta area in functional vs organic mitral regurgitation using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:912–21.
de Agustin JA, Marcos-Alberca P, Fernandez-Golfin C, et al. Direct measurement of proximal isovelocity surface area by single-beat three-dimensional color Doppler echocardiography in mitral regurgitation: a validation study. J Am Soc Echocardiogr. 2012;25:815–23.
Altiok E, Hamada S, van Hall S, et al. Comparison of direct planimetry of mitral valve regurgitation orifice area by three-dimensional transesophageal echocardiography to effective regurgitant orifice area obtained by proximal flow convergence method and vena contracta area determined by color Doppler echocardiography. Am J Cardiol. 2011;107:452–8.
Shanks M, Siebelink HM, Delgado V, et al. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2010;3:694–700.
Flachskampf FA, Badano L, Daniel WG, et al. Recommendations for transoesophageal echocardiography: update 2010. Eur J Echocardiogr. 2010;11:557–76.
Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42:S1–44.
Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation. 2012;126:2005–17.
Biaggi P, Jedrzkiewicz S, Gruner C, et al. Quantification of mitral valve anatomy by three-dimensional transesophageal echocardiography in mitral valve prolapse predicts surgical anatomy and the complexity of mitral valve repair. J Am Soc Echocardiogr. 2012;25:758–65.
Ben Zekry S, Lang RM, Sugeng L, et al. Mitral annulus dynamics early after valve repair: preliminary observations of the effect of resectional vs nonresectional approaches. J Am Soc Echocardiogr. 2011;24:1233–42.
Langer F, Kunihara T, Hell K, et al. RING + STRING: successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation. 2009;120:S85–91.
Greenhouse DG, Dellis SL, Schwartz CF, et al. Regional changes in coaptation geometry after reduction annuloplasty for functional mitral regurgitation. Ann Thorac Surg. 2012;93:1876–80.
Rim Y, Laing ST, Kee P, McPherson DD, Kim H. Evaluation of mitral valve dynamics. JACC Cardiovasc Imaging. 2013;6:263–8.
Zamorano J, Cordeiro P, Sugeng L, et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004;43:2091–6.
Zamorano J, Perez de Isla L, Sugeng L, et al. Noninvasive assessment of mitral valve area during percutaneous balloon mitral valvuloplasty: role of real-time 3D echocardiography. Eur Heart J. 2004;25:2086–91.
Messika-Zeitoun D, Brochet E, Holmin C, et al. Three-dimensional evaluation of the mitral valve area and commissural opening before and after percutaneous mitral commissurotomy in patients with mitral stenosis. Eur Heart J. 2007;28:72–9.
Schlosshan D, Aggarwal G, Mathur G, Allan R, Cranney G. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc Imaging. 2011;4:580–8.
Min SY, Song JM, Kim YJ, et al. Discrepancy between mitral valve areas measured by two-dimensional planimetry and three-dimensional transoesophageal echocardiography in patients with mitral stenosis. Heart. 2013;99:253–8.
Thompson KA, Shiota T, Tolstrup K, Gurudevan SV, Siegel RJ. Utility of three-dimensional transesophageal echocardiography in the diagnosis of valvular perforations. Am J Cardiol. 2011;107:100–2.
Hansalia S, Biswas M, Dutta R, et al. The value of live/real time three-dimensional transesophageal echocardiography in the assessment of valvular vegetations. Echocardiography. 2009;26:1264–73.
•• Krim SR, Vivo RP, Patel A, et al. Direct assessment of normal mechanical mitral valve orifice area by real-time 3D echocardiography. JACC Cardiovasc Imaging. 2012;5:478–83. The first study of 3D TEE measurement of mechanical mitral valve area.
Sugeng L, Shernan SK, Weinert L, et al. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347–54.
Kronzon I, Sugeng L, Perk G, et al. Real-time 3-dimensional transesophageal echocardiography in the evaluation of postoperative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009;53:1543–7.
Naqvi TZ. Echocardiography in percutaneous valve therapy. JACC Cardiovasc Imaging. 2009;2(10):1226–37.
Tsang W, Lang RM, Kronzon I. Role of real-time three dimensional echocardiography in cardiovascular interventions. Heart. 2011;97:850–7.
Cavalcante JL, Rodriguez LL, Kapadia S, Tuzcu EM, Stewart WJ. Role of echocardiography in percutaneous mitral valve interventions. JACC Cardiovasc Imaging. 2012;5:733–46. A current review of mitral interventional echocardiography.
Perk G, Kronzon I. Interventional echocardiography in structural heart disease. Curr Cardiol Rep. 2013;15:338.
Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. J Am Soc Echocardiogr. 2011;24:937–65.
Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Percutaneous repair of paravalvular prosthetic regurgitation: acute and 30-day outcomes in 115 patients. Circ Cardiovasc Interv. 2011;4:314–21.
Kim MS, Casserly IP, Garcia JA, Klein AJ, Salcedo EE, Carroll JD. Percutaneous transcatheter closure of prosthetic mitral paravalvular leaks: are we there yet? JACC Cardiovasc Interv. 2009;2:81–90.
Rihal CS, Sorajja P, Booker JD, Hagler DJ, Cabalka AK. Principles of percutaneous paravalvular leak closure. JACC Cardiovasc Interv. 2012;5:121–30.
Binder RK, Webb JG. Percutaneous mitral and aortic paravalvular leak repair: indications, current application, and future directions. Curr Cardiol Rep. 2013;15:342.
Mauri L, Garg P, Massaro JM, et al. The EVEREST II Trial: design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am Heart J. 2010;160:23–9.
Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol. 2005;46:2134–40.
Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406.
Altiok E, Becker M, Hamada S, Reith S, Marx N, Hoffmann R. Optimized guidance of percutaneous edge-to edge repair of the mitral valve using real-time 3-D transesophageal echocardiography. Clin Res Cardiol. 2011;100:675–81.
Altiok E, Hamada S, Brehmer K, et al. Analysis of procedural effects of percutaneous edge-to-edge mitral valve repair by 2D and 3D echocardiography. Circ Cardiovasc Imaging. 2012;5:748–55.
Faletra FF, Pedrazzini G, Pasotti E, Moccetti T. Side-by-side comparison of fluoroscopy, 2D and 3D TEE during percutaneous edge-to-edge mitral valve repair. JACC Cardiovasc Imaging. 2012;5:656–61.
• Faletra FF, Pedrazzini G, Pasotti E, et al. Role of real-time three dimensional transoesophageal echocardiography as guidance imaging modality during catheter based edge-to-edge mitral valve repair. Heart. 2013;99(16):1204–15. An uptodate review of 3D TEE in percutaneousedge-to-edge mitral valve repair.
Settergren M, Back M, Shahgaldi K, Jacobsen P, Winter R. 3D TEE with stereovision for guidance of the transcatheter mitral valve repair. JACC Cardiovasc Imaging. 2012;5:1066–9. A unique study that suggests the potential for stereovision in 3D echo.
Delgado V, Kapadia S, Marsan NA, Schalij MJ, Tuzcu EM, Bax JJ. Multimodality imaging before, during, and after percutaneous mitral valve repair. Heart. 2011;97:1704–14.
Cheung A, Webb JG, Wong DR, et al. Transapical transcatheter mitral valve-in-valve implantation in a human. Ann Thorac Surg. 2009;87:e18–20.
Nunez-Gil IJ, Goncalves A, Rodriguez E, et al. Transapical mitral valve-in-valve implantation: a novel approach guided by three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2011;12:335–7.
Theron A, Gariboldi V, Grisoli D, et al. Three-dimensional transesophageal echocardiography assessment of a successful transcatheter mitral valve in valve implantation for degenerated bioprosthesis. Echocardiography. 2013;30(6):E152–5.
Bonow RO, Carabello BA, Chatterjee K, et al. Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–661.
Langerveld J, Valocik G, Plokker HW, et al. Additional value of three-dimensional transesophageal echocardiography for patients with mitral valve stenosis undergoing balloon valvuloplasty. J Am Soc Echocardiogr. 2003;16:841–9.
Applebaum RM, Kasliwal RR, Kanojia A, et al. Utility of three-dimensional echocardiography during balloon mitral valvuloplasty. J Am Coll Cardiol. 1998;32:1405–9.
Compliance with Ethics Guidelines
Conflict of Interest
Sonia Jain declares that she has no conflict of interest. Joseph F. Malouf declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Valvular Heart Disease
Electronic supplementary material
Below is the link to the electronic supplementary material.
Live 3D Zoom three-dimensional transesophageal echocardiography ‘surgeons view’ from the left atrial perspective of the mitral valve, demonstrating a P2 prolapse with a ruptured chord. © Mayo Foundation (MPG 683 kb)
Rights and permissions
About this article
Cite this article
Jain, S., Malouf, J.F. Incremental Value of 3-D Transesophageal Echocardiographic Imaging of the Mitral Valve. Curr Cardiol Rep 16, 439 (2014). https://doi.org/10.1007/s11886-013-0439-2
Published:
DOI: https://doi.org/10.1007/s11886-013-0439-2