Abstract
Purpose of review
This review presents the risks and benefits of very low LDL cholesterol and the safety of using lipid-lowering therapy to achieve these levels.
Recent findings
A growing body of literature suggests that lower LDL cholesterol levels are associated with a reduced risk of cardiovascular disease. Further, achieving these levels with pharmaceuticals is remarkably safe. Although statins may slightly increase the risk of diabetes mellitus and hemorrhagic stroke, the benefits outweigh the risks.
Summary
While recommendations from professional societies are increasingly aggressive, additional risk reduction could be achieved by setting more even ambitious LDL cholesterol goals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While elevated low-density lipoprotein (LDL) cholesterol has been the primary concern for cardiologists and lipidologists for many years, the cardiovascular outcomes of patients achieving very low levels of LDL cholesterol (e.g., less than 50 mg/dL) are gaining interest. This is for two reasons. First, novel lipid-lowering therapies can now significantly reduce LDL cholesterol to levels previously unattainable. Second, there is an increased recognition of syndromes with very low cholesterol levels and a better understanding of the outcomes of patients with these disorders.
In this review, we will focus on evaluating and managing patients with very low LDL cholesterol levels (i.e., less than the 5th percentile or less than 50 mg/dL) rather than low levels of total cholesterol. The purpose of this review is to present the risks and benefits of living with very low levels of LDL cholesterol and the safety of driving LDL cholesterol to these levels with the increasingly potent arsenal of lipid-lowering therapy.
Measurement
Investigators have used a wide range of values for defining hypocholesterolemia. In the literature, definitions have included total cholesterol levels ranging from 100 mg/dL to 190 mg/dL1 or an LDL cholesterol less than the 5th percentile [2,3,4,5,6]. Depending on the definition, hypocholesterolemia is estimated to occur in 2 to 3% of patients in the general population and up to 6% of hospitalized patients [1]. In the National Health and Nutrition Examination Survey, a total cholesterol less than 30 mg/dL was found in 1.8% of self-identified white patients and in twice as many self-identified black patients (3.6%). Similar racial/ethnic differences were found in a study of firefighters in which 3.6% of self-identified black firefighters had hypocholesterolemia compared to 2.9% of self-identified white firefighters [1].
While a majority of studies use the total cholesterol to define hypocholesterolemia, this does not provide sufficient detail for the provider. It is much more important to understand if the hypocholesterolemia is driven by low high-density lipoprotein (HDL) cholesterol or low LDL cholesterol. Typically, the LDL cholesterol is estimated rather than measured with direct techniques, such as ultras centrifugation (i.e., beta quantification), because this is time-consuming and costly [7]. The most commonly used method for estimating LDL cholesterol has traditionally been the Friedewald Eq. [8]. Unfortunately, this equation can significantly underestimate the LDL cholesterol if the triglyceride level is above 400 mg/dL or in patients with LDL cholesterol less than 70 mg/dL [7, 9]. More recently, the Martin-Hopkins method has been introduced, which calculates LDL cholesterol more accurately when it is less than 70 mg/dL and when the patient has significantly elevated triglyceride levels [10, 11]. For those with severely elevated triglyceride levels, the Sampson equation may be appropriate as it improves estimation up to 800 mg/dL.7 However, at the extremes, it is likely prudent to measure the LDL cholesterol directly.
Role of Cholesterol
To understand the potential complications and benefits of very low LDL cholesterol levels, it is important to review the role of cholesterol in homeostasis. Cholesterol is an essential component of a number of substances that are critical for normal development and health. For instance, it is a component of vitamin D, estrogen, testosterone, cell membranes, and myelin [12]. Most of the body’s cholesterol is produced by the liver, which is responsible for approximately 80% of circulating cholesterol. This is from endogenous production, LDL cholesterol uptake, or the recycling of bile acids [13]. The remainder of the cholesterol comes from the diet. Notably, the brain is responsible for producing its own supply of cholesterol, which is nearly entirely independent of the systemic circulation secondary to the presence of the blood-brain barrier [14, 15].
The majority of circulating cholesterol is carried in LDL because of its relatively long half-life (i.e., 2 to 3 days) compared to other lipoproteins [14]. Studies have demonstrated that LDL has a remarkably high affinity for its receptor and that saturation of these receptors occurs at a level of 12.5 mg/dL.14 As discussed in the manuscript, this is a much lower level than most patients can hope to achieve. Other than returning cholesterol to the liver, it remains unclear if LDL performs other essential functions.
There are several lines of evidence that levels much higher than 12.5 mg/dL are not necessary. A growing body of literature suggests that the “normal” levels may range between 25 and 60 mg/dL, rather than the much higher levels sign in clinical practice [15]. For instance, newborns have plasma LDL cholesterol of 50–70 mg/dL, while levels in children average 95 mg/dL.7,16 In addition, ethnic groups that have maintained a hunter-gatherer lifestyle have LDL cholesterol levels well below 50 mg/dL with no clear known health consequences [13, 17, 18]. Similarly, those who adhere to a vegetarian diet can drive their LDL cholesterol below the 5th percentile without evidence of adverse effects [19]. Even in children who adhere to a vegetarian diet, complications related to the low LDL cholesterol have not been reported [20].
Benefits of Low LDL Cholesterol
It is well-established that LDL cholesterol values are associated with CVD [7, 21]. Trials have consistently demonstrated that the degree to which LDL cholesterol is lowered correlates with CVD event incidence [21,22,23]. With the publication of the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) [24], the benefits of LDL cholesterol lowering were demonstrated to occur in patients taking lipid-lowering therapy other than statins. This landmark trial provided the most substantial evidence that it is the amount of LDL cholesterol reduction that is important, rather than some specific mechanism unique to statins.
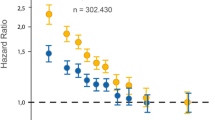
Further, trials have shown that aggressive lowering of the LDL cholesterol with high-intensity statins can yield even better results. For example, the Treating to New Targets (TNT) [16, 25] study showed that using high-intensity statins was more effective than moderate-intensity statins at reducing CVD events. Additional trials also have found that there does not appear to be a floor in which LDL cholesterol lowering is no longer beneficial, although there may be diminishing returns [26]. Studies like the Further Cardiovascular Outcomes Research With PCSK9 inhibitors in Subjects With Elevated Risk (FOURIER) and several statin trials [21] have shown that patients who achieved LDL cholesterol values less than 50 mg/dL had a lower risk of CVD events than those with LDL cholesterol values of 75 mg/dL [27].
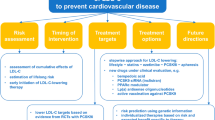
Based on an expected reduction of LDL cholesterol of 30 to 50% (approximately 77 mg/dL [2 mmol/L]) with a high-dose statin, studies suggest it is not unreasonable to suggest that patients could have a 40 to 50% reduction in CVD risk [13, 16, 21]. Based on these studies, the European Society of Cardiology recommends achieving LDL cholesterol values less than 70 mg/dL in high-risk patients and less than 55 mg/dL in very high-risk patients [28]. While the American Heart Association/American College of Cardiology did not include a specific LDL cholesterol goal for most patients, it still recommends that high-risk patients start additional lipid-lowering therapy even when LDL values are 70 mg/dL.29
Syndromes
Abetalipoproteinemia and Familial Hypobetalipoproteinemia
Monogenetic diseases that lead to severe hypocholesterolemia are ideal for studying the effects of very low LDL cholesterol levels. In these disorders, the total cholesterol often is less than 30 mg/dL, and the LDL cholesterol is negligible. The three most severe forms of hypocholesterolemia are abetalipoproteinemia, familial hypobetalipoproteinemia (FHBL), and chylomicron retention disease. Each of these disorders are rare, occurring in approximately one in one million people [30]. While caused by two different mutations, the diseases are clinically indistinguishable. In abetalipoproteinemia, a mutation in the MTTP gene, which encodes the microsomal triglyceride transfer protein (MTP), leads to improper folding of the apoB protein in the endoplasmic reticulum. The improperly formed apoB does not acquire triglycerides and is quickly degraded, which results in the inability to secrete triglycerides as either chylomicrons (in the intestine) or very low density lipoprotein (in the liver).
FHBL is most commonly caused by mutations in the gene encoding apoB, but it can also be caused by proprotein convertase subtilisin/kexin type 9 (PCSK9) mutations; other genes are likely involved but not yet characterized. This leads to a “truncated” apoB that is dysfunctional and cannot accept triglycerides or cholesterol. Unlike abetalipoproteinemia, FHBL is inherited in a codominant manner, and less severe heterozygous forms exist.
Chylomicron retention disease is a slightly less severe of hypocholesterolemia that is caused by mutations in the SAR1B gene. The SAR1B protein is critical for assembly of Coat Protein Complex (COPII)-coated vesicles that assist in the transport of proteins critical for chylomicron formation. While sharing similar gastrointestinal and neurological complications of FHBL and abetalipoproteinemias, it is distinguished from these disorders by a normal triglyceride level and often having an elevated creatine kinase level [19].
Abetalipoproteinemia and FHBL typically present in infancy with symptoms related to fat malabsorption. As fats accumulate in the enterocytes, they swell and absorption is impaired. Patients develop diarrhea, vomiting, abdominal distention, and failure to thrive. Over time, neurological symptoms develop related to vitamin deficiencies, particularly vitamin E. In addition, deficiencies in essential fatty acids develop, such as skin abnormalities, poor growth and development, and immunodeficiencies, among others [31]. The neurologic symptoms typically present in adolescence, but evidence of neurological abnormalities may actually be present in the first year of life. In severe cases, respiratory failure has been described as secondary to respiratory muscle weakness. Ophthalmological disease is a common finding in adulthood but less so until the third or fourth decades.
Heterozygous FHBL
Patients with the heterozygous form of FHBL also have very low levels of LDL cholesterol, but they typically have values of at least 30 mg/dL. The primary concern in these patients is the risk of developing hepatic steatosis. Studies suggest up to 25% of patients with hypolipoproteinemia progress to hepatitis and that one in five of those patients develops cirrhosis. Studies have shown that patients with FHBL have hepatic triglyceride levels three times higher than those in normal controls. Interestingly, the relationship between insulin resistance and non-alcoholic fatty liver disease found in the general population is absent in these patients [30].
Familial Combined Hypolipidemia
In a disorder with a similar clinical profile as heterozygous FHBL, Familial Combined Hypolipidemia (FCH) is an autosomal recessive disorder that results from mutations in the ANGPTL3 gene, which is important in regulating lipoprotein lipase and endothelial lipase [6, 14, 32, 33]. Inhibition of ANGPLT3 leads to increased clearance of triglyceride-rich lipoproteins and, subsequently, lower levels of LDL cholesterol [6, 14, 32, 33]. This is a relatively rare form of hypocholesterolemia, occurring in about 10% of patients [32]. Clinically, there is a 60 to 70% reduction in both the LDL cholesterol and triglyceride levels. While generally also considered to decrease HDL cholesterol, this was not found in the Dallas Heart Study [32, 34]. Interest in this disease is its absence of adverse consequences compared to the other forms of hypolipoproteinemia. In fact, it even has a reduced risk of diabetes mellitus [32]. In a study by Mincocci [35], there was an increase in carotid-intima medial thickening in patients who were homozygous for ANGPLT3 mutations, despite the lower LDL cholesterol. However, in a pooled analysis of patients with FH, cardiovascular disease was absent in those with homozygous mutations in ANGPLT3 [36]. Currently, this disease requires no treatment or follow-up.
Treatment of Inherited Hypocholesterolemia
While a discussion of the treatment of these syndromes is not the purpose of this review, understanding the approach to the management of these patients provides critical insight to the consequences of very low levels of LDL cholesterol. For instance, even though LDL cholesterol is nearly undetectable in the homozygous forms, cholesterol supplementation is not part of the treatment. In fact, the cornerstone of treatment in homozygous hypocholesterolemia is fat restriction and the supplementation of fat-soluble vitamins. In general, a diet containing less than 30% of calories from fat will reduce gastrointestinal symptoms and improve absorption [32], with reductions as much as 10 to 15% recommended in some patients [31].
The second component of the treatment of homozygous hypocholesterolemias is vitamin supplementation with fat-soluble vitamins. While patients with these disorders often require extremely high doses of vitamins (e.g., vitamin E doses as high as 100 to 300 international units per kilogram per day or 15,000 international units per day of Vitamin A), vitamin deficiencies are rarely seen in patients whose LDL cholesterol is driven to very low levels pharmacologically [7, 30]. It is worth noting that only vitamin E is associated with LDL cholesterol levels [13]. While initial clinical trials of PCSK9 inhibitors found total vitamin E levels were reduced in those achieving very low LDL cholesterol, more sophisticated measurements (i.e., vitamin E concentrations in red blood cells) demonstrate that tissue delivery remains unaffected [13, 14, 37].
For heterozygous FHBL, the management largely relies on monitoring for fat-soluble vitamin deficiencies and the management of non-alcoholic fatty liver disease. In general, patients remain asymptomatic throughout their lives and can be considered to have a reduced risk of CVD.
Familial Hypercholesterolemia
Similar to the insights garnered from understanding hypobetalioproteinemias regarding the function of LDL cholesterol, conditions characterized by persistently elevated levels of LDL cholesterol, such as familial hypercholesterolemia, can similarly yield valuable information. The heterozygous form of familial hypercholesterolemia is the most common autosomal dominant disorder in the United States and affects approximately 1 in 250 people; the homozygous form is much rarer and found in 1 in 1 million individuals [38, 39]. It is most often caused by a defect in the LDL receptor but also can be caused by mutations in apolipoprotein B and PCSK9. There also is a form of familial hypercholesterolemia that is inherited in an autosomal recessive pattern caused by mutations in the gene encoding for low-density lipoprotein receptor adaptor protein (1LDLRAP1). While patients with heterozygous forms of familial hypercholesterolemia only have a reduced capacity to update LDL cholesterol, those with most forms of homozygous familial hypercholesterolemia have a near-total inability to uptake circulating LDL cholesterol through the LDL receptor. However, this does not appear to be problematic. For instance, there is normal fetal development during a period of relatively rapid cellular replication and growth. In addition, there is no evidence of ill effects on bile acid formation or adrenal hormone synthesis. However, it should be noted that there are a few reports of suboptimal responses to adrenocorticotropic hormone in some patients with homozygous familial hypercholesterolemia [14]. Even following LDL apheresis, in which LDL cholesterol rapidly decreases to levels less than 30 mg/dL, patients tolerate the rapid fluctuations without problems [7]. Interestingly, as opposed to patients with low LDL cholesterol, patients with familial hypercholesterolemia appear to be at a reduced risk of developing diabetes mellitus, but this mechanism is not fully understood.
Potential Complications Related to Low LDL Cholesterol
As described, the benefits of lowering LDL cholesterol are clear. However, concerns about excessive lowering have arisen based on the outcomes of patients with genetic forms of hypocholesterolemia and the results of clinical trials and Mendelian randomization studies. The following reviews these concerns.
Diabetes Mellitus
One of the first off-target effects of statins was their effect on the risk of diabetes mellitus. Initially, it appeared that statins may offer protection against the development of diabetes mellitus based on the results of the West of Scotland Coronary Prevention Study (WOSCOPS) [40]. However, later randomized clinical trials of statins, Mendelian randomization studies [21], and several meta-analyses [40,41,42,43,44] have suggested a small but clinically significant increased risk of diabetes mellitus depending on the statin and its dose.
The exact mechanism by which statins increase the risk of diabetes mellitus has not been determined. However, there is evidence that mutations in 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA reductase), LDL receptor gene (LDLRI), and others may be involved [45]. In the Metabolic Syndrome in Men (METSIM) cohort, various measures of insulin secretion and sensitivity suggested that both of these proteins were negatively affected by statins [45]. This may be achieved by activation of AKT in skeletal muscle or by reducing the activity of HMG CoA reductase [45]. Similarly, mutations in the PCSK9 gene may also lead to excess cholesterol within the cell, which may disrupt glucose homeostasis [16, 46]. Others have suggested that it is related to hepatic-induced insulin resistance through Niemann-Pick C1-Like 1 [13, 47]. At this time, there are ongoing studies to better understand the interactions between cholesterol metabolism and insulin resistance.
While there appears to be a dose-response relationship between statin dose and risk of diabetes mellitus [21], the relationship is not necessarily related to the degree of LDL cholesterol lowering. For instance, there was no increased risk of diabetes mellitus in those who achieved an LDL cholesterol of less than 50 mg/dL in JUPITER [21]. Evidence that the risk of diabetes mellitus is from the mechanism of action of statins rather than a low LDL cholesterol is further emphasized by the absence of an association with other classes of lipid-lowering therapy despite achieving very low levels of LDL cholesterol [13]. Neither OSLER or FOURIER found a difference in new onset diabetes mellitus nor was glycemic control affected [48]. This was true even in those achieving the lowest LDL cholesterol levels in FOURIER [7, 49]. Further, a meta-analysis of studies using PCSK9 inhibitors, including over 160,000 patients, did not find a more significant reduction in LDL cholesterol associated with an increased risk of diabetes mellitus [7, 49, 50].
While Mendelian randomization studies [16] indicate that PCSK9 inhibition may promote insulin resistance, PCSK9 inhibitors block plasma (i.e., circulating) PCSK9 rather than intracellular PCSK9. Mendelian randomization studies cannot differentiate between these locations since they arise from the same gene. This suggests that intracellular inhibition of PCSK9 may increase the risk of diabetes mellitus through a similar pathway as the inhibition of HMG-CoA reductase, while inhibition of circulating PCSK9 may not. Further, patients with monogenic hypobetalipoproteinemia caused by PCSK9 mutations do not have an increased risk of diabetes mellitus either [6]. This suggests that the genetic mutations that lead to very low levels of LDL cholesterol may contribute to raising the diabetes mellitus risk, but that the low LDL cholesterol levels are not a causal risk factor.
It should be noted that even with this increased risk of diabetes mellitus, statins are still recommended even in those at the highest risk of diabetes mellitus, and statins are not discontinued if it develops. Using data from meta-analyses, for approximately every 250 patients treated with a statin, one excess case of type 2 diabetes mellitus would be expected, which is about a 9% increased risk [40]. As others have suggested, this suggests that 5 to 10 CVD events would be prevented for each new case of diabetes mellitus [51].
Hemorrhagic Stroke
While the evidence for reducing ischemic stroke with aggressive lipid-lowering is well-established, the association between low LDL cholesterol and the risk of hemorrhagic stroke has been more challenging to establish. Several observational studies, along with two Mendelian randomization studies, have suggested an inverse association between LDL cholesterol and risk for hemorrhagic stroke. However, clinical trials and meta-analyses have been inconsistent [52,53,54,55]. In the largest and most recent meta-analysis, a small but statistically significant association between the use of statins and hemorrhagic stroke was noted [54]. Interestingly, other forms of lipid-lowering therapy were not associated with an increased risk of hemorrhagic stroke in this meta-analysis. In a previous American Heart Association Scientific Statement, the authors concluded that there may be a risk but that this risk is likely limited to a subset of patients at higher risk for hemorrhagic stroke [15].
While statins may play a role, no clearly established pathway has linked LDL cholesterol with hemorrhagic stroke. As mentioned, cholesterol homeostasis in the brain is largely independent of the serum cholesterol [15], and there is no clear role of LDL cholesterol in developing hemorrhagic strokes. Even in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study, while the number of hemorrhagic strokes increased, it was unrelated to the magnitude of LDL cholesterol reduction [56]. As in the case of low levels of LDL cholesterol and the risk of diabetes mellitus, Mendelian randomization studies may suffer from the same inability to accurately reflect in vitro drug action. As was the case for diabetes mellitus, trials of PCSK9 inhibitors have not replicated the findings of statin trials. For instance, FOURIER and ODYSSEY did not find a relationship. Further, patients in these trials, as well as in IMPROVE-IT, were not at an increased risk of hemorrhagic stroke even when achieving extremely low LDL-cholesterol [13]. Similarly, in the previously mentioned meta-analysis, only statins were found to have an association, and other classes of lipid-lowering therapy (i.e., PCSK9 inhibitors, ezetimibe, and omega-3 fatty acid supplements) did not [54].
Further evidence that LDL cholesterol is not related to hemorrhagic stroke risk comes from a secondary prevention study of high-risk subjects from France and South Korea with a history of ischemic stroke. In this study of patients with a history of ischemic stroke, the risk of hemorrhagic stroke did not differ by degree of LDL cholesterol lowering and there was a lower risk of CVD in those achieving lower LDL cholesterol levels [7, 57]. Based on this evidence, a conservative conclusion would be that there is a slight increase in the risk of hemorrhagic stroke. Still, it is limited to a subgroup of patients taking statins and is not likely related to the LDL cholesterol level.
Cancer
An additional concern for aggressive lipid lowering is the possibility of an increased risk of cancer. In the Pravastatin in elderly individuals at risk of vascular disease (PROSPER) [58], there was an increased number of cases of cancer in those treated with pravastatin compared to placebo. Similarly, in the Cholesterol And Recurrent Events (CARE) trial [59], there was an increased risk of breast cancer with lipid-lowering therapy. Patients in the Copenhagen City Heart Study who had an LDL cholesterol lower than the 10th percentile had a 43% increased risk of cancer [1].
However, these findings have yet to be supported by Mendelian randomization studies, other clinical trials, or meta-analyses [13, 14]. For instance, Mendelian randomization studies found that mutations in HMG-CoA reductase may be protective for cancer [14]. In addition, Mendelian randomization studies have not found an association between cancer and PCSK9 [14]. The Atherosclerosis Risk in Communities Study (ARIC) study also did not find that patients with the PCSK9 variant had any increased risk of cancer [19, 60]. Findings from a meta-analysis of statin trials by the Cholesterol Treatment Trialists’ (CTT) Collaboration [61] did not find an association between cancer and lipid-lowering therapy use, regardless of the intensity of the statin, the reduction in LDL cholesterol, or the baseline LDL cholesterol. While earlier results of the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial [62] suggested an increased risk of cancer, longer-term follow-up of the subjects did not find an association [63]. Therefore, while early studies suggested a relationship may exist between the risk of cancer and LDL cholesterol, genetic studies and more recent clinical trials have failed to find that LDL cholesterol is associated with the risk of cancer.
Cataracts
As the lens of the eye requires substantial cholesterol, lowering the LDL cholesterol could theoretically lead to lens dysfunction (i.e., cataracts). A slight increase in the risk of cataracts was found in those receiving rosuvastatin in the Heart Outcomes Prevention Evaluation (HOPE-3) trial [64]. A pooled analysis of alirocumab trials found the risk increased in those with LDL cholesterol levels less than 25 mg/dL.37 However, a majority of evidence suggests no association between either pharmacologic lowering of LDL cholesterol or very low levels of LDL cholesterol with cataracts [52, 65, 66]. Larger and more recent meta-analyses of statins and PCSK9 inhibitors have failed to find an association [66, 67]. For instance, JUPITER did not find an association between LDL cholesterol and cataracts, even in those achieving very low LDL cholesterol levels. One criticism of HOPE-3 and JUPITER was that the presence of cataracts was self-reported. However, the Expanded Clinical Evaluation of Lovastatin (EXCEL) [68] and Scandinavian Simvastatin Survival Study (4 S) [69] trials obtained routine ophthalmologic exams, and neither found a relationship between cataracts and statin use [65, 66]. Based on these results, the risk of cataracts seems exceedingly small.
Overall Mortality
While a majority of studies demonstrate a reduction in CVD events and a decrease in all-cause mortality, observational studies have occasionally linked very low LDL cholesterol with an increase risk of mortality. Several observational studies of patients with low LDL cholesterol have found that these patients have an increased susceptibility to infections and overall mortality [1]. However, these studies likely reflect the influence of inflammation on the lipid panel, which often drives the LDL cholesterol lower, rather than an active role of low LDL cholesterol on outcomes. For instance, cancer, infections, and even gastrointestinal diseases have been shown to lead to hypocholesterolemia [1, 70]. However, in JUPITER, rosuvastatin was associated with a lower instance of pneumonia [14]. Similarly, studies have found that PCSK9 inhibition improved survival in sepsis [13].
Conclusions
The ability to drive LDL cholesterol to unprecedented levels has opened the door to substantially reduce CVD. It also has prompted concerns that extremely low levels of LDL cholesterol may have adverse effects. While statins may affect glucose metabolism and increase the risk of hemorrhagic stroke at very low levels (i.e., less than 50 mg/dL), this is not clearly associated with LDL cholesterol. Further, the results of clinical trials, Mendelian randomization studies, and meta-analyses suggest that using other forms of lipid-lowering therapy, such as PCSK9 inhibitors, ezetimibe, and evinacumab to achieve very low LDL cholesterol levels is remarkably safe. While recommendations from professional societies are increasingly aggressive, additional risk reduction could be achieved by setting more even ambitious LDL cholesterol goals and helping providers be more comfortable caring for patients with very low levels.
Data Availability
No datasets were generated or analysed during the current study.
References
Elmehdawi RR, Hypolipidemia. A word of caution. Libyan J Med. 2008;3:84–90.
Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, Zhao Y, Barrett PH, Hegele RA, van Bockxmeer FM, et al. Missense mutations in APOB within the betaalpha1 domain of human APOB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J Biol Chem. 2007;282:24270–83. https://doi.org/10.1074/jbc.M702442200.
Hooper AJ, Burnett JR. Recent developments in the genetics of LDL deficiency. Curr Opin Lipidol. 2013;24:111–5. https://doi.org/10.1097/MOL.0b013e32835ca0d9.
Sankatsing RR, Fouchier SW, de Haan S, Hutten BA, de Groot E, Kastelein JJ, Stroes ES. Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol. 2005;25:1979–84. https://doi.org/10.1161/01.Atv.0000176191.64314.07.
Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941–7. https://doi.org/10.1194/jlr.M300508-JLR200.
Welty FK, Lahoz C, Tucker KL, Ordovas JM, Wilson PW, Schaefer EJ. Frequency of ApoB and ApoE gene mutations as causes of hypobetalipoproteinemia in the framingham offspring population. Arterioscler Thromb Vasc Biol. 1998;18:1745–51.
Karagiannis AD, Mehta A, Dhindsa DS, Virani SS, Orringer CE, Blumenthal RS, Stone NJ, Sperling LS. How low is safe? The frontier of very low (< 30 mg/dL) LDL cholesterol. Eur Heart J. 2021;42:2154–69. https://doi.org/10.1093/eurheartj/ehaa1080.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of Low-Density Lipoprotein Cholesterol in plasma, without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18:499–502.
Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem. 2002;48:236–54.
Martin SS, Giugliano RP, Murphy SA, Wasserman SM, Stein EA, Ceska R, Lopez-Miranda J, Georgiev B, Lorenzatti AJ, Tikkanen MJ, et al. Comparison of Low-Density Lipoprotein Cholesterol Assessment by Martin/Hopkins Estimation, Friedewald Estimation, and Preparative Ultracentrifugation: insights from the FOURIER Trial. JAMA Cardiol. 2018;3:749–53. https://doi.org/10.1001/jamacardio.2018.1533.
Whelton SP, Meeusen JW, Donato LJ, Jaffe AS, Saenger A, Sokoll LJ, Blumenthal RS, Jones SR, Martin SS. Evaluating the atherogenic burden of individuals with a Friedewald-estimated low-density lipoprotein cholesterol < 70 mg/dL compared with a novel low-density lipoprotein estimation method. J Clin Lipidol. 2017;11:1065–72. https://doi.org/10.1016/j.jacl.2017.05.005.
Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–64. https://doi.org/10.1007/s13238-014-0131-3.
Masana L, Girona J, Ibarretxe D, Rodríguez-Calvo R, Rosales R, Vallvé JC, Rodríguez-Borjabad C, Guardiola M, Rodríguez M, Guaita-Esteruelas S, et al. Clinical and pathophysiological evidence supporting the safety of extremely low LDL levels—the zero-LDL hypothesis. J Clin Lipidol. 2018;12:292–e299293. https://doi.org/10.1016/j.jacl.2017.12.018.
Olsson A, Angelin B, Assmann G, Binder C, Björkhem I, Cedazo-Minguez A, Cohen J, von Eckardstein A, Farinaro E, Müller-Wieland D, et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. J Intern Med. 2017;281:534–53. https://doi.org/10.1111/joim.12614.
Goldstein LB, Toth PP, Dearborn-Tomazos JL, Giugliano RP, Hirsh BJ, Pena JM, Selim MH, Woo D, American Heart Association Council on, Arteriosclerosis T, Vascular B et al. Aggressive LDL-C Lowering and the Brain: Impact on Risk for Dementia and Hemorrhagic Stroke: A Scientific Statement From the American Heart Association. Arteriosclerosis, thrombosis, and vascular biology. 2023;43:e404-e442. https://doi.org/10.1161/ATV.0000000000000164
Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348–54. https://doi.org/10.1016/j.tcm.2017.12.011.
O’Keefe JH Jr., Cordain L. Cardiovascular disease resulting from a diet and lifestyle at odds with our Paleolithic genome: how to become a 21st-century hunter-gatherer. Mayo Clinic proceedings. 2004;79:101–108. https://doi.org/10.4065/79.1.101
Cordain L, Eaton SB, Miller JB, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(Suppl 1):S42–52. https://doi.org/10.1038/sj.ejcn.1601353.
Tarugi P, Averna M. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem. 2011;54:81–107.
Desmond MA, Sobiecki JG, Jaworski M, Płudowski P, Antoniewicz J, Shirley MK, Eaton S, Książyk J, Cortina-Borja M, De Stavola B, et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am J Clin Nutr. 2021;113:1565–77. https://doi.org/10.1093/ajcn/nqaa445.
LaRosa J, Pedersen T, Somaratne R, Wasserman S. Safety and effect of very low levels of low-density lipoprotein cholesterol on cardiovascular events. Am J Cardiol. 2013;111:1221–9. https://doi.org/10.1016/j.amjcard.2012.12.052.
Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. https://doi.org/10.1016/s0140-6736(12)60367-5.
Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW, Group SS. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* trial). Am J Cardiol. 2003;92:152–60. https://doi.org/10.1016/s0002-9149(03)00530-7.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after Acute Coronary syndromes. N Engl J Med. 2015;372:2387–97. https://doi.org/10.1056/NEJMoa1410489.
LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart J-C, Gotto AM, Greten H, Kastelein JJP, Shepherd J, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. https://doi.org/10.1056/NEJMoa050461.
Wiviott SD, Cannon CP, Morrow DA, Ray KK, Pfeffer MA, Braunwald E. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy. J Am Coll Cardiol. 2005;46:1411–6.
Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, Larosa JC, Waters DD, Demicco DA, Simes RJ, et al. Very low levels of atherogenic lipoproteins and risk of cardiovascular events; a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–94. https://doi.org/10.1016/j.jacc.2014.02.615.Very.
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida J-M, Capodanno D, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–337. https://doi.org/10.1093/eurheartj/ehab484.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;140:e596–646. https://doi.org/10.1161/CIR.0000000000000678.
Welty F. Hypobetalipoproteinemia and abetalipoproteinemia: liver disease and cardiovascular disease. Curr Opin Lipidol. 2020;31:49–55. https://doi.org/10.1097/MOL.0000000000000663.
Bredefeld C, Hussain M, Averna M, Black D, Brin M, Burnett J, Charrière S, Cuerq C, Davidson N, Deckelbaum R, et al. Guidance for the diagnosis and treatment of hypolipidemia disorders. J Clin Lipidol. 2022;16:797–812. https://doi.org/10.1016/j.jacl.2022.08.009. This is an excellent review of the options for diagnosing and treating monogenetic hypobetalipoproteinemias.
Hooper AJ, Burnett JR. Update on primary hypobetalipoproteinemia. Curr Atheroscler Rep. 2014;16. https://doi.org/10.1007/s11883-014-0423-3.
Musunuru K, Pirruccello JP, Ron D, Peloso GM, Guiducci C, Sougnez C, Garimella KV, Fisher S, Abreu J, Barry AJ, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–7.
Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, Cohen JC. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119:70–9. https://doi.org/10.1172/JCI37118.
Minicocci I, Cantisani V, Poggiogalle E, Favari E, Zimetti F, Montali A, Labbadia G, Pigna G, Pannozzo F, Zannella A, et al. Functional and morphological vascular changes in subjects with familial combined hypolipidemia: an exploratory analysis. Int J Cardiol. 2013;168:4375–8. https://doi.org/10.1016/j.ijcard.2013.05.053.
Minicocci I, Santini S, Cantisani V, Stitziel N, Kathiresan S, Arroyo JA, Martí G, Pisciotta L, Noto D, Cefalù AB, et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J Lipid Res. 2013;54:3481–90. https://doi.org/10.1194/jlr.P039875.
Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L, Miller K, Kastelein JJP. Safety of very low low-density lipoprotein cholesterol levels with Alirocumab: Pooled Data from Randomized trials. J Am Coll Cardiol. 2017;69:471–82. https://doi.org/10.1016/j.jacc.2016.11.037.
Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–94. https://doi.org/10.1093/eurheartj/ehw028.
de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133:1067–72. https://doi.org/10.1161/circulationaha.115.018791.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. https://doi.org/10.1016/S0140-6736(09)61965-6.
Coleman CI, Reinhart K, Kluger J, White CM. The effect of statins on the development of new-onset type 2 diabetes: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2008;24:1359–62. https://doi.org/10.1185/030079908x292029.
Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, Caputo S, Grzesk G, Kubica A, Swiatkiewicz I, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123–30. https://doi.org/10.1016/j.amjcard.2012.12.037.
Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64. https://doi.org/10.1001/jama.2011.860.
Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016;25:1131–49. https://doi.org/10.1002/pds.4020.
Laakso M, Fernandes Silva L. Statins and risk of type 2 diabetes: mechanism and clinical implications. Front Endocrinol (Lausanne). 2023;14:1239335. https://doi.org/10.3389/fendo.2023.1239335.
Carugo S, Sirtori CR, Corsini A, Tokgozoglu L, Ruscica M. PCSK9 inhibition and risk of diabetes: should we worry? Curr Atheroscler Rep. 2022;24:995–1004. https://doi.org/10.1007/s11883-022-01074-y.
Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, Luan Ja, Ardanaz E, Arriola L, Balkau B, et al. Association between Low-Density Lipoprotein cholesterol–lowering genetic variants and risk of type 2 diabetes: a Meta-analysis. JAMA. 2016;316:1383–91. https://doi.org/10.1001/jama.2016.14568.
Schonck WAM, Stroes ESG, Hovingh GK, Reeskamp LF. Long-term efficacy and tolerability of PCSK9 targeted therapy: a review of the literature. Drugs. 2024. https://doi.org/10.1007/s40265-024-01995-9. A thorough review of the evidence for the efficacy of PCSK9-inhibitors and associated adverse effects.
Sabatine MS. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol. 2019;16:155–65. https://doi.org/10.1038/s41569-018-0107-8.
Khan SU, Rahman H, Okunrintemi V, Riaz H, Khan MS, Sattur S, Kaluski E, Lincoff AM, Martin SS, Blaha MJ. Association of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering therapies and risk of diabetes Mellitus: a systematic review and Meta-analysis. J Am Heart Assoc. 2019;8:e011581. https://doi.org/10.1161/jaha.118.011581.
Maki KC, Dicklin MR, Baum SJ. Statins and diabetes. Endocrinol Metab Clin North Am. 2016;45:87–100. https://doi.org/10.1016/j.ecl.2015.09.006.
Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, De Backer G, Hegele RA, Hovingh GK, Jacobson TA, et al. Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39:2526–39. https://doi.org/10.1093/eurheartj/ehy182.
Masson W, Lobo M, Siniawski D, Masson G, Lavalle-Cobo A, Molinero G. LDL-C levels below 55 mg/dl and risk of hemorrhagic stroke: a Meta-analysis. J Stroke Cerebrovasc Dis. 2021;30:105655. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105655.
Betrisey S, Haller ML, Efthimiou O, Speierer A, Del Giovane C, Moutzouri E, Blum MR, Aujesky D, Rodondi N, Gencer B. Lipid-lowering therapy and risk of hemorrhagic stroke: a systematic review and Meta-analysis of Randomized controlled trials. J Am Heart Assoc. 2024;13:e030714. https://doi.org/10.1161/JAHA.123.030714.
Sanz-Cuesta BE, Saver JL. Lipid-lowering therapy and hemorrhagic stroke risk: comparative Meta-analysis of statins and PCSK9 inhibitors. Stroke. 2021;52:3142–50. https://doi.org/10.1161/STROKEAHA.121.034576.
Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. https://doi.org/10.1056/NEJMoa061894.
Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha J-K, Ducrocq G, Giroud M, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2019;382:9–19. https://doi.org/10.1056/NEJMoa1910355.
Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. https://doi.org/10.1016/s0140-6736(02)11600-x.
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335:1001–9. https://doi.org/10.1056/nejm199610033351401.
Folsom AR, Peacock JM, Boerwinkle E. Sequence variation in proprotein convertase subtilisin/kexin type 9 serine protease gene, low LDL cholesterol, and cancer incidence. Cancer Epidemiol Biomarkers Prev. 2007;16:2455–8. https://doi.org/10.1158/1055-9965.Epi-07-0502.
Cholesterol Treatment Trialists C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. https://doi.org/10.1016/S0140-6736(10)61350-5.
Green A, Ramey DR, Emneus M, Iachina M, Stavem K, Bolin K, McNally R, Busch-Sørensen M, Willenheimer R, Egstrup K, et al. Incidence of cancer and mortality in patients from the simvastatin and ezetimibe in aortic stenosis (SEAS) trial. Am J Cardiol. 2014;114:1518–22. https://doi.org/10.1016/j.amjcard.2014.08.016.
McCormack T, Dent R, Blagden M. Very low LDL-C levels may safely provide additional clinical cardiovascular benefit: the evidence to date. Int J Clin Pract. 2016;70:886–97. https://doi.org/10.1111/ijcp.12881.
Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, et al. Cholesterol lowering in Intermediate-Risk persons without Cardiovascular Disease. N Engl J Med. 2016;374:2021–31. https://doi.org/10.1056/NEJMoa1600176.
Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL 2nd, Goldstein LB, Chin C, Tannock LR, Miller M, Raghuveer G et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:e38-e81. https://doi.org/10.1161/ATV.0000000000000073
Yu S, Chu Y, Li G, Ren L, Zhang Q, Wu L. Statin use and the risk of cataracts: a systematic review and Meta-analysis. J Am Heart Assoc. 2017;6. https://doi.org/10.1161/JAHA.116.004180.
**Li J, Du H, Wang Y, Aertgeerts B, Guyatt G, Hao Q, Shen Y, Li L, Su N, Delvaux N et al. Safety of proprotein convertase subtilisin/kexin 9 inhibitors: a systematic review and meta-analysis. Heart. 2022;108:1296–1302. doi: 10.1136/heartjnl-2021-320556. A meta-analysis demonstrating the safety of PCSK9 inhibitors, which suggests that low LDL cholesterol is safe and that other adverse effects may be associated with the cause of the low LDL cholesterol.
Laties AM, Shear CL, Lippa EA, Gould AL, Taylor HR, Hurley DP, Stephenson WP, Keates EU, Tupy-Visich MA, Chremos AN. Expanded clinical evaluation of lovastatin (EXCEL) study results. II. Assessment of the human lens after 48 weeks of treatment with lovastatin. Am J Cardiol. 1991;67:447–53. https://doi.org/10.1016/0002-9149(91)90002-3.
Pedersen TR, Berg K, Cook TJ, Faergeman O, Haghfelt T, Kjekshus J, Miettinen T, Musliner TA, Olsson AG, Pyörälä K, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the scandinavian simvastatin survival study. Arch Intern Med. 1996;156:2085–92.
Ahmed A, Keeffe EB. Asymptomatic Elevation of Aminotransferase Levels and fatty liver secondary to heterozygous hypobetalipoproteinemia. AJG. 1998;93.
Funding
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL145109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
JH wrote the initial drafts, edited the drafts, and was responsible for all content.
Corresponding author
Ethics declarations
Studies with Animals
This article does not contain any studies with human or animal subjects.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hartz, J. Low LDL-C: Is It all Good News?. Curr Atheroscler Rep (2024). https://doi.org/10.1007/s11883-024-01238-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-024-01238-y