Abstract
Purpose of Review
Cardiometabolic diseases, which include obesity, type 2 diabetes, and cardiovascular diseases, constitute a worldwide health crisis of unparalleled proportions. The human gut microbiota has emerged as a prominent topic of inquiry in the search for novel treatment techniques. This review summarizes current research on the potential of addressing the gut microbiota to treat cardiometabolic disease.
Recent Findings
Recent studies have highlighted a complex link between the gut microbiota and host physiology, shedding light on the several processes through which gut microorganisms impact metabolic health, inflammation, and cardiovascular function. Furthermore, a growing corpus of research is available on microbiome-based therapies such as dietary interventions, probiotics, prebiotics, synbiotics, and fecal microbiota transplantation. These therapies show promise as methods for reshaping the gut microbiota and, as a result, improving cardiometabolic outcomes. However, hurdles remain, ranging from the intricacies of microbiome research to the necessity for tailored treatments that take individual microbial variations into consideration, emphasizing the significance of furthering research to bridge the gap between microbiome science and clinical practice.
Summary
The gut microbiome is a beacon of hope for improving the management of cardiometabolic disease in the age of precision medicine, since its association with their pathophysiology is constantly being unraveled and strengthened. Available studies point to the potential of gut microbiome-based therapeutics, which remains to be tested in appropriately designed clinical trials. Further preclinical research is, however, essential to provide answers to the existing obstacles, with the ultimate goal of enhancing patient care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiometabolic diseases, encompassing a spectrum of disorders such as obesity, type 2 diabetes (T2DM), and cardiovascular diseases (CVD), have reached epidemic proportions worldwide, posing a significant public health challenge [1]. While traditional therapeutic approaches have made substantial strides in managing these conditions, their increasing prevalence calls for innovative strategies that address the root causes of these interconnected ailments.
In recent years, the human gut microbiome (GM) has emerged as a promising frontier in understanding and potentially treating cardiometabolic diseases [1]. The intricate relationship between the trillions of microorganisms residing in the gastrointestinal tract and host physiology has unveiled a new realm of therapeutic possibilities. This review article delves into the burgeoning field of targeting the gut microbiome as a novel therapeutic avenue for managing and mitigating cardiometabolic diseases. We explore the multifaceted interactions between gut microbes and host metabolism, shedding light on the potential mechanisms by which modulating the microbiome composition can influence cardiometabolic health. Additionally, we delve into the latest advancements in GM-based interventions, including dietary modifications, prebiotics, probiotics, and fecal microbiota transplantation, all aimed at harnessing the power of these microbial communities to improve cardiometabolic outcomes. By critically evaluating the existing research landscape, we aim to provide a comprehensive overview of the potential of gut microbiome-targeted therapies and their implications for reshaping the future of cardiometabolic disease management.
Gut Microbiome and Cardiometabolic Disease
Overview of the Gut Microbiome Composition and Function
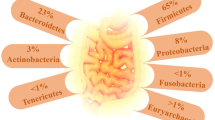
The GM, a vast and diverse ecosystem of microorganisms residing in the gastrointestinal tract, plays a crucial role in maintaining human health and well-being. Their genetic material is referred to as the “microbiome” [2]. Comprising approximately 100 trillion microorganisms, this complex ecosystem primarily consists of anaerobic bacteria, alongside archaea, fungi, and viruses [3]. The colonization process begins at birth and steadily diversifies until it reaches an adult configuration around the age of 3, remaining relatively stable throughout adulthood [4]. The most abundant bacterial phyla include Firmicutes, Bacteroidetes, Actinobacteria, Verrucomicrobia, and Proteobacteria [5], with Firmicutes and Bacteroidetes making up more than 90% of the total population in healthy bacterial community [6]. However, factors such as host genetics, diet, lifestyle, medication, and environmental exposures are leading to a distinct GM composition in each individual [7].
The GM serves numerous essential functions that have a profound impact on human physiology. Among its numerous functions, the intestinal microflora plays a vital role in nutrient and xenobiotic metabolism, the maintenance of gut mucosal integrity, bile salt metabolism, vitamin synthesis, and immune system regulation [8, 9]. Additionally, certain bacterial species synthesize short-chain fatty acids (SCFA), known for their positive impact on human health, with butyrate serving as a potent anti-inflammatory molecule and the primary energy source for enterocytes [10]. Moreover, the GM has also demonstrated its ability to metabolize cardiovascular drugs, affecting their bioavailability and actions [11]. It is essential to maintain a balance in the composition and functions of the intestinal microbiota; otherwise, a departure from homeostasis could lead to a condition known as gut “dysbiosis,” which research data has shown to be involved in various human disorders, such as CVD [12].
Role and Mechanisms of the Gut Microbiome in Cardiometabolic Disease Development and Progression
As aforementioned, alterations in the composition and metabolic capacity of GM have been linked to various chronic diseases, including CVD. The connection between bacterial dysbiosis and CVD may be rooted in diverse cellular and molecular pathways, primarily involving alterations in host bile acid and lipid metabolism, leakage of harmful endotoxins across the gut epithelial barrier, and the generation of potentially atherogenic bacterial metabolites [13].
Gut bacterial functions play a role in lipid metabolism, particularly with regard to low-density lipoprotein-cholesterol (LDL-C) [14]. The interaction between the host and gut microbiota in lipid metabolism involves nuclear peroxisome proliferator-activated receptors (PPAR), with species-specific effects [15]. For instance, PPAR responds to butyrate production by gut bacteria, promoting oxidation while suppressing nitric oxide (NO) synthesis, which helps maintain the anaerobic environment in the colon and prevent dysbiosis [13, 15]. Additionally, a high-fat diet can cause changes in the gut microbiota and dysregulation of PPAR signaling, leading to alterations in the spatial distribution of bacteria in the ileum of mice [16]. However, administration of a PPAR agonist was able to reverse these effects.
Several significant metabolites, such as trimethylamine N-oxide (TMAO), lipopolysaccharides (LPS), SCFAs, secondary bile acids, and phenylacetylglutamine (PAGln), have been identified and associated with the onset and progression of cardiovascular diseases (Fig. 1). TMAO is formed through a multi-step enzymatic process involving the conversion of L-carnitine, choline, and betaine found in certain foods by the gut microbiota’s Firmicute species [17]. Once absorbed into the bloodstream, TMA is transported to the liver, where it is converted into TMAO by the enzyme flavin-dependent monooxygenase 3 (FMO3) [18]. Normally, around 95% of TMA is excreted in the urine as TMAO [19]. Any disruption in this metabolic pathway, from dietary intake to liver function and FMO3 activity, could lead to increased TMAO levels and related complications, such as atherosclerotic CVD [20]. Among the main mechanisms implicated in TMAO’s cardiovascular consequences are endothelial dysfunction, foam cell, and thrombus formation [21].
The effects of gut microbiome metabolites on atherosclerosis. Abbreviations: TMA, trimethylamine; FMO3, flavin-containing monooxygenase 3; TMAO, trimethylamine N-oxide; ED, endothelial dysfunction; TLR4, toll-like receptor 4; MD-2, myeloid differentiation protein 2; LBP, lipopolyssacharide-binding protein; IRAK-1, interleukin-1 receptor-associated kinas; MyD88, myeloid differentiation factor 88; NF-kB, nuclear factor-kB; PPA, phenylpyruvic acid; PAA, phenylacetic acid; PAGln, phenylacetylglutamine; SCFA, short-chain fatty acid; HDAC, histone deacetylase; BA, bile acid; FXR, farnesoid X receptor; TGR5, Takeda G protein-coupled receptor 5. Green-colored words indicate the gut microbiome metabolites. Red font words indicate pro-atherosclerotic effects. Blue font words indicate anti-atherosclerotic effects
LPS are endotoxins found on the outer membrane of Gram-negative bacteria and play a role in the pathogenesis of CVD. These endotoxins are recognized by toll-like receptor 4 (TLR4), triggering a pro-inflammatory response with increased cytokine and chemokine production [22]. Other receptors, such as LPS-binding protein (LBP), myeloid differentiation protein 2 (MD-2), and cluster of differentiation 14 (CD14), also identify LPS, leading to activation of protein kinases like IL-1 receptor-associated kinase (IRAK-1) and myeloid differentiation factor 88 (MyD88) [23]. This activation subsequently triggers nuclear factor-kB (NF-kB) and various pro-atherosclerotic inflammatory pathways. LPS induce endothelial dysfunction, raise oxidative stress via reactive oxygen species (ROS) production, and generate pro-inflammatory cytokines like tumor necrosis factor-a (TNF-a), interleukin (IL) -1, IL-6, and IL-8, all contributing to CVD development through inflammation propagation [24,25,26].
SCFAs play a protective role against atherosclerosis and are produced by gut bacteria, including A. butyraticus., F. prausnitzii, and R. intestinalis, through the digestion of complex carbohydrates [27]. The most common SCFAs are acetate, butyrate, and propionate, which as aforementioned serve various functions, with their primary role being the modulation of the host immune system [28]. They increase the production of regulatory T cells and suppress histone deacetylases (HDACs), leading to inhibition of inflammatory pathways and reduced production of pro-inflammatory cytokines. SCFAs also contribute to enhanced intestinal barrier stability and protection against pathogen invasion [29, 30]. Thus, SCFAs protect against atherosclerosis by modulating inflammatory pathways.
Primary bile acids are produced in the liver from cholesterol and converted into cholic acid and chenodeoxycholic acid through conjugation with glycine. In the gut, the microbiota deconjugates primary bile acids, leading to the formation of secondary bile acids in the distal ileum [31]. Secondary bile acids aid in the absorption of lipid nutrients and fat-soluble vitamins and activate two key receptors, farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5), which modulate glucose and cholesterol metabolism [32]. TGR5 also contributes to improved glucose tolerance and possesses anti-inflammatory properties. Inhibition of both FXR and TGR5 exacerbates atherosclerotic formation, highlighting their potential benefits in disease control [20]. Secondary bile acids exert anti-inflammatory and anti-atherogenic effects by suppressing TNF-a and NF-kB signaling pathways and reducing the secretion of pro-inflammatory cytokines [33, 34]. Overall, secondary bile acids play a significant role in inhibiting major atherosclerotic pathways.
PAGln is a recently discovered metabolite positively associated with CVD [35]. It is derived from phenylalanine and undergoes transformations, including microbial porA gene-mediated conversion to phenylacetic acid and hepatic metabolization into PAGln [36]. PAGln has been correlated with ASCVD and overall mortality in patients with chronic kidney disease (CKD) [37]. Mechanisms explaining this correlation include PAGln’s ability to increase platelet activation and responsiveness, promoting thrombosis potential, while transmitting cellular events through specific adrenergic receptors [38]. Carvedilol, a common β-blocker, was found to inhibit PAGln’s prothrombotic effects [36]. Overall, PAGln is implicated in the occurrence of atherosclerotic CVD through an accelerated rate of thrombus generation and vessel occlusion, potentially leading to acute myocardial infarction [38].
Gut Microbiome Modulation Strategies
Increasing clinical evidence highlights the significant contribution of GM modulation on various cardiometabolic diseases. Concerning common medications used in cardiometabolic disease such as lipid-lowering therapy, there is controversial evidence on their GM-modulating effects. Beginning with statins, reports have suggested that they may positively influence GM composition and reduce the secretion of hazardous metabolites such as TMAO [39,40,41,42,43]. However, few earlier studies pointed to the potential statin-induced GM dysbiosis [44, 45]. Limited evidence on ezetimibe suggests a favorable effect on GM composition [46, 47]. No evidence is available to date on the impact of proprotein convertase subtilisin/kexin type 9 inhibitors.
As previously mentioned high-fiber diets, probiotics, prebiotics, synbiotics, and other interventions that modulate GM-host interactions were investigated in different studies to shed light on this interesting association [7].
High-fiber diet
High-fiber diets have received considerable scientific attention due to their favorable cardiometabolic effects as a result of GM modulation. To begin with, high-fiber diets could lead to enhanced generation of SCFAs by bacterial fermentation [48]. Lower levels of SCFAs have been associated with the development of essential hypertension in preclinical and clinical models, while administration of SCFAs may possess blood pressure-lowering effects [48]. Additional studies have proven that administration of magnesium acetate, propionate, and butyrate could lower the blood pressure of experimental hypertensive models [49,50,51]. Experimental models of cardiometabolic disease have proven the positive metabolic properties of various fiber sources, such as white kidney bean [52], psyllium fiber [53], pawpaw fruit fiber [54], banana peel fiber [55], flaxseed fiber [56], kiwifruit [57], and konjac glucomannan [58, 59]. As far as atherosclerosis development is concerned, when germ-free ApoE−/− mice were colonized with fecal samples of human donors and were fed with either fermentable fiber diet or cellulose control diet, the reduced atherosclerotic burden observed with the fermentable fiber diet was linked to the GM changes (higher abundance of butyrate-producing species) [60]. Other than the upregulation of SCFAs, diet rich in fiber may also lead to lower levels of TMAO, thus providing cardiovascular benefits [61].
High-fiber diets have shown variable results in the clinical setting concerning their cardiometabolic effects. In a clinical trial of 70 healthy subjects, supplementation of inulin fiber led to an increased concentration of Bifidobacteria, corresponding to enhanced SCFA production and ameliorated cardiovascular risk markers (serum cholesterol, very low-density lipoprotein triglyceride) [62]. In another small-scale randomized controlled trial of 17 patients with T2DM, a high-fiber diet resulted in enhanced glycemic control, a more favorable lipid profile, and suppressed systemic inflammation [63]. These findings were accompanied by an increased abundance of Lactobacillus, Akkermansia, Bifidobacterium, and Bacteroides, among others [63].
Probiotics
Probiotics are live microorganisms that are administered in adequate amounts to induce health effects. Numerous preclinical studies have supported their cardiometabolic benefits. In a study of hypercholesterolemic rats, the administration of single cholesterol-lowering probiotic strains (Limosilactobacillus reuteri TF-7, Enterococcus faecium TF-18, and Bifidobacterium animalis TA-1) could affect weight gain, lipidemic indices (total cholesterol, triglycerides, high-density lipoprotein-cholesterol, LDL-C), and hepatic steatosis [64]. The effect was more pronounced in the mixed probiotic formulation [64]. We should also state that probiotic administration not only increased the abundance of the single strain, but also the concentration of other beneficial bacteria (Lactobacillus, Enterococcus, Akkermansia, and Ruminococcaceae) [64]. Apart from the established metabolic benefits, probiotic anti-atherosclerotic effects were proven in a meta-analysis of studies using mouse models of atherosclerosis, as they were efficacious in reducing atherosclerotic plaque burden [65].
Multiple clinical studies suggest that probiotic administration may offer CVD benefits. In a recently published randomized trial involving 77 patients with dyslipidemia, the combined use of Lactobacillus plantarum and the lipid-lowering drug simvastatin led to substantial improvements in the lipid profile and a notable reduction in calculated cardiovascular risk, as compared to using simvastatin alone [66]. A mixed probiotic formulation by the name of Probio-X (L. casei Zhang, B. lactis V9, B. lactis Probio-M8, L. rhamnosus Probio-M9, L. plantarum P-8) also ameliorated the lipid profile of hyperlipidemic patients compared to placebo [67].
Moreover, in a double-blind, randomized study involving 44 patients with CAD, supplementation with Lactobacillus rhamnosus along with caloric restriction for 12 weeks resulted in noteworthy weight loss and demonstrated anti-inflammatory effects (reduction of LPS levels and IL1-Beta concentration, p = 0.0016 and p = 0.027, respectively) surpassing the effects of caloric restriction alone [68]. In a randomized, double-blind, placebo-controlled trial involving 60 patients with CAD, the probiotic strain Bifidobacterium lactis Probio-M8, when combined with conventional treatment, showed significant improvement in anginal (improved Seattle Angina Questionnaire), anxiety, and depressive symptoms, as well as reduced interleukin-6 and LDL-C levels compared to the control group. The investigator mentioned that the probiotic treatment led to alterations in gut microbial composition, increased bioactive microbial metabolites, and decreased TMAO and proatherogenic amino acids, which may have contributed to the observed improvements in quality of life and the reported anti-inflammatory and hypolipidemic effects [69].
Meta-analytic evidence has suggested that probiotics could emerge as the most potent nutritional intervention in ameliorating the glycemic profile (fasting blood glucose, fasting insulin level, homeostatic model assessment of insulin resistance) of overweight or obese patients, as demonstrated in the Bayesian network meta-analysis by Yu et al. [70]. However, these results should be interpreted with caution and deserve further validation, since glycated hemoglobin remained unaffected [70]. Moreover, probiotics may lead to weight loss and reduction in body mass index, as well as with slight reduction in LDL-C [71].
Prebiotics
Prebiotics, a class of dietary compounds, have emerged as a compelling area of research in the context of cardiometabolic disease. These non-digestible food components play a pivotal role in nurturing beneficial gut bacteria and have been linked to potential benefits for individuals at risk of or living with cardiometabolic conditions. The cardiometabolic effects have been examined preclinically, as in the study of Catry et al. [72]. The investigators supplemented apoE−/− mice fed a diet poor in n-3 polyunsaturated fatty acids with or without inulin-type fructans and detected an improvement in endothelial function by ameliorating NO bioavailability [72]. These findings were accompanied by an increased abundance of beneficial bacteria (Erysipelotrichaceae, Akkermansia) and lower concentrations of deleterious species (Lachnospiraceae, Ruminococcaceae, Desulfovibrionales) ultimately affecting the production of secondary bile acids [72]. In another study in diabetic Wistar rats, the administration of β-glucan, isolated from Saccharomyces cerevisiae, leads to improvements in glycemic and lipidemic indices and suppression of inflammation compared to the n-3 supplementation [72].
There are few clinical studies directly addressing the impact of prebiotics on human cardiometabolic diseases. In a small pilot clinical study, beta glycans treatment was found to protect against ischemia/reperfusion injury (lower leakage of myocardial enzymes postoperatively) in 48 patients who underwent coronary artery bypass grafting [73]. In another human trial involving CAD patients, the administration of chitosan oligosaccharides led to improvements in left ventricular ejection fraction, lipid profiles (the serum levels of TG, TC, and LDL-C were reduced while high-density lipoprotein-cholesterol (HDL-C) was increased) and increased the abundance of specific genera like Faecalibacterium, Alistipes, Escherichia, Lactobacillus, Lactococcus, and Phascolarctobacterium [74].
Synbiotics
Synbiotics, a cutting-edge approach in the realm of nutrition and gut health, consists of a combination of prebiotics and probiotics. Their aim is to offer a synergistic approach to fostering a healthy gut microbiome, making them a promising avenue for addressing and preventing cardiometabolic conditions.
Limited data is available on the preclinical level regarding the cardiometabolic effects of synbiotics. In the in vivo study of Méndez-Albiñana et al., a symbiotic mixture containing fructooligosaccharides, a mixture of Lactobacillus casei PXN 37, Lactobacillus rhamnosus PXN 54, Streptococcus thermophilus PXN 66, Bifidobacterium breve PXN 25, Lactobacillus acidophilus PXN 35, Bifidobacterium infantis PXN 27, and Lactobacillus bulgaricus PXN 39, was administered to male spontaneously hypertensive rats. This intervention led to ameliorated blood pressure levels that were accompanied by enhanced production of SCFAs, arterial nitric oxide release, and suppressed oxidative stress [75]. This formulation was also proven effective in improving serum triglycerides, insulin resistance, and blood pressure, along with improved endothelial function in another study performed in male Wistar rats on a high-fat diet [76]. However, no changes in other lipid parameters such as LDL-C or weight gain were noted [76].
As far as the in-human data of synbiotic administration is concerned, we should begin by stating that the existing clinical evidence is scarce. In a randomized, double-blind, among 60 patients with T2DM and CAD, a 12-week intervention using a synbiotic mixture resulted in improved glycemic status and HDL-C levels, while other cardiovascular risk factors remained unchanged [77]. Efforts to reduce hyperglycemia in diabetes through similar studies yielded varying outcomes [78]. In one small randomized, placebo-controlled human trial, the 12-week use of a synbiotic led to a modest reduction in total cholesterol and LDL-C [79]. Similarly, another randomized controlled trial also demonstrated benefits in lipid metabolism [80]. A meta-analysis of randomized control trials revealed that the use of symbiotic supplementation in individuals with metabolic syndrome resulted in significant reductions in serum insulin levels, TG, TC, LDL-C, body weight, and systolic blood pressure, while increasing HDL-C levels and reducing serum IL-6 [81••]. Finally, it is hypothesized that the blood pressure-lowering effect of synbiotics may be proportional to the stimulation of SCFA production, which may represent a potential surrogate marker of treatment effectiveness [82].
Fecal Transplantation
Fecal microbiota transplantation (FMT), a groundbreaking medical procedure, has gained significant attention in recent years for its potential to impact cardiometabolic disease. This innovative approach involves the transfer of healthy gut bacteria from a donor into a recipient’s digestive system, offering promising insights into the intricate relationship between the gut microbiome and cardiometabolic health. In the preclinical setting, FMT in diabetic db/db mice enhanced the abundance of certain bacterial species (Ruminococaceae, Porphyromonadaceae), restored the intestinal barrier integrity, and ameliorated inflammation [83]. Moreover, FMT administration in BTBRob/ob mice, a model of diabetic kidney disease, abrogated weight gain, inflammation, and insulin resistance compared to the control group, a finding that was accompanied by an increased abundance of Odoribacteraceae [84]. As the cardiometabolic effects of FMT are beginning to be unveiled, future preclinical studies are expected to examine its efficacy in lipid metabolism, arterial hypertension, and atherosclerosis, among others.
Concerning FMT in men with metabolic syndrome, the transfer of gut microbiota from lean donors led to improved insulin sensitivity, accompanied by an increase in butyrate-producing intestinal bacteria such as Roseburia intestinalis and Eubacterium hallii [85]. However, a subsequent randomized controlled trial involving male patients with metabolic syndrome, who received either vegan donor or their own fecal transplant, did not show significant reductions in TMAO levels or vascular inflammation, despite observing changes in the gut microbiota toward a vegan type [86]. No obvious clinical effects of FMT were noted in female patients with metabolic syndrome who were randomized to this intervention compared to the control group in the study of da Ponte Neto et al. [87]. We should also note the results of a recent meta-analysis assessing the role of FMT in patients with obesity or metabolic disorders [88]. The investigators observed an improvement in obesity metrics, insulin resistance, and glycemia [88], a finding which could be critical should it be replicated in large-scale, appropriately designed, randomized controlled trials. Moreover, it should be further clarified whether repeated FMTs are required, as this strategy was shown to be more effective in providing a greater percentage of the donor microbiota [89•].
Challenges and Future Directions
Despite the rapid advancements in our understanding of the gut microbiome’s role in cardiometabolic disease, several challenges remain on the path toward translating this knowledge into effective clinical practice. To begin with, the gut microbiome is a highly complex ecosystem comprising trillions of microorganisms and thousands of species. Characterizing this diversity and understanding its functional relevance is a formidable task. High inter-individual variability in microbiome composition further complicates efforts to identify consistent disease-associated patterns. Moreover, standardization of sampling, sequencing, and analysis methods is essential for reliable comparisons across studies. Lack of consistency in these approaches can introduce variability and hinder the reproducibility of findings.
We should also state that, while numerous associations between the gut microbiome and cardiometabolic diseases have been identified, establishing causality remains challenging. Mechanistic insights explaining how specific microbial populations or metabolites influence disease pathogenesis are often lacking. Multiple approaches are often used to strengthen the evidence. To begin with, longitudinal studies may be warranted to demonstrate the impact of changes in the microbiome over time on the progression of atherosclerosis. Moreover, studies on interventions that modulate gut microbiome can provide further evidence on its effect in disease development. Mendelian randomization studies may also play a role by using genetic variants associated with the microbiome as instrumental variables to assess causality. However, this approach assumes that the genetic variants affect the outcome only through the exposure of interest, and it may not be applicable if the microbiome is influenced by other factors that also impact atherosclerosis. In the future, integrating evidence from multiple approaches will provide a more robust understanding of the relationship between the microbiome and atherosclerosis.
Concerning the optimal timing and duration of microbiome-targeted interventions, it also represents an ongoing challenge. The dynamic nature of the microbiome, which can be influenced by diet, lifestyle, and medications, makes it difficult to pinpoint the most opportune moments for therapeutic interventions. Finally, as microbiome-based therapies emerge, ethical and regulatory issues must be addressed. Questions regarding safety, informed consent, and long-term effects of microbiome interventions require careful consideration.
The remarkable variability in individual gut microbiomes presents both a challenge and an opportunity in the pursuit of personalized medicine for cardiometabolic disease. Each person’s microbiome is unique, shaped by genetics, environment, diet, and other factors. This variability extends to the microbiome’s response to interventions, complicating efforts to develop one-size-fits-all treatments. To overcome this challenge, future research must focus on tailoring interventions to individual patients based on their microbiome profiles, genetics, and clinical characteristics. Personalized approaches may involve selecting specific dietary interventions, probiotics, or other treatments based on a patient’s microbiome composition. Advanced omics technologies, such as metagenomics and metabolomics, hold promise for characterizing individual microbiomes and predicting patient responses to interventions. Machine learning and artificial intelligence techniques can help identify patterns and correlations within large datasets, aiding in the development of personalized treatment strategies.
The rapidly evolving field of gut microbiome research opens up exciting avenues for future investigation. Integrating multiple omics data (genomics, metagenomics, transcriptomics, proteomics, metabolomics) can provide a more comprehensive understanding of microbiome-host interactions. This holistic approach can uncover novel therapeutic targets and biomarkers. The development of microbiome-based diagnostics, including biomarkers for disease risk and progression, is an emerging area of research. These diagnostics could revolutionize early disease detection and monitoring. In all, the integration of microbiome data with a patient’s clinical history and genetic information may offer a more complete picture of disease risk and treatment response. This interdisciplinary approach could lead to highly personalized treatment strategies. Tailoring microbiome interventions to individual patients may involve analyzing their microbiome profiles and selecting interventions that target specific microbial imbalances or functions associated with their disease. Critically, advances in technology may allow for real-time monitoring of a patient’s microbiome, enabling healthcare providers to adjust treatments as needed and track the effectiveness of interventions.
Ongoing clinical trials and research initiatives are exploring the feasibility and efficacy of personalized microbiome-based approaches in cardiometabolic disease management. These studies provide valuable insights into the practicality of personalized interventions. As we navigate these challenges and explore these exciting future directions, the potential for personalized medicine targeting the gut microbiome in cardiometabolic disease remains a tantalizing prospect. With continued research and innovation, we inch closer to harnessing the full therapeutic power of this intricate microbial ecosystem.
Conclusion
In conclusion, the intricate interplay between the gut microbiome and cardiometabolic disease presents a captivating frontier in modern medicine. While challenges persist, including the complexity of the microbiome and the need for personalized approaches, our expanding knowledge offers immense therapeutic potential. As we delve deeper into microbiome research, the prospect of tailored interventions based on individual microbiome profiles becomes increasingly attainable. By harnessing the power of this microbial world within us, we hold the promise of transforming the landscape of cardiometabolic disease treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cheng X, Ma T, Ouyang F, Zhang G, Bai Y. Trends in the prevalence of cardiometabolic multimorbidity in the United States, 1999–2018. Int J Environ Res Public Health. 2022;19(8):4726.
Tang WHW, Backhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(16):2089–105.
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–40.
Katsimichas T, Antonopoulos AS, Katsimichas A, Ohtani T, Sakata Y, Tousoulis D. The intestinal microbiota and cardiovascular disease. Cardiovasc Res. 2019;115(10):1471–86.
Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–44.
Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–9.
Tousoulis D, Guzik T, Padro T, et al. Mechanisms, therapeutic implications, and methodological challenges of gut microbiota and cardiovascular diseases: a position paper by the ESC Working Group on Coronary Pathophysiology and Microcirculation. Cardiovasc Res. 2022;118(16):3171–82.
Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–9.
Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70.
Salvi PS, Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. 2021;10(7):1775.
Tuteja S, Ferguson JF. Gut microbiome and response to cardiovascular drugs. Circ Genomic Precis Med. 2019;12(9):421–9.
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79.
Katsimichas T, Theofilis P, Tsioufis K, Tousoulis D. Gut microbiota and coronary artery disease: current therapeutic perspectives. Metabolites. 2023;13(2):256.
Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817–24.
Hasan AU, Rahman A, Kobori H. Interactions between host PPARs and gut microbiota in health and disease. Int J Mol Sci. 2019;20(2):387.
Tomas J, Mulet C, Saffarian A, et al. High-fat diet modifies the PPAR-gamma pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci USA. 2016;113(40):E5934–43.
Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–86.
Theofilis P, Vordoni A, Kalaitzidis RG. Trimethylamine N-oxide levels in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Metabolites. 2022;12(12):1243.
Fennema D, Phillips IR, Shephard EA. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos: Biol Fate Chem. 2016;44(11):1839–50.
Al Samarraie A, Pichette M, Rousseau G. Role of the gut microbiome in the development of atherosclerotic cardiovascular disease. Int J Mol Sci. 2023;24(6):5420.
Canyelles M, Borras C, Rotllan N, Tondo M, Escola-Gil JC, Blanco-Vaca F. Gut microbiota-derived TMAO: a causal factor promoting atherosclerotic cardiovascular disease? Int J Mol Sci. 2023;24(3):1940.
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94.
Gorabi AM, Kiaie N, Khosrojerdi A, et al. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc Med. 2022;32(8):525–33.
Chen T, Huang W, Qian J, et al. Macrophage-derived myeloid differentiation protein 2 plays an essential role in ox-LDL-induced inflammation and atherosclerosis. EBioMedicine. 2020;53:102706.
Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501.
Diks SH, van Deventer SJ, Peppelenbosch MP. Lipopolysaccharide recognition, internalisation, signalling and other cellular effects. J Endotoxin Res. 2001;7(5):335–48.
Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277.
Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 2018;7(4):198–206.
Chen X, He Y, Fu W, et al. Histone deacetylases (HDACs) and atherosclerosis: a mechanistic and pharmacological review. Front Cell Dev Biol. 2020;8:581015.
Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3(3):1191–212.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Yoo JY, Sniffen S, McGill Percy KC, Pallaval VB, Chidipi B. Gut Dysbiosis and immune system in atherosclerotic cardiovascular disease (ACVD). Microorganisms. 2022;10(1):108.
Miyazaki-Anzai S, Masuda M, Kohno S, et al. Simultaneous inhibition of FXR and TGR5 exacerbates atherosclerotic formation. J Lipid Res. 2018;59(9):1709–13.
Ottosson F, Brunkwall L, Smith E, et al. The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J Hypertens. 2020;38(12):2427–34.
Nemet I, Saha PP, Gupta N, et al. A Cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862-877 e822.
Poesen R, Claes K, Evenepoel P, et al. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol. 2016;27(11):3479–87.
Liu Y, Liu S, Zhao Z, Song X, Qu H, Liu H. Phenylacetylglutamine is associated with the degree of coronary atherosclerotic severity assessed by coronary computed tomographic angiography in patients with suspected coronary artery disease. Atherosclerosis. 2021;333:75–82.
Khan TJ, Ahmed YM, Zamzami MA, et al. Effect of atorvastatin on the gut microbiota of high fat diet-induced hypercholesterolemic rats. Sci Rep. 2018;8(1):662.
Kummen M, Solberg OG, Storm-Larsen C, et al. Rosuvastatin alters the genetic composition of the human gut microbiome. Sci Rep. 2020;10(1):5397.
Vieira-Silva S, Falony G, Belda E, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581(7808):310–5.
Li DY, Li XS, Chaikijurajai T, et al. Relation of statin use to gut microbial trimethylamine N-oxide and cardiovascular risk. Am J Cardiol. 2022;178:26–34.
Hu X, Li H, Zhao X, et al. Multi-omics study reveals that statin therapy is associated with restoration of gut microbiota homeostasis and improvement in outcomes in patients with acute coronary syndrome. Theranostics. 2021;11(12):5778–93.
Nolan JA, Skuse P, Govindarajan K, et al. The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G488–97.
Caparros-Martin JA, Lareu RR, Ramsay JP, et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5(1):95.
Catry E, Pachikian BD, Salazar N, Neyrinck AM, Cani PD, Delzenne NM. Ezetimibe and simvastatin modulate gut microbiota and expression of genes related to cholesterol metabolism. Life Sci. 2015;132:77–84.
Jin J, Wang J, Cheng R, et al. Orlistat and ezetimibe could differently alleviate the high-fat diet-induced obesity phenotype by modulating the gut microbiota. Front Microbiol. 2022;13:908327.
Xu C, Marques FZ. How Dietary Fibre, Acting via the Gut Microbiome. Lowers Blood Pressure Curr Hypertens Rep. 2022;24(11):509–21.
Bartolomaeus H, Balogh A, Yakoub M, et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019;139(11):1407–21.
Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond). 2018;132(6):701–18.
Marques FZ, Nelson E, Chu PY, et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135(10):964–77.
Feng Q, Niu Z, Zhang S, et al. Protective Effects of White Kidney Bean (Phaseolus vulgaris L.) against Diet-Induced Hepatic Steatosis in Mice Are Linked to Modification of Gut Microbiota and Its Metabolites. Nutrients. 2023;15(13):3033.
Bretin A, Yeoh BS, Ngo VL, et al. Psyllium fiber protects mice against western diet-induced metabolic syndrome via the gut microbiota-dependent mechanism. Gut Microbes. 2023;15(1):2221095.
Chen K, Wu S, Guan Y, et al. Changes in gut microbiota linked to a prevention of cardiac remodeling induced by hypertension in spontaneously hypertensive rats fed a pawpaw fruit diet. Heliyon. 2023;9(5):e15576.
Wang M, Yang F, Yan X, et al. Anti-diabetic effect of banana peel dietary fibers on type 2 diabetic mellitus mice induced by streptozotocin and high-sugar and high-fat diet. J Food Biochem. 2022;46(10):e14275.
Zhao M, Wang B, Li L, Zhao W. Anti-obesity effects of dietary fibers extracted from flaxseed cake in diet-induced obese mice. Nutrients. 2023;15(7):1718.
Wang K, Wang Y, Chen S, Gu J, Ni Y. Insoluble and soluble dietary fibers from kiwifruit (Actinidia deliciosa) modify gut microbiota to alleviate high-fat diet and streptozotocin-induced type 2 diabetes in rats. Nutrient. 2022;14(16):3369.
Liu Q, Fang J, Huang W, et al. The intervention effects of konjac glucomannan with different molecular weights on high-fat and high-fructose diet-fed obese mice based on the regulation of gut microbiota. Food Res Int. 2023;165:112498.
Jayachandran M, Christudas S, Zheng X, Xu B. Dietary fiber konjac glucomannan exerts an antidiabetic effect via inhibiting lipid absorption and regulation of PPAR-gamma and gut microbiome. Food Chem. 2023;403:134336.
Hutchison ER, Kasahara K, Zhang Q, Vivas EI, Cross TL, Rey FE. Dissecting the impact of dietary fiber type on atherosclerosis in mice colonized with different gut microbial communities. NPJ Biofilms Microbiomes. 2023;9(1):31.
Evans M, Dai L, Avesani CM, Kublickiene K, Stenvinkel P. The dietary source of trimethylamine N-oxide and clinical outcomes: an unexpected liaison. Clin Kidney J. 2023;16(1):1804–12.
Vijay A, Astbury S, Panayiotis L, et al. Dietary interventions reduce traditional and novel cardiovascular risk markers by altering the gut microbiome and their metabolites. Front Cardiovasc Med. 2021;8:691564.
Chen L, Liu B, Ren L, et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front Cell Infect Microbiol. 2023;13:1069954.
Puttarat N, Kasorn A, Vitheejongjaroen P, Chantarangkul C, Tangwattanachuleeporn M, Taweechotipatr M. Beneficial effects of indigenous probiotics in high-cholesterol diet-induced hypercholesterolemic rats. Nutrients. 2023;15(12):2710.
Khalili L, Centner AM, Salazar G. Effects of berries, phytochemicals, and probiotics on atherosclerosis through gut microbiota modification: a meta-analysis of animal studies. Int J Mol Sci. 2023;24(4):3084.
Neverovskyi A, Chernyavskyi V, Shypulin V, et al. Probiotic Lactobacillus plantarum may reduce cardiovascular risk: an experimental study. ARYA Atheroscler. 2021;17(4):1–10.
Wang H, Ma C, Li Y, et al. Probio-X relieves symptoms of hyperlipidemia by regulating patients’ gut microbiome, blood lipid metabolism, and lifestyle habits. Microbiol Spectr. 2023;11(3):e0444022.
Moludi J, Kafil HS, Qaisar SA, Gholizadeh P, Alizadeh M, Vayghyan HJ. Effect of probiotic supplementation along with calorie restriction on metabolic endotoxemia, and inflammation markers in coronary artery disease patients: a double blind placebo controlled randomized clinical trial. Nutr J. 2021;20(1):47.
Sun B, Ma T, Li Y, et al. Bifidobacterium lactis probio-M8 adjuvant treatment confers added benefits to patients with coronary artery disease via target modulation of the gut-heart/-brain axes. mSystems. 2022;7(2):e0010022.
Yu Z, Zhao D, Liu X. Nutritional supplements improve cardiovascular risk factors in overweight and obese patients: a Bayesian network meta-analysis. Front Nutr. 2023;10:1140019.
Mayta-Tovalino F, Diaz-Arocutipa C, Piscoya A, Hernandez AV. Effects of probiotics on intermediate cardiovascular outcomes in patients with overweight or obesity: a systematic review and meta-analysis. J Clin Med. 2023;12(7):2554.
Catry E, Bindels LB, Tailleux A, et al. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67(2):271–83.
Aarsaether E, Rydningen M, Einar Engstad R, Busund R. Cardioprotective effect of pretreatment with beta-glucan in coronary artery bypass grafting. Scand Cardiovasc J. 2006;40(5):298–304.
Jiang T, Xing X, Zhang L, Liu Z, Zhao J, Liu X. Chitosan oligosaccharides show protective effects in coronary heart disease by improving antioxidant capacity via the increase in intestinal probiotics. Oxid Med Cell Longev. 2019;2019:7658052.
Mendez-Albinana P, Martinez-Gonzalez A, Camacho-Rodriguez L, et al. Supplementation with the Symbiotic formulation prodefen((R)) increases neuronal nitric oxide synthase and decreases oxidative stress in superior mesenteric artery from spontaneously hypertensive rats. Antioxidants (Basel). 2022;11(4):680.
Llevenes P, Rodrigues-Diez R, Cros-Brunso L, et al. Beneficial Effect of a multistrain synbiotic prodefen((R)) plus on the systemic and vascular alterations associated with metabolic syndrome in rats: the role of the neuronal nitric oxide synthase and protein kinase A. Nutrients. 2020;12(1):117.
Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, et al. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes. 2017;125(1):21–7.
Nikbakht E, Khalesi S, Singh I, Williams LT, West NP, Colson N. Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials. Eur J Nutr. 2018;57(1):95–106.
Ooi LG, Ahmad R, Yuen KH, Liong MT. Lactobacillus gasseri [corrected] CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J Dairy Sci. 2010;93(11):5048–58.
Shakeri H, Hadaegh H, Abedi F, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701.
Arabi SM, Bahrami LS, Rahnama I, Sahebkar A. Impact of synbiotic supplementation on cardiometabolic and anthropometric indices in patients with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2022;176:106061. The use of synbiotics in the presence of metabolic syndrome may ameliorate markers of glycemia, dyslipidemia, obesity, and inflammation, together with a reduction of systolic blood pressure according to this meta-analysis.
Bartolomaeus H, Avery EG, Bartolomaeus TUP, et al. Blood pressure changes correlate with short-chain fatty acid production potential shifts under a synbiotic intervention. Cardiovasc Res. 2020;116(7):1252–3.
Chen L, Guo L, Feng S, et al. Fecal microbiota transplantation ameliorates type 2 diabetes via metabolic remodeling of the gut microbiota in db/db mice. BMJ Open Diabetes Res Care. 2023;11(3):e003282.
Bastos RMC, Simplicio-Filho A, Savio-Silva C, et al. Fecal microbiota transplant in a pre-clinical model of type 2 diabetes mellitus, obesity and diabetic kidney disease. Int J Mol Sci. 2022;23(7):3842.
Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913-916 e917.
Smits LP, Kootte RS, Levin E, et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018;7(7):e008342.
da Ponte Neto AM, Clemente ACO, Rosa PW, et al. Fecal microbiota transplantation in patients with metabolic syndrome and obesity: a randomized controlled trial. World J Clin Cases. 2023;11(19):4612–24.
Hu D, Zhao J, Zhang H, Wang G, Gu Z. Fecal microbiota transplantation for weight and glycemic control of obesity as well as the associated metabolic diseases: meta-analysis and comprehensive assessment. Life (Basel). 2023;13(7):1488.
Ng SC, Xu Z, Mak JWY, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71(4):716–23. In this randomized controlled trial, repeated fecal microbiota transplantation promoted a lean-associated microbiota, that was associated with favorable lipid profile changes and liver stiffness.
Author information
Authors and Affiliations
Contributions
P.T. and P.K.V. performed the literature search and wrote the main manuscript text. P.T. prepared figure 1. E.O., K.T. and D.T. supervised the writing of the paper and critically revised the manuscript. All authors have read and agreed to the submission of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Theofilis, P., Vlachakis, P.K., Oikonomou, E. et al. Targeting the Gut Microbiome to Treat Cardiometabolic Disease. Curr Atheroscler Rep 26, 25–34 (2024). https://doi.org/10.1007/s11883-023-01183-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01183-2