Abstract
Purpose of Review
Cardiovascular disease is an escalating cause of maternal morbidity and mortality. Women are at risk for acute myocardial infarction (MI), and more are living with risk factors for ischemic heart disease (IHD). The purpose of this review is to describe the evaluation and management of women at risk for and diagnosed with IHD in pregnancy.
Recent Findings
Pregnancy can provoke MI which has been estimated as occurring in 1.5–10/100, 000 deliveries or 1/12,400 hospitalizations, with a high inpatient mortality rate of approximately 5–7%. An invasive strategy may or may not be preferred, but fetal radiation exposure is less of a concern in comparison to maternal mortality. Common medications used to treat IHD may be continued successfully during pregnancy and lactation, including aspirin, which has an emerging role in pregnancy to prevent preeclampsia, preterm labor, and maternal mortality. Hemodynamics can be modulated during pregnancy, labor, and postpartum to mitigate risk for acute decompensation in women with IHD.
Summary
Cardiologists can successfully manage IHD in pregnancy with obstetric partners and should engage women in a lifetime of cardiovascular care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
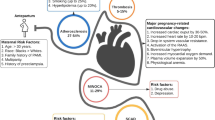
Cardiovascular disease, already the major cause of death in the USA, is a rising cause of maternal morbidity and mortality, responsible for over 1/3 of deaths [1••]. Non-Hispanic black and Native American/Alaska Native women have the highest all-cause maternal mortality in the USA, 3.3- and 2.5-fold that of non-Hispanic white women [1••]. Analysis of pregnancy-related deaths indicates these groups are disproportionately affected by multiple factors which contribute to worse outcomes. These include less access to medical care, inadequate insurance coverage, lack of provider training or continuity, limitations in their built environment leading to poor housing or transportation, and less health education [1••]. In response, obstetricians and cardiologists across professional societies have galvanized to address, evaluate, and manage risk, advocating for improved research, education, and treatment to avert tragic outcomes in young women, which has family and community-wide impact [2, 3•]. The aim of this review is to summarize current knowledge relating to the management of ischemic heart disease in pregnancy (Fig. 1) and where there are urgent opportunities for interdisciplinary endeavors.
a Highlights of the management of women with ischemic heart disease (IHD) in pregnancy. ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor inhibitor, MI = myocardial infarction. 1From ref. 4. b Composition of the Cardio-Obstetrics Heart Team at our institution. Cardiology representation is most frequently from general/imaging and adult congenital heart disease. Additional services are involved depending on maternal need
More Women in Their Reproductive Years Are Living with Risk Factors for Ischemic Heart Disease
While cardiovascular disease (CVD) has declined across the population, there has been a paradoxical rise in women of reproductive age, with less awareness of risk factors in women of color [5, 6]. More young women in the USA are living with obesity, hypertension, and diabetes, a classic trifecta of risk factors for ischemic heart disease (IHD). Physicians must be attuned to this evidence and routinely estimate risk and screening for overt disease in young women. Obstetric and pre-conception care encounters are occasions for global assessment that will affect women beyond the three trimesters of pregnancy. These are moments for anticipatory guidance about cardiovascular health which can influence and engage women in care for a lifetime.
A broad scope of risk factors for IHD in women of reproductive age must be considered. Sex-specific CVD risk factors are under-evaluated at physician visits [7]. In the general population, for adults 20–39 with low-to-borderline 10-year risk assessment, periodic monitoring (every 4–6 years) is recommended [8]. However, not all risk factors, like female-preponderant or female-specific risk factors, are considered by widely used scoring calculators like the Atherosclerotic Cardiovascular Disease (ASCVD) Risk Algorithm or Framingham 10-year risk score [9, 10]. More frequent monitoring might be useful with increasing risk factors, but at present, specific recommendations for women are lacking, thus relying on obstetricians, cardiologists, and other physicians to tailor care.
With regard to women of reproductive age, physicians may take into account advanced maternal age, obesity, physical inactivity, undiagnosed hypertension, metabolic (thyroid, polycystic ovarian syndrome, diabetes) and rheumatic disorders (lupus and rheumatoid arthritis), race-ethnic, immigration, and socioeconomic characteristics, psychosocial stress (domestic violence, depression), symptoms of untreated sleep apnea, and substance use. This is in addition to classical biomarkers like elevated serum total, low density lipoprotein (LDL) cholesterol, triglycerides, and potentially lipoprotein (a), as well as low serum high density lipoprotein (HDL) cholesterol, prothrombotic factors, and inflammation [11, 12]. Use of estrogen-containing contraceptives must be evaluated, as high estrogen, > 50 μg, increases risk of myocardial infarction (MI) and stroke, 1.6–1.7-fold, respectively [13].
Evidence indicates the importance of reproductive history in the full CVD risk assessment of women. The total number of lifetime ovulations matters with respect to a woman’s risk of MI; the more lifetime ovulations, the higher the risk. IHD risk decreases with older age at menarche. In a study of the National Health and Nutrition Examinations Survey (NHANES), for each 1-year delay in menarche, there was a 17% increase in the odds of having ideal cardiovascular health [14]. In a small prospective cohort, IHD risk rose with older age at first birth (age-adjusted relative risk ratio (RR) 2.90 for ages 33–43 as compared with 25–29 years) and later age at last birth (age-adjusted RR 3.79 for ages ≥40 as compared with 35–39 years) [15]. In a large prospective Japanese study, a U-shaped association between parity and CVD mortality was described [16]. For women with ≥3 deliveries, older maternal age ≥28 years at first delivery was associated with a 1.2- to 1.5-fold increase in CVD mortality [16]. With respect to the postpartum period, there is mixed evidence on the effects of lactation on cardiovascular health. Some studies indicate that longer breastfeeding may be associated with reduced odds of maternal metabolic syndrome, but a recent systematic review was inconclusive, potentially due to between-study heterogeneity [17].
Nulliparity has been identified as a long-term risk factor for cardiovascular disease, but studies do not usually distinguish if this is due to women’s life choices or infertility, per se. Polycystic ovarian syndrome, but not necessarily all-cause infertility, has been associated with cardiovascular risk and diabetes [18]. Premature ovarian failure, recurrent miscarriage, or early menopause contributes a 1.5- to 1.9-fold increased risk of MI [15]. While ovulation-inducing agents used in reproductive endocrine treatments of infertility have not been shown to increase the risk of cancer, the relationship between fertility medications and MI is understudied [19]. An infrequent complication from infertility treatment, ovarian hyperstimulation syndrome (OSH), leads to increased vascular permeability, inflammation, and hypercoagulability. It has been associated with stroke and acute MI [20]. Individuals with predisposition to CVD or preexisting cardiovascular conditions may be at greater risk of MI with OSH.
A woman’s obstetric history is a window to her cardiovascular future. Lifetime prevalence of hypertension and diabetes are predicted by hypertensive disease of pregnancy (gestational hypertension, preeclampsia/eclampsia), diabetes of pregnancy (both pre-gestational and gestational), and adverse pregnancy outcomes (placental abruption, fetal demise, intrauterine growth restriction (IUGR)). The total contribution of mild-to-moderately elevated risk factors should be considered as describing a fuller picture of the pregnant patient, with the view towards establishing an intrapartum and long-term plan for interval monitoring. A cardio-obstetric or similar heart team approach is useful in assessing and monitoring at-risk pregnant patients, and recommended for those with overt CVD, like preexisting IHD [3•, 21, 22].
Pregnancy is a physiologic stress test, with increased demand to the cardiovascular system from doubling of blood volume and cardiac output and weight carrying [23]. Distinguishing between normal pregnancy and occult heart disease can therefore be challenging, and screening with electrocardiogram (EKG) and echocardiography may yield normal studies. However, in a study of maternal mortality in California, cardiovascular disease, primarily cardiomyopathy, was detected in 25% of women who died and retrospective screening of abnormal signs and symptoms revealed that 88% of cases could have been identified using an algorithmic approach [24, 25]. The Centers for Disease Control and Prevention estimates 60% of maternal deaths as preventable [1••]. While the optimal threshold for screening of women without a prior diagnosis of IHD requires further investigation, physicians may consider EKG, echocardiography, and referral to cardiology for pregnant women with abnormal vital signs, like hypoxia, hypertension, tachycardia >100 bpm, or unexpected changes in their exercise tolerance [24]. For women with a diagnosis of IHD, risk stratification scores for adverse outcomes from cardiac disease in pregnancy, like CARPREG, ZAHARA, or WHO, ascribe higher risk to worse New York Heart Association Class and left ventricular ejection fraction, but in the newer CARPREG II, women with a prior diagnosis of IHD also receive points for a diagnosis of CAD [26••]. Women with CAD alone had higher odds of adverse cardiac events in pregnancy, OR 3.0 (95% CI 1.1–7.6); hence, scoring tools may be useful for pre-pregnancy risk stratification as well as patient counseling [26••]. Obesity also portends a poor prognosis [4].
Women in Their Reproductive Years and in Pregnancy Can Experience Acute Myocardial Infarction
The rate of acute myocardial infarction (MI) in women of reproductive age was <100/100,000 patient-years for ages 30–39, rising to >100/100,000 over the age of 45 years, in a study of the National Inpatient Sample (NIS), with black women having a higher rate of MI than white women [27]. Young women as a group had higher in-hospital mortality than men and longer length of stay [27]. There is up to a 4-fold higher risk of MI during pregnancy and while MI is a rare event, it is associated with a high in-hospital mortality rate [28•]. Risk of MI in pregnancy was estimated at 3–10/100,000 deliveries or 1/12,400 hospitalizations in the NIS and the in-hospital maternal fatality rate was astoundingly high at 4.5–5.1% and up to 7.3% in a California-based cohort [28•, 29, 30].
Hypercoagulability is a frequent etiology of MI in women of reproductive age, from antiphospholipid syndrome and protein S and factor XII deficiencies. In a small series, mechanisms of MI in pregnancy were most often spontaneous coronary artery dissection (SCAD), followed by atherosclerosis and, less often, stress (takotsubo) cardiomyopathy or non-iatrogenic coronary spasm [31]. Less common are embolization or ischemia from coronary artery anomalies. Mortality from ST elevation MI (STEMI) was not significantly different than non-STEMI (NSTEMI) and NSTEMI accounted for most cases. In the NIS, slightly less than half of MI in pregnancy occurred antepartum or during labor and delivery (L&D) [28•]. Older maternal age, tobacco use, hypertension, diabetes mellitus, and thrombophilia were independent risk factors. Approximately half of MI cases were managed invasively, and of those, >3/4 underwent percutaneous coronary intervention while <1/4 underwent coronary artery bypass grafting. Invasive management was associated with lower in-hospital mortality than medical management with an adjusted odds ratio (OR) of 0.17 (95% CI, 0.07–0.42) [28•].
The 2018 European Society of Cardiology guidelines for the management of cardiovascular disease in pregnancy give coronary angiography and PCI a Class I recommendation, Level of evidence (LOE) C for STEMI and invasive management for NSTEMI with high risk features a Class IIa, LOE C recommendation [21]. When do the benefits of an invasive strategy in pregnant women outweigh potential risks? Besides STEMI, cardiologists might consider an invasive strategy for hemodynamic instability, when a left anterior descending or left main culprit is likely, and when no alternative diagnosis is suspected. Invasive diagnosis can change management and while radiation exposure in pregnancy should be minimized, maternal health must be prioritized. A case report of PCI in pregnancy reported an estimated fetal absorbed dose = 0.79 milliGray (mGy), modeled retrospectively. By comparison, a chest X-ray has an estimated fetal absorbed dose of 0.1–0.9 mGy [32]. For perspective, doses of 50 mGy increase fetal loss within the first 2 weeks of pregnancy and the risk of congenital abnormality increases at doses >150 mGy, well above what might be expected from a single cardiac catheterization [33]. Technical choices such as radial artery access, using 4-Fr catheters and 5-Fr guides; ensuring coaxial injection and lowering the injection rate; collimation; placing lead under the patient; and saving fluoroscopy images rather than using higher radiation dose cine acquisition are strategies interventional cardiologists can use to help mitigate risk to pregnant women undergoing catheterization. Pregnant women should be positioned with a slight leftwards tilt to relieve pressure on the inferior vena cava to increase filling to the right heart and avoid syncope. This position is especially important in emergent cases when cardiopulmonary resuscitation (CPR) must be performed. If CPR duration is longer than 5 min, emergent bedside evacuation of the uterus by an obstetrician is recommended for maternal survival [34].
Noninvasive testing may not provide additional risk stratification, may be non-diagnostic, or may delay care for MI in pregnancy. Echocardiography is useful in assessing wall motion abnormalities which may aide in decision-making but can be limited by poor windows. Computed tomography angiography requires a heart rate <100 bpm, weight <350–400 lbs (usual table limit), 100–150 mL of contrast, and radiation dose of 1–2 mSV, and if positive, cardiac catheterization may still be recommended. Conversely, there may be equipoise for an invasive strategy when EKG changes are equivocal or normal or co-morbidities make the procedure riskier, like renal dysfunction or contrast dye allergy. Additionally, the frequency of complications during invasive catheterization has been reported as higher in pregnant women than might be expected for non-pregnant patients, including emergent coronary artery bypass grafting (CABG) for iatrogenic dissection [31]. Thus, a thorough informed consent discussion and shared decision-making with patients are important, as is an interdisciplinary team approach involving obstetricians, interventional cardiologists, and other physicians.
Pregnancy increases vessel fragility, and this may contribute to SCAD, in which a false lumen results from disruption of the coronary tunica media [35]. Two pathophysiologies have been proposed: the “inside-out” and “outside-in” models. The former is thought to begin with an intimal tear from shear stress, with blood penetrating the internal elastic lamina to accumulate in the tunica media, the latter resulting from bleeding of the vasa vasorum and medial hemorrhage [36]. The Saw classification groups SCAD into four types based on angiographic appearance: Type 1 (29–48%), characterized by a linear flap with contrast dye staining; Type 2 (52–67%), a long smooth stenosis; Type 3 (0–3.9%), a short, focal stenosis, like atherosclerosis; and Type 4, an acute total occlusion [37]. SCAD can be a manifestation of fibromuscular dysplasia, a diffuse disorder of small and medium arteries characterized by disorganized architecture of the arterial wall [38]. Individuals presenting with SCAD should undergo evaluation of the cerebral, renal, and thoracic vasculature to rule out this disorder [38].
Treatment options for SCAD are medical therapy, balloon angioplasty, percutaneous coronary intervention (PCI), and/or CABG. Selection depends on hemodynamic stability, ischemia, and SCAD classification. For non-flow limiting dissection, medical management, especially beta-blockers, is generally preferred as the vessel wall commonly heals [39]. Dissection can propagate and extended inpatient monitoring has been suggested for this reason [39]. With increasing obstruction to flow and active ischemia, balloon angioplasty and/or PCI may be preferred or necessary when the patient is unstable [39]. Low-pressure cutting balloon angioplasty may be useful to fenestrate the dissection flap and has been described in case series and reports [40]. If PCI is desired, the interventionalist might consider stenting the distal and proximal ends of the dissection and then the mid-section, if a dual stent strategy is required to cover the entire length of the lesion, so as not to propagate the dissection down the vessel [41]. Intravascular imaging during cardiac catheterization with optical coherence tomography (OCT) or intravascular ultrasound (IVUS) may be useful in distinguishing SCAD from atherosclerosis, particularly the Type III variety. The risk of passing catheters through an abnormal vessel and a larger-volume injection in the case of OCT should be weighed against information gained. Dissected vessels may be challenging to size by visual estimation alone which might contribute to the high in-stent restenosis and thrombosis rates reported in PCI for SCAD [39]. Intravascular image-guided PCI for atherosclerosis generally leads to increased minimal luminal stent area from better stent sizing, more frequent post-dilation and less in-stent restenosis [42]. It is not clear if this is also true in PCI for SCAD but is a hypothesis for study. CABG for left main or multivessel disease is rare during pregnancy but has been documented [28•]. It is generally avoided as placental perfusion may be poor during bypass, leading to fetal bradycardia, hypoxia, or acidemia [43]. Nonetheless, maternal survival must be prioritized.
Aspirin Is Beneficial in the Treatment of Ischemic Heart Disease and Prevention of Preeclampsia in Pregnancy
Aspirin is safe and effective in women for the prevention and treatment of MI and ischemic stroke, but aspirin also has a unique and emerging role in pregnancy [44, 45]. Use of 81–162 mg of daily oral aspirin after the 9th week of pregnancy is salutorious for the placenta by improving invasion of the uterine spiral arteries and reduces placental vascular resistance [46]. Women with CVD are more likely to have fetal growth abnormalities due to placental insufficiency or fetal demise [47]. As a result of improved placental health, at-risk women on aspirin initiated between 6 and 13 weeks of pregnancy have less early-onset preeclampsia, preterm labor, and mortality around the time of delivery [48, 49••]. Aspirin may also prevent recurrent miscarriage in women with antiphospholipid antibodies [50]. It is recommended for women with a combination of risk factors, including obesity and hypertension, for example, but also socioeconomic disadvantage and African American race-ethnicity [51•]. Cardiologists should be aware of the dual importance of aspirin in pregnancy for women with IHD.
Labor and Delivery Considerations for Women with Ischemic Heart Disease
Delivery planning should be multidisciplinary for women with CVD (Fig. 1b). At our institution, cardio-obstetrics team meetings most often include representatives from Maternal Fetal Medicine (MFM), Cardiology (including Adult Congenital Heart Disease), Internal Medicine/Critical Care, L&D plus Cardiac Intensive Care Unit (CICU) Nursing, and Obstetric Anesthesia. A heart team approach is recommended by consensus [21]. A standardized approach is helpful in organizing data and communicating the plan, and can be incorporated into the electronic medical record to inform stakeholders [22]. The team reviews cardiac studies and discusses concerns and contingencies during labor and delivery to decide the timing and mode of delivery, location (L&D vs. CICU), and monitoring. The pregnant patient with IHD should be stabilized and medically optimized prior to delivery. MFM physicians recommend planned delivery between 37 and 39 weeks when the fetus is full term to avoid preeclampsia and better control circumstances of L&D [52].
Avoiding preeclampsia from prolonged pregnancy, avoiding high afterload, reducing cardiac workload, and controlling tachycardia, as well as lowering volume and oxygen demand, are paramount in women with IHD, particularly in the second and third trimesters when cardiac output peaks [53]. A vaginal delivery is usually ideal and safe in the stable patient with IHD, but avoiding Valsalva and pain control should be considered to decrease left ventricular wall stress and demand ischemia. The patient may require operative assisted vaginal delivery, assistance in the second stage of labor with vacuum or forceps, or in certain cases, expedited delivery with Cesarean (C) section. Besides maternal indications, there can be obstetric indications for C-section which are not foreseeable. Therefore, in the delivery planning process, the cardio-obstetric team must review risks associated with the maternal cardiac stress of a surgical procedure.
Continuous maternal and fetal monitoring, maternal oxygen saturation, and telemetry are required during L&D for women with IHD. Of note, this requires cardiac nursing to be available on the L&D floor, as obstetric nurses are not often trained to interpret EKGs and may have less familiarity with advanced cardiac life support. Maternal implantable cardioverter defibrillators (ICDs) should be turned off and automatic external defibrillator pads placed on the chest with continuous monitoring from the device in case of arrhythmia. Medications, like beta-blockers, calcium channel antagonists, and intravenous nitroglycerin, should be readily available to treat cardiac ischemia during labor and are commonly located on L&D floors. Importantly, some common obstetric vasoconstrictors should be avoided, like methergine, neostigmine, epinephrine, and other vasopressors.
Neuraxial anesthesia is preferred for pain relief of vaginal and C-section deliveries. Hypotension due to anesthesia can be avoided with careful obstetric technique, lower-dose anesthesia, slower induction, and intravenous fluid administration. However, spinal hematomas are a risk for those patients on anti-platelet or anti-coagulants. It is generally preferred that thienopyridines and anti-coagulants be ceased ahead of labor to prevent this complication [54]. General anesthesia is more commonly used for patients who are fully anti-coagulated and require emergent delivery where there is no time for reversal.
The placenta is delivered during the third stage of labor immediately after the neonate is born. Release of blood back into the circulation from the involuting uterus and release of pressure on the inferior vena cava is equivalent to a large-volume bolus which may provoke acute congestive heart failure in some women. Diuretics, venodilation with nitrates, antihypertensives, and continuing anesthesia are strategies to decrease acute volume return.
Medications for the Treatment of Ischemic Heart Disease Can Be Used in Pregnancy and Women Who Breastfeed
There may be hesitancy or unfamiliarity regarding medications to treat IHD during pregnancy. Some of the medications cardiologists are accustomed to using in non-pregnant patients can be initiated or continued in pregnancy and breastfeeding [55]. In addition to aspirin, which has dual roles, clopidogrel is used in pregnancy for patients needing dual anti-platelet therapy. Ticagrelor and prasugrel have less data and are not usually preferred for this reason. There is concern for statin use in pregnancy, as HMG-coA reductase inhibitors could affect embryogenesis with case reports on limb defects and severe central nervous system anomalies [56]. Metoprolol is well known for its benefits in IHD, and twice-daily dosing of metoprolol succinate might be preferable given a higher metabolic rate in pregnancy, although unproven. However, beta-blockers can be associated with IUGR; thus, fetal growth must be monitored. As IUGR is more frequent in women with CVD due to worse placental health, it may be difficult to distinguish between placental versus medication effects on the fetus. Labetalol and methyldopa are frequently used antihypertensives, but so are hydralazine and isosorbide, often favored for heart failure with reduced ejection fraction, particularly due to benefit in African Americans. Angiotensin-converting enzyme inhibitors are not used during pregnancy to avoid damage to fetal kidneys, but enalapril is often used in breastfeeding when the neonate is not premature [57]. The website https://www.reprotox.org/ is a resource for cardiologists and obstetricians needing updated information on use of medications in pregnancy and lactation.
The “Fourth Trimester”: an Opportunity to Prevent or Capture Acute Decompensation and Engage in Lifelong Cardiac Care
The postpartum period is characterized by rapid reversal of pregnancy physiology, both immediately and over the subsequent 3 months. Postpartum preeclampsia, a sudden rise in blood pressure associated with pulmonary edema, stroke, or seizure, in addition to postpartum cardiomyopathy, may appear during this period. Routine follow-up for healthy women is commonly at 6 weeks postpartum, but for women with IHD or at higher risk, it may be useful to schedule follow-up in the first week, either in-person or by telehealth visit with home blood pressure or even oxygen saturation monitoring. Symptoms of shortness of breath, chest discomfort, or new-onset hypertension >140/90 mmHg should trigger referral to the emergency department, for example [24]. In one study, women at risk for severe postpartum hypertension were followed via telehealth for remote blood pressure monitoring, of which, 16% developed severe hypertension post discharge from L&D and 53% at 6 weeks postpartum [58]. The advantages of telehealth for women with CVD is an area for future investigation.
Postpartum, cardiologists should ensure that women of reproductive age with IHD are referred for contraceptive counseling. For women with IHD, non-hormonal or progesterone-alone contraception avoids exposure to the prothrombotic effects of estrogen. There is a wide array of methods from which women may select, grouped into reversible and non-reversible methods. Long-acting reversible contraception (LARC) includes the non-hormonal copper intrauterine device (IUD), progesterone-containing IUD (Liletta, AbbVie, Inc., Chicago, IL), medroxyprogesterone acetate subcutaneous injection (Depo-Provera, Pfizer, Inc. New York, NY), or implant (Nexplanon, Merck & Co., Inc., Kenilworth, NJ). Tubal ligation, IUD placement, or subcutaneous implant can be performed immediately postpartum. Barrier methods (diaphragm, female/male condoms) or progesterone-alone oral contraception (“minipill”) are other options. Progesterone-containing products are compatible with breastfeeding and not considered to decrease milk supply [59]. Suppressing menses with progesterone-containing IUDs may be particularly useful for women in which menorrhagia and/or pregnancy can be life-threatening, such as those requiring dual anti-platelet therapy or anticoagulation due to mechanical valves or left ventricular assist devices. A plan for long-term retention in cardiac care should be established.
Conclusions
Cardiologists must be informed about the management of IHD in pregnancy in an era of rising US maternal mortality and morbidity. Cardiologists bring a wealth of expertise to partner effectively with obstetricians and others, for the benefit of women during and after pregnancy. Reducing the burden of cardiovascular disease risk factors in young women, especially women of color, using best practices to treat acute myocardial infarction in pregnancy, and retaining high-risk women in lifelong cardiac care, are areas for continued research and education to curb this alarming trend.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Petersen EE, et al. Vital signs: pregnancy-related deaths, United States, 2011-2015, and strategies for prevention, 13 states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423–9. Data from the Center for Disease Control indicating elevated mortality for black and indigenous women in the USA. https://doi.org/10.15585/mmwr.mm6818e1.
Bond RM, Gaither K, Nasser SA, Albert MA, Ferdinand KC, Njoroge JN, et al. Working agenda for black mothers: a position paper from the Association of Black Cardiologists on solutions to improving black maternal health. Circ Cardiovasc Qual Outcomes. 2021;14(2):e007643. https://doi.org/10.1161/CIRCOUTCOMES.120.007643.
• Mehta L, et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation. 2020;141(23):e884–903. Consensus document with recommendations for women with high-risk cardiac disease in pregnancy. https://doi.org/10.1161/CIR.0000000000000772.
Pfaller B, et al. Impact of obesity on outcomes of pregnancy in women with heart disease. J Am Coll Cardiol. 2021;77(10):1317–26. https://doi.org/10.1161/CIR.0b013e318287cf2f.
Williams RA. Cardiovascular disease in African American women: a health care disparities issue. J Natl Med Assoc. 2009;101(6):536–40. https://doi.org/10.1016/s0027-9684(15)30938-x.
Cushman M, Shay CM, Howard VJ, Jiménez MC, Lewey J, McSweeney JC, et al. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American Heart Association National Survey: a special report from the American Heart Association. Circulation. 2021;143(7):e239–48. https://doi.org/10.1161/CIR.0000000000000907.
Harvey RE, Coffman KE, Miller VM. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health (Lond). 2015;11(2):239–57. https://doi.org/10.2217/whe.14.64.
Arnett DK, Khera A, Blumenthal RS. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: part 1, lifestyle and behavioral factors. JAMA Cardiol. 2019;4(10):1043–4. https://doi.org/10.1161/CIR.0000000000000677.
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. https://doi.org/10.1161/01.cir.0000437741.48606.98.
Andersson C, Johnson AD, Benjamin EJ, Levy D, Vasan RS. 70-year legacy of the Framingham Heart Study. Nat Rev Cardiol. 2019;16(11):687–98. https://doi.org/10.1038/s41569-019-0202-5.
Grundy SM, Pasternak R, Greenland P, Smith S Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100(13):1481–92. https://doi.org/10.1161/01.CIR.100.13.1481.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596. https://doi.org/10.1161/CIR.0000000000000757.
Roach RE, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;8:CD011054. https://doi.org/10.1002/14651858.CD011054.
Zheng Y, Wen TS, Shen Y, Hu H. Age at menarche and cardiovascular health: results from the NHANES 1999-2016. Menopause. 2020;28(1):18–24. https://doi.org/10.1097/GME.0000000000001653.
Cooper GS, et al. Menstrual and reproductive risk factors for ischemic heart disease. Epidemiology. 1999;10(3):255–9. https://doi.org/10.1056/NEJM198909073211004.
Tanigawa K, Ikehara S, Kimura T, Imano H, Muraki I, Shirai K, et al. Relationships between reproductive history and mortality from cardiovascular diseases among Japanese women: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) study. J Epidemiol. 2020;30(11):509–15. https://doi.org/10.2188/jea.JE20190020.
Tørris C, Bjørnnes AK. Duration of lactation and maternal risk of metabolic syndrome: a systematic review and meta-analysis. Nutrients. 2020;12(9). https://doi.org/10.3390/nu12092718.
Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017;34(2):167–77. https://doi.org/10.1007/s10815-016-0836-8.
Momenimovahed Z, Taheri S, Tiznobaik A, Salehiniya H. Do the fertility drugs increase the risk of cancer? A review study. Front Endocrinol (Lausanne). 2019;10:313. https://doi.org/10.3389/fendo.2019.00313.
Al-Sadawi M, et al. Ovarian Hyperstimulation Syndrome and Myocardial Infarction: A Systematic Review. Int J Clin Endocrinol Metab. 2019;5(1):009–12. https://doi.org/10.17352/ijcem.000035.
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, de Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–241. https://doi.org/10.1093/eurheartj/ehy340.
Wolfe DS, Hameed AB, Taub CC, Zaidi AN, Bortnick AE. Addressing maternal mortality: the pregnant cardiac patient. Am J Obstet Gynecol. 2018;220:167.e1–8. https://doi.org/10.1016/j.ajog.2018.09.035.
Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–8. https://doi.org/10.1161/CIRCULATIONAHA.114.009029.
Hameed AB, et al. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol. 2015;213(3):379 e1–10. https://doi.org/10.1016/j.ajog.2015.05.008.
Hameed A, Morton CH, Moore A. Improving health care response to cardiovascular disease in pregnancy and postpartum developed under contract #11-10006 with the California Department of Public Health, Maternal, Child and Adolescent Health Division. California Department of Public Health, 2017. https://www.cmqcc.org/resources-toolkits/toolkits/improving-health-care-response-cardiovascular-disease-pregnancy-and. Accessed 12 July 2021.
•• Silversides CK, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71(21):2419–30 Risk stratification system which specifically identifies coronary artery disease as a significant risk factor for adverse cardiac events in pregnancy in a validated multivariate model.
Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64(4):337–45. https://doi.org/10.1016/j.jacc.2014.04.054.
• Smilowitz NR, et al. Acute myocardial infarction during pregnancy and the puerperium in the United States. Mayo Clin Proc. 2018;93(10):1404–14. Data from the National Inpatient Sample documenting high in-hospital maternal mortality from myocardial infarction. https://doi.org/10.1016/j.mayocp.2018.04.019.
James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113(12):1564–71. https://doi.org/10.1161/CIRCULATIONAHA.105.576751.
Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105(3):480–4. https://doi.org/10.1097/01.AOG.0000151998.50852.31.
Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129(16):1695–702. https://doi.org/10.1161/CIRCULATIONAHA.113.002054.
Kuba K, Wolfe D, Schoenfeld AH, Bortnick AE. Percutaneous coronary intervention in pregnancy: modeling of the fetal absorbed dose. Case Rep Obstet Gynecol. 2019;2019:8410203–5. https://doi.org/10.1155/2019/8410203.
Dauer LT, Thornton RH, Miller DL, Damilakis J, Dixon RG, Marx MV, et al. Radiation management for interventions using fluoroscopic or computed tomographic guidance during pregnancy: a joint guideline of the Society of Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe with Endorsement by the Canadian Interventional Radiology Association. J Vasc Interv Radiol. 2012;23(1):19–32. https://doi.org/10.1016/j.jvir.2011.09.007.
Morris S, Stacey M. Resuscitation in pregnancy. BMJ. 2003;327(7426):1277–9. https://doi.org/10.1136/bmj.327.7426.1277.
Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1):R27–44. https://doi.org/10.1530/JOE-16-0340.
Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee, al-Hussaini A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39(36):3353–68. https://doi.org/10.1093/eurheartj/ehy080.
Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84(7):1115–22. https://doi.org/10.1002/ccd.25293.
Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2019;37(2):229–52. https://doi.org/10.1177/1358863X18821816.
Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137(19):e523–57. https://doi.org/10.1161/CIR.0000000000000564.
Main A, Lombardi WL, Saw J. Cutting balloon angioplasty for treatment of spontaneous coronary artery dissection: case report, literature review, and recommended technical approaches. Cardiovasc Diagn Ther. 2019;9(1):50–4. https://doi.org/10.21037/cdt.2018.10.11.
Yip A, Saw JJ. Spontaneous coronary artery dissection—a review. 2015;5(1):37–48. https://doi.org/10.3978/j.issn.2223-3652.2015.01.08.
Darmoch F, Alraies MC, al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(5):e013678. https://doi.org/10.1161/JAHA.119.013678.
Patel A, et al. Cardiac surgery during pregnancy. Tex Heart Inst J. 2008;35(3):307–12. https://doi.org/10.1016/j.athoracsur.2010.11.037.
Dalen JE. Aspirin to prevent heart attack and stroke: what's the right dose? Am J Med. 2006;119(3):198–202. https://doi.org/10.1016/j.amjmed.2005.11.013.
Ittaman SV, VanWormer JJ, Rezkalla SH. The role of aspirin in the prevention of cardiovascular disease. Clin Med Res. 2014;12(3-4):147–54. https://doi.org/10.3121/cmr.2013.1197.
Haapsamo M, Martikainen H, Rasanen J. Low-dose aspirin reduces uteroplacental vascular impedance in early and mid gestation in IVF and ICSI patients: a randomized, placebo-controlled double-blind study. Ultrasound Obstet Gynecol. 2008;32(5):687–93. https://doi.org/10.1002/uog.6215.
Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S745–61. https://doi.org/10.1016/j.ajog.2017.11.577.
Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–20 e6. https://doi.org/10.1016/j.ajog.2016.09.076.
•• Hoffman MK, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):285–93. Randomized controlled trial demonstrating benefit of aspirin for preventing of pregnancy complications and maternal mortality. https://doi.org/10.1016/S0140-6736(19)32973-3.
Verheugt FW, Bolte AC. The role of aspirin in women’s health. Int J Women's Health. 2011;3:151–66. https://doi.org/10.2147/IJWH.S18033.
• ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018;132(1):e44–52. Society recommendations for aspirin to prevent pregnancy complications. https://doi.org/10.1097/AOG.0000000000002708.
Grobman WA, Caughey AB. Elective induction of labor at 39 weeks compared with expectant management: a meta-analysis of cohort studies. Am J Obstet Gynecol. 2019;221(4):304–10. https://doi.org/10.1016/j.ajog.2019.02.046.
Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52(3):171–80. https://doi.org/10.1016/j.jacc.2008.03.049.
Yarrington CD, Valente AM, Economy KE. Cardiovascular management in pregnancy: antithrombotic agents and antiplatelet agents. Circulation. 2015;132(14):1354–64. https://doi.org/10.1161/CIRCULATIONAHA.114.003902.
American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776–89. https://doi.org/10.1542/peds.108.3.776.
Taguchi N, Rubin ET, Hosokawa A, Choi J, Ying AY, Moretti ME, et al. Prenatal exposure to HMG-CoA reductase inhibitors: effects on fetal and neonatal outcomes. Reprod Toxicol. 2008;26(2):175–7. https://doi.org/10.1016/j.reprotox.2008.06.009.
Redman CW, Kelly JG, Cooper WD. The excretion of enalapril and enalaprilat in human breast milk. Eur J Clin Pharmacol. 1990;38(1):99. https://doi.org/10.1007/BF00314815.
Hoppe KK, Williams M, Thomas N, Zella JB, Drewry A, Kim KM, et al. Telehealth with remote blood pressure monitoring for postpartum hypertension: a prospective single-cohort feasibility study. Pregnancy Hypertens. 2019;15:171–6. https://doi.org/10.1016/j.preghy.2018.12.007.
Phillips SJ, Tepper NK, Kapp N, Nanda K, Temmerman M, Curtis KM. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2016;94(3):226–52. https://doi.org/10.1016/j.contraception.2015.09.010.
Acknowledgments
AEB recognizes support from an American Heart Association Mentored and Clinical Population Research Award 17MCPRP33630098 and K23 HL146982 from the National Heart, Lung and Blood Institute (NHLBI). The authors thank Emmanuel Gee for assistance in preparing Figure 1a.
Author information
Authors and Affiliations
Contributions
PC, DW, and AEB: manuscript writing, manuscript editing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no financial relationships or conflicts of interest regarding the content herein. AEB is a site PI for trials by Abbott, Inc. and CSL-Behring for which her institution receives compensation. AEB also reports honorarium from Clearview Healthcare Partners, LLC, and S2N Healthcare, LLC outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Chavez, P., Wolfe, D. & Bortnick, A.E. Management of Ischemic Heart Disease in Pregnancy. Curr Atheroscler Rep 23, 52 (2021). https://doi.org/10.1007/s11883-021-00944-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-021-00944-1