Abstract
Purpose of Review
Establishing a diagnosis of ischemic heart disease (IHD) in women, including assessment for coronary microvascular dysfunction (CMD) when indicated, can be challenging. Access to performance of invasive testing when appropriate may be limited, and noninvasive imaging assessments have evolved. This review will summarize the various noninvasive imaging modalities available for the diagnosis of IHD and CMD in women, outlining indications, performance modalities, advantages, and limitations.
Recent Findings
While stress echocardiography and single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) are widely available and can detect IHD in women, their ability to specifically identify CMD is limited. Novel developments in cardiac magnetic resonance (CMR) imaging, including spectroscopy, and positron emission tomography (PET) have changed the diagnostic landscape. Coronary computed tomographic angiography (CCTA), while unable to diagnose CMD, is developing an emerging role in the risk stratification of ischemic syndromes.
Summary
Despite the discovery of increased CMD prevalence in symptomatic women and technological advances in diagnostic imaging, practitioners are limited by user expertise and center availability when choosing a diagnostic imaging modality. Knowledge of this evolving field is imperative as it highlights the need for sex-specific assessment of cardiovascular syndromes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
This contemporary review will summarize the current knowledge concerning noninvasive diagnostic strategies for IHD in women, with an additional focus on assessment of microvascular disease. This has current relevance in that mortality from cardiovascular diseases, which had been overall progressively declining over the last decades for both sexes has recently increased [1]. In addition, there is evidence that younger women with acute coronary syndromes have worse outcomes when compared with men [2, 3]. It has been postulated that these worsening mortality statistics and outcomes gap may be due to lifestyle-induced increased cardiovascular risk [1] compounded by sex differences in symptom presentation and pathophysiology of ischemic syndromes, inclusive of more microvascular, or “small vessel” versus “epicardial vessel” coronary disease in women as compared with men, a phenomenon which has been under-recognized [4]. Further understanding, awareness, and diagnosis of microvascular coronary disease, also termed “coronary microvascular dysfunction” (CMD), is essential in order to provide appropriate treatment and impact cardiovascular outcomes. Sex-specific aspects of IHD clinical presentations and the strengths and limitations of available and evolving diagnostic testing strategies will be reviewed. Awareness and implementation of tailored clinical diagnostic sex-specific approaches to IHD, inclusive of assessment of CMD, must occur if cardiovascular outcomes are to improve for women.
Background
Coronary Microvascular Dysfunction in the Context of Ischemic Heart Disease in Women
IHD is a term which has traditionally been used to refer to obstructive (> 50% stenosis) coronary atherosclerosis, also termed “coronary artery disease (CAD)” or “coronary heart disease (CHD).” However, recent observations characterized by sex differences in myocardial ischemic syndromes have expanded the definition of IHD to include a broader spectrum of etiologies. Myocardial infarction (MI) in the absence of obstructive coronary artery disease (MINOCA) is a recently coined term, now included in the 4th Universal Definition of MI which includes myocardial infarction resulting from nonobstructive CAD, spontaneous coronary artery dissection, coronary artery spasm, thromboembolism, and coronary microvascular dysfunction (CMD) [5••]. Women, presenting with both stable and unstable chest pain syndromes, are at least twice as likely as men to have nonobstructive CAD on coronary angiography [6, 7••], termed ANOCA (angina with no obstructive coronary arteries) or INOCA (ischemia with no obstructive coronary arteries) [8, 9].
Of concern is that this entity is not benign; such patients may have a 5-year combined fatal and nonfatal cardiovascular event rate of up to 25% [10]. Factors observed to be associated with increased morbidity and mortality in the absence of obstructive CAD are CMD, coronary atherosclerotic plaque composition (increased lipid core density, regardless of degree of calcification), and variations in arterial remodeling [11•]. There is an urgency to recognize and diagnose IHD in women, since they have higher morbidity and mortality associated with MI from any etiology as compared with men; establishing a diagnosis facilitates risk reduction and treatment [12]. With respect to factors predisposing to IHD, women not only have a unique risk profile which changes across the lifespan as related to pregnancy-related complications, ovarian disorders, and menopausal status [13, 14], but traditional risk factors have different magnitudes of burden. There is an exponential summative effect on cardiac risk with diabetes, smoking, and hypertension playing a larger role than dyslipidemia in women compared with men [15]. The differing pathophysiology of IHD in women may be postulated to be related to the different structural, physiologic, and epiphenomenal exposures women experience across their lifespan.
What Is Coronary Microvascular Dysfunction and How Does It Impact Women?
Coronary microvascular dysfunction (CMD) is a term which refers to impaired blood flow in the coronary microcirculation. In the absence of myocardial diseases, the spectrum of mechanisms includes microvascular remodeling, endothelial dysfunction, smooth muscle dysfunction, and microembolization from epicardial arteries [16]. These abnormalities, with or without abnormal vasoreactivity of epicardial arteries, result in reduced blood flow to the myocardium. While the role of CMD in the etiology of MINOCA is uncertain [5••], an established role in causation of stable ischemic chest pain is supported, with a sex preference for females [8]. One-half of the patients undergoing invasive coronary evaluation for angina have no significant CAD. Additionally, nearly 75% of these patients had CMD or vasospasm [17••]. Moreover, in one large cohort study of stable angina in the absence of obstructive CAD, more female than male patients had coronary vasomotor dysfunction defined either as epicardial artery vasospasm or CMD, 70.2% versus 43.1% (p < 0.001) [18•]. Of the 458 patients specifically with CMD, females were disproportionately represented (75% vs 25% males). It has been hypothesized that anatomical differences such as the smaller, thin walled, tortuous architecture of coronary arteries in women make them more susceptible to CMD. Further, female hormonal changes may be associated with decreased capillary density, increased remodeling, and arterial stiffness, factors which have been proposed to explain the increased prevalence of CMD in post-menopausal women [18•].
The Coronary Artery Vasospastic Disorders Summit (COVADIS) has established diagnostic criteria for vasospastic angina and microvascular angina; evidence of impaired coronary microvascular function must be proven by either (a) impaired coronary flow reserve, (b) abnormal coronary resistance indices, (c) coronary microvascular spasm, or (d) coronary slow flow phenomena [19•]. Coronary flow reserve (CFR) is defined as the ratio between coronary blood flow at maximal hyperemia and at baseline condition [20] and expresses the capacity of the coronary circulation (both epicardial and microvascular compartments) to respond to a physiological increase in oxygen demands with a corresponding increase in blood flow. In ANOCA patients, the average CFR is 2.7 + 0.6 [21]; thus, a cutoff value of 2.0 is generally accepted as the lower threshold for normal CFR. Abnormal CFR measurements can reflect focal, diffuse, and/or small vessel (microcirculatory) coronary artery disease resulting in vasomotor dysfunction and alterations in myocardial perfusion [22].
The Conundrum of Clinical Presentation and the Need for Consideration of Sex-Specific Cardiovascular Assessments
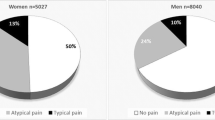
It is commonly acknowledged that women with IHD can present similarly but also differently compared with men. Historically, angina symptoms in women have thus been referred to as “atypical.” For example, women with IHD are more likely to experience dyspnea, nausea, vomiting, fatigue, malaise, back or jaw pain, and excessive fatigue, sometimes even in the absence of chest pain [23]. Misdiagnosis is amplified by poorly characterized and underdiagnosed CMD in women. This underscores the need for sex-specific cardiovascular risk stratification as an opportunity to move away from a male centric diagnosis and management model.
Findings from the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial demonstrated that, despite the fact that symptomatic women were more often than men (adjusted OR: 1.21, 95% CI: 1.01–1.44) referred for diagnostic stress imaging (echocardiography or nuclear) tests, they were less likely to have anatomically obstructive CAD compared with men (10% vs 15%; p < 0.001) [6]. This discordance raises the possibility that traditional noninvasive testing is potentially missing the diagnosis of CMD as the etiology of symptoms. Furthermore, the same study found that although women had an increased number of risk factors, multiple traditional risk stratification scoring systems (Framingham, ASCVD Pooled Cohort, Diamond-Forrester) classified them as low risk [6]. The traditional risk scores often underestimate risk as these stratification scores do not include sex-specific risk factors known to be important in women. Thus, sex not only influences risk factors and clinical presentations but also affects a patient’s course through the diagnostic algorithm for IHD assessment.
What Is the Role of Noninvasive Imaging for IHD Diagnosis in Women and What Tests Are Available?
Ischemic heart disease (IHD) in women can be assessed noninvasively by both functional and anatomic testing, and the characteristics, advantages, and disadvantages of each of these modalities will be reviewed in this section. Table 1 summarizes the noninvasive imaging techniques available for the diagnosis of obstructive coronary artery disease.
Functional Testing
Stress testing to establish the presence of IHD has traditionally focused on functional modalities for the detection of ischemia due to obstructive CAD. Widely employed modalities are exercise stress electrocardiography (ECG), exercise or pharmacologic stress echocardiography, and single-photon emission computed tomography (SPECT) exercise or pharmacologic myocardial perfusion imaging (MPI). More recently, MPI with positron emission tomography (PET) and cardiac magnetic resonance imaging (CMR) have been shown to be accurate in diagnosing ischemia due to obstructive CAD but vary in availability to clinicians; perfusion imaging is also possible with stress echocardiography when using ultrasound enhancing agents [29], but expertise in using this technique is not widespread.
A comparative meta-analysis [24] found that both the sensitivity and specificity of stress ECG were lower in women compared with men for the diagnosis of IHD. While stress ECG is not an imaging test, it is an appropriate initial test for symptomatic patients who are classified as low-intermediate risk and are able to perform adequate physical activity (treadmill or bicycle) as the stressor. The caveat is that women are more likely to have nonspecific ST segment and T-wave changes at baseline, resulting in ambiguous interpretation of an exercise ECG [11•]. Interestingly, a large cohort study of over 5000 women found that exercise stress-induced ST depression did not correlate with cardiovascular mortality or morbidity hence offering limited prognostic information [25]. A review also noted that the sensitivity for exercise stress ECG in women ranged from 31 to 71%, with specificity from 66 to 86% [26]. These limitations highlight the difficulties of relying on this diagnostic test, especially in women.
Stress Echocardiography
Stress echocardiography identifies ischemia by detecting left ventricular (LV) wall motion abnormalities induced by exercise or pharmacologic stress typically with dobutamine. In a historic meta-analysis, the sensitivity and specificity of this modality in women was 79 and 83%, respectively [27•]. In the event of poor or inadequate endocardial visualization, ultrasound enhancing “contrast” agents, comprised of microbubbles which reflect ultrasound, improve both feasibility, reproducibility, and accuracy of test performance [28, 29]. Although stress ECG has lower specificity in diagnosing obstructive CAD, it compared favorably with contrast enhanced stress echocardiography in a prospective single-center study for the prediction of major adverse events (AE) in low-intermediate risk early menopausal women [30].
Stress echocardiography has an advantage over other stress modalities, especially in the dyspneic patient, by its ability to detect valvular heart disease, pericardial or pleural effusions, and hemodynamic assessments of the change in LV filling and/or pulmonary artery pressures induced by exercise [31]. Moreover, multi-pulse ultrasound imaging techniques, specifically “very low mechanical index” imaging now standard on all echocardiographic equipment, allows for routine myocardial perfusion imaging when used with ultrasound enhancing agents during dobutamine or vasodilator stress and can provide simultaneous myocardial function and perfusion assessment [29] with enhanced diagnostic and prognostic capability [32], especially in women [33•].
Wall motion assessments for ischemia cannot differentiate between obstructive and coronary microvascular disease. Indeed it is not unusual, especially in female patients, to have a report termed “false positive stress echo” when coronary angiography fails to demonstrate obstructive CAD. However, it has been underappreciated that these “false positives stress echocardiograms” may indeed represent CMD and associated ANOCA or INOCA [34].
SPECT MPI
SPECT, in combination with either exercise or pharmacologic stress, is the conventional approach for myocardial perfusion imaging by many clinicians [35••]. A meta-analysis assessing SPECT MPI in females found that among the 1148 females and 1142 males studied, mean sensitivity was 86% and 89%, respectively, while specificity was 83% vs 71% [36]. While normal or low-risk results (< 5% ischemia or a summed stress score < 4) prognostically have a 0.3–1% chance of nonfatal MI or CAD death [37, 38], testing may not capture microvascular disease and result in a lost opportunity for treatment [39]. However, the WOMEN study evaluating chest pain symptoms in low-intermediate risk women demonstrated that stress MPI results had no prognostic advantage compared with stress ECG alone at 2 years of follow-up [40].
More recently, the CE-MARC study compared CMR and SPECT MPI evaluation of CAD and demonstrated that SPECT in women vs men had worse sensitivity (50.9% versus 70.8%; P = 0.007) but comparable specificity (84.1% versus 81.3%; P = 0.48) [41]. Since this data, new SPECT cadmium-zinc-telluride (CZT) technology has emerged with the additional advantage of lower radiation exposure [42]. A recent meta-analysis found that while it has similar sensitivity to traditional SPECT (84% vs 82–91%), it has poorer specificity (69% vs 79–90%) [43]. In another study, solid-state camera offer improved image quality with lower radiation exposure compared with conventional SPECT imaging, with similar accuracy in women (82%) and men (88%) [44]. Limitations of SPECT specific to women include photon attenuation due to dense breast tissue and the difficulty to detect perfusion differences in the smaller sized heart muscle of women [35••, 45]. Relative radiation exposure may also be higher in women, especially younger women, when compared with men [27•, 46].
Cardiac MRI
Although cardiac magnetic resonance imaging (cMRI) cannot reliably image specific coronary anatomy, it can be used to detect obstructive CAD, impaired coronary vasoreactivity, and endothelial dysfunction [47••]. First-pass contrast-enhanced cMRI can assess myocardial perfusion, and pharmacologic stress-induced wall motion abnormalities can be assessed. In women with ACS due to MINOCA, cMRI can be used to detect and further characterize myocardial edema and scarring, providing insight into potential pathophysiologic mechanisms [48].
Dobutamine cMRI stress can yield useful information about wall motion abnormalities, while vasodilators, such as adenosine, dipyridamole, and regadenoson, can identify subendocardial ischemia; failure of perfusion to increase appropriately in response to stress has been observed in INOCA subjects [49]. Stress cMRI detection of ischemia has been shown to have prognostic value in women [50, 51]. However, despite the better temporal spatial resolution of cMRI compared with PET [52], it is unclear whether this translates into improved accuracy, specificity, or sensitivity among women. Perhaps one of the most important benefits of cMRI is lack of radiation which is particularly advantageous for young women with chest pain syndromes requiring diagnostic work-up.
PET MPI
Positron emission tomography (PET) is a highly specialized nuclear noninvasive metabolic imaging technique that can evaluate left ventricular function, myocardial perfusion, and CFR. PET measures physiologic function by assessing blood flow and metabolism and can assess a spectrum of IHD etiologies in women and has been assigned class IB recommendation in the 2014 American Heart Association Recommendations on the Role of Noninvasive Testing in the Clinical Evaluation of Women with Suspected IHD [7••]. However, emerging evidence suggests that PET is a superior imaging modality; in a study comparing CCTA, SPECT, and PET, in the diagnosis of IHD, PET had the greatest diagnostic accuracy (85%) compared with CCTA (74%) and SPECT (77%); there was no incremental increase in accuracy by combining modalities [53]. However, no sex stratification was done, and the cohort was 64% men. Since women have a higher prevalence of single vessel CAD [54], and PET has a higher sensitivity (92%) for identifying single vessel disease CAD, this may be of particular benefit in women [55].
Compared with other modalities, added benefits are depth-independent attenuation correction measurements which reduce false positives, especially common in women due to breast tissue or large body mass [35••]. Moreover, improved spatial contrast resolution translates to reduced false negatives which can arise in women due to small left ventricular size [35••]. Another advantage, especially important for younger women whom are more susceptible to adverse radiation effects, is that PET offers lower radiation exposure than SPECT [27•].
PET is a validated technique to also assess for CMD. Ratio of myocardial blood flow (MBF) during hyperemia and rest is termed myocardial flow reserve (MFR). Reduction in MFR is commonly seen in patients with CMD [35••].
Anatomical Testing
Over the last decade, computed coronary tomographic angiography (CCTA) has emerged as a new option for the noninvasive performance of anatomical, rather than functional, assessment of chest pain patients. Indeed, 64-slice CCTA is the recommended first-line guideline-directed diagnostic investigation in the UK [56] followed by functional imaging as second line. An emerging feature of CCTA is the calculation of fractional flow reserve (FFR), a parameter traditionally invasively calculated using angiography to determine the likelihood that an observed stenosis is associated with a significant functional flow limitation causing myocardial ischemia. In a review that summarized 3 prospective trials examining 609 patients and 1050 vessels comparing FFR derived from CCTA versus angiography on average, the FFR CCTA sensitivity was 89% and specificity 71%; no sex differences were described [57].
Diagnostic Tools and Strategies Useful in the Diagnosis of CMD
Evaluation of IHD in women with chest pain syndromes in the absence of obstructive CAD, or other coronary anatomical explanation for symptoms, and thus presumed CMD, is a particularly challenging diagnostic and management dilemma. Of course, it must be recognized that visualization of the coronary microvasculature is beyond the limits of coronary angiographic resolution, which is limited to vessels 100 μm in diameter or greater [58]. Performance of invasive testing of coronary physiology using graded doses of acetylcholine and adenosine to assess for endothelial-dependent and endothelial-independent dysfunction, respectively, in an assessment of the components of CMD, is considered the “gold standard” for determination of CFR and CMD [8].
However, despite the presence of persistent symptoms, frequently accompanied by ECG and/or biomarker changes, most often this additional invasive coronary physiology testing is not performed in the clinical setting, due to logistical or feasibility reasons, leaving a frequently confused patient and provider with no diagnosis other than “there is no CAD,” or “your heart is normal,” and reassurance without guidance, resulting in a missed opportunity for optimization of medical and preventive therapies for a potentially missed diagnosis of CMD. At the current time, although noninvasive nonimaging techniques such as those assessing peripheral reactive hyperemia [59] are available, there are no rigorously validated nonimaging noninvasive alternatives for assessment of CMD. But there are several features of the noninvasive imaging options already discussed above which can be leveraged to assess for the presence of CMD, and these are reviewed below and summarized in Table 2.
Stress Echocardiography
Vasodilator stress echocardiography using dipyridamole, adenosine, or regadenoson enables noninvasive CFR measurement, either through direct transthoracic Doppler assessment of primarily the left anterior descending coronary artery or quantitative 2-D video densitometric assessment of myocardial contrast perfusion and assessment of myocardial blood flow reserve (MBFR) using ultrasound enhancing agents, also described as myocardial contrast echocardiography. [29, 32, 44]. However, these techniques are not currently routinely employed in North America, although CFR assessment using transthoracic Doppler Coronary Flow Velocity Reserve assessment in the left anterior descending coronary artery has been deemed readily feasible and proposed as a standard component of stress echocardiography practice in Europe [60•, 61].
SPECT MPI
The role of SPECT in CMD is investigational, and not currently clinically applicable. Most SPECT laboratories are unable to perform quantitative assessment of MBF. A recent but small study of 31 patients (only 13% women) comparing SPECT vs PET found MBF values had significant variability and large standard errors (2% ± 32%) and similar for MBR (2% ± 28%) [62]. Limitations include that larger studies need to be done for further assessment, and this technology is not widely available.
Preferred Emerging Noninvasive Techniques for CMD Evaluation
PET and cardiac magnetic resonance imaging (cMRI), and specifically magnetic resonance spectroscopy (MRS), are more recently developed techniques that are not routinely utilized as first-line diagnostic procedures for IHD diagnosis but have unique capabilities to characterize myocardial metabolism and tissue characterization, which are valuable in the differential diagnosis of IHD, unexplained by routine conventional noninvasive testing summarized above. PET can evaluate myocardial blood flow and is considered the gold standard in the noninvasive assessment of CMD. Stress cMRI and magnetic resonance spectroscopy (MRS) can determine myocardial function, perfusion, and energetics and has implications for CMD. However, due to the requirement for highly specialized experts and facilities with advanced equipment, accessibility for PET, cMRI, and MRS is not as widespread as echocardiography, nuclear perfusion imaging, and CCTA.
Stress PET MPI
PET has the ability to measure both CFR and MBF to yield information about ischemic disease that traditional imaging cannot detect. Studies have noted that a CFR < 2 carries a 3.4-fold increased risk of cardiac death and a CFR < 1 5. a 5.6-fold increase [63]. The greatest limitation is that since PET examines hyperemic myocardial blood flow of both epicardial and microvascular dysfunctions, if both are present, it is hard to discern if there is just one or both contributing to the positive result.
Cardiac MRI and MRS (Magnetic Resonance Imaging and Spectroscopy)
Myocardial blood flow measurements and CFR can be determined with cMRI, but significant variability has been observed when comparing with PET [52, 64]. Small studies of semi-quantitative perfusion analysis during adenosine stress cMRI have trended with the presence of CMD in INOCA patients [65] suggesting that CMD may be an underlying pathological substrate predisposing to MINOCA. Magnetic resonance spectroscopy (MRS) utilizes magnetic resonance signals from nuclei such as phosphorus-31 to provide information regarding the biochemical composition and metabolic state of cardiac muscle and provides insight into the role of cardiac energetics in IHD.
In a subset of women enrolled in the Women’s Ischemic Syndrome Evaluation (WISE) study, and angiographically characterized as having ANOCA, abnormal decrease in myocardial phosphocreatine-to-adenosine triphosphate ratios with hand-grip exercise was observed, similar to that seen in those with obstructive CAD [66], and, moreover, was predictive of increased IHD events (most were chest pain hospitalization) at 3 years of follow up [67]. Although this study was provocative, practical application of MRS techniques for CMD evaluation in ANOCA patients has not been observed in the ensuing decade. An advantage in contrast to PET is that cMRI and MRS techniques do not require the application of external radioactive tracers.
Coronary CTA
Although CCTA is not currently suitable for the detection of CMD, there is emerging evidence regarding assessment of plaque characteristics to determine and prognosticate associated atherosclerotic disease vulnerability, which could enhance functional assessment of nonobstructive CAD. In a secondary data analysis of 472 of 1000 total patients, including 47% women, presenting with acute coronary syndrome (ACS) symptoms and randomized to the coronary CTA arm of the ROMICAT-II trial, high-risk nonobstructive plaque characteristics (positive remodeling, low < 30 Hounsfield units plaque, napkin-ring sign, spotty calcium) on CCTA were demonstrated to predict ACS (odds ratio [OR] = 8.9; 95% confidence interval 1.8–43.3) [68]. More recently, a secondary pre-specified nested observational cohort analysis of the PROMISE trial, including 52% women, found that there was a greater correlation of MACE in women with high-risk plaque on CCTA than in men (adjusted hazard ratio, aHR 2.41 vs 1.4, respectively) and in younger patients than older (aHR 2.33 vs 1.36, respectively) [69•]. This technique may have particular relevance for improving diagnosis in women with angina symptomatology and nonobstructive IHD, especially those who are younger and have increased diagnostic challenges and poorer prognosis.
Summary Illustration: (Fig. 1)
Assessment of IHD (Obstructive and Nonobstructive) in Women: Role of Different Modalities
The figure displays the comprehensive spectrum of different modalities for assessment of both obstructive and nonobstructive coronary artery disease in women. The invasive tests are circled in yellow. Abbreviations: CAD, coronary artery disease; CFR, coronary flow reserve; CT, computed tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; MPI, myocardial perfusion imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography
Conclusions
The noninvasive evaluation of at-risk women with stable symptoms of cardiac ischemia is a rapidly evolving field, and we are still learning more about their unique physiology, presentations, and outcomes. Recent advances have occurred in the understanding of ischemic heart disease in women, including expansion of the spectrum to include both obstructive and nonobstructive disease, the latter primarily represented by microvascular disease; Fig. 1 summarizes the different available imaging modalities and their position in the spectrum of IHD diagnosis in women. Developments in noninvasive diagnostic and prognostic tools may improve cardiovascular outcomes in women by providing increasingly accurate diagnosis and prediction of future major adverse cardiac events. A noninvasive testing strategy using a stepwise approach may be followed (Fig. 2), with newer modalities such as PET and CMR utilized when our conventional stress testing or anatomic imaging methods do not provide answers in women with significant risk factors and ongoing symptoms, especially if they persist despite a trial of guideline-directed risk-reduction and symptom-relief therapies.
Suggested Diagnostic Work-Up for A Woman with Suspected Stable IHD. The image encompasses a comprehensive diagnostic algorithm for a woman with suspected stable IHD (chest (and/or other upper body) pain, dyspnea, and/or excessive fatigue on exertion). Imaging for obstructive epicardial coronary artery disease is depicted in orange boxes and for nonobstructive IHD is in purple boxes; management options are depicted in yellow boxes. *If resting ECG is normal, woman is able to perform > 5 METS; otherwise, proceed to “Intermediate” pathway, and if a functional option is chosen, perform pharmacologic rather than exercise stress testing. IHD, ischemic heart disease; ECG, electrocardiogram; Echo, echocardiogram; SPECT, single-photon emission tomography; MPI, myocardial perfusion imaging; CMR, cardiac magnetic resonance imaging; CCTA, coronary computed tomographic angiography; CMD, coronary microvascular dysfunction; OMT, optimal medical therapy; Revasc, coronary revascularization; CFR, coronary flow reserve; MFR, myocardial flow reserve; Ach, acetylcholine; NTG, nitroglycerin
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 Update: a report from the American Heart Association. Vol 141; 2020. https://doi.org/10.1161/CIR.0000000000000757.
Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CR, Kopec J, et al. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Women's Health. 2014;23(1):10–7. https://doi.org/10.1089/jwh.2013.4507.
Mehta LS, Beckie TM, DeVon HA, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Vol 133; 2016. https://doi.org/10.1161/CIR.0000000000000351.
Norris CM, Yip CYY, Nerenberg KA, Clavel MA, Pacheco C, Foulds HJA, et al. State of the science in women’s cardiovascular disease: a Canadian perspective on the influence of sex and gender. J Am Heart Assoc. 2020;9(4):1–18. https://doi.org/10.1161/JAHA.119.015634.
•• Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):E891–908. https://doi.org/10.1161/CIR.0000000000000670A contemporary statement that defines MINOCA (myocardial infarction without obstructive disease) and provides a comprehensive framework and algorithm for diagnostic evaluation and management of these patients.
Hemal K, Pagidipati NJ, Coles A, Dolor RJ, Mark DB, Pellikka PA, et al. Sex differences in demographics, risk factors, presentation, and noninvasive testing in stable outpatients with suspected coronary artery disease insights from the PROMISE trial. JACC Cardiovasc Imaging. 2016;9(4):337–46. https://doi.org/10.1016/j.jcmg.2016.02.001.
•• Mieres JH, Gulati M, Merz NB, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease a consensus statement from the American Heart Association. Circulation. 2014;130:350–79. https://doi.org/10.1161/CIR.0000000000000061This consensus statement by the American Heart Association, is the only clinical statement specific to women with ischemic heart disease, and guides the reader through various imaging tests and recommended algorithms in female patients with anginal symptoms covering the gamut of obstructive and nonobstructive disease.
Noel Bairey Merz C, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–92. https://doi.org/10.1161/CIRCULATIONAHA.116.024534.
Pacheco Claudio C, Quesada O, Pepine CJ, Noel Bairey Merz C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol 2018;41(2):185–193. doi:https://doi.org/10.1002/clc.22894.
Gulati M, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease. Arch Intern Med. 2009;169(9):843–50.
• Baldassarre LA, Raman SV, Min JK, et al. Noninvasive imaging to evaluate women with stable ischemic heart disease. JACC Cardiovasc Imaging. 2017;9(4):421–35. https://doi.org/10.1016/j.jcmg.2016.01.004.NoninvasiveAn inclusive paper summarizing the importance of sex-specific tagrets in non-invasive imaging of CAD as well as anatomical and functional differences between modalities.
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das S. Heart disease and stroke statistics — 2017 update a report from the American Heart Association. Circulation. 2017;135:146–603. https://doi.org/10.1161/CIR.0000000000000485.
Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol. 2018;41(2):239–46. https://doi.org/10.1002/clc.22887.
Maffei S, Guiducci L, Cugusi L, Cadeddu C, Deidda M, Gallina S, et al. Women-specific predictors of cardiovascular disease risk - new paradigms. Int J Cardiol. 2019;286:190–7. https://doi.org/10.1016/j.ijcard.2019.02.005.
Young L, Cho L. Unique cardiovascular risk factors in women. Heart. 2019;105:1656–60. https://doi.org/10.1136/heartjnl-2018-314268.
Camici PG, Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. https://doi.org/10.1038/nrcardio.2014.160.
•• Ford TJ, Yii E, Sidik N, et al. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv. 2019;12(12). https://doi.org/10.1161/CIRCINTERVENTIONS.119.008126This multicenter study encompassing 391 patients with angina, demonstrated patients without obstructive disease (47%) had similar angina burden, were more likely to be female, and often had microvascular and vasospastic angina, and had reduced quality of life compared to those with obstructive disease.
• Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. JACC. 2017;70(19):2349–58. https://doi.org/10.1016/j.jacc.2017.09.016Using an ACH test to assess for vasospasm or coronary microvascular dysfunction (CMD) in patients with stable non-obstructive angina, this retrospecive study of 1379 patients identifies a higher prevalence of vasomotor dysfunction (especially CMD) in females compared with male patients.
• Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. https://doi.org/10.1016/j.ijcard.2017.08.068This landmark paper which emerged from Coronary Vasomotion Disorders International Study Group (COVADIS) Summits held in August 2014, establishes an international diagnostic criteria for ischemic symptoms due to coronary microvascular dysfunction (CMD).
Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34(1):48–55. https://doi.org/10.1016/0002-9149(74)90092-7.
Kern MJ, Bach RG, Mechem CJ, Caracciolo EA, Aguirre FV, Miller LW, et al. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28(5):1154–60. https://doi.org/10.1016/S0735-1097(96)00327-0.
Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2(8):1009–23. https://doi.org/10.1016/j.jcmg.2009.06.004.
Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307(8):813–22.
Kwok Y, Kim C, Grady D, Segal M, Redberg R. Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol. 1999;83(5):660–6. https://doi.org/10.1016/S0002-9149(98)00963-1.
Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation. 2003;108(13):1554–9. https://doi.org/10.1161/01.CIR.0000091080.57509.E9.
Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation. 2010;122(24):2570–80. https://doi.org/10.1161/CIRCULATIONAHA.109.914754.
• Garuba HA, Erthal F, Stadnick E, Alzahrani A, Chow B, Beanlands RS. Optimizing risk stratification and noninvasive diagnosis of ischemic heart disease in women. Can J Cardiol. 2018;34(4):400–12. https://doi.org/10.1016/j.cjca.2018.01.026A comprehensive summary that highlights sex specific differences in pathophysiology, risk factors, presentation of CAD along with diagnostic modalities that can help risk stratify women.
Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, et al. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21(11):1179–201. https://doi.org/10.1016/j.echo.2008.09.009.
Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018;31(3):241–74. https://doi.org/10.1016/j.echo.2017.11.013.
Abdelmoneim SS, Ball CA, Mantovani F, Hagen ME, Eifert-Rain S, Wilansky S, et al. Prognostic utility of stress testing and cardiac biomarkers in menopausal women at low to intermediate risk for coronary artery disease (SMART study): 5-year outcome. J Women's Health. 2018;27(5):542–51. https://doi.org/10.1089/jwh.2017.6506.
Hardegree EL, Sachdev A, Fenstad ER, Villarraga HR, Frantz RP, McGoon MD, et al. Impaired left ventricular mechanics in pulmonary arterial hypertension identification of a cohort at high risk. Circ Heart Fail. 2013;6(4):748–55. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000098.
Laiq Z, Smith LM, Xie F, Chamsi-Pasha M, Porter TR. Differences in patient outcomes after conventional versus real time perfusion stress echocardiography in men versus women: a prospective randomised trial. Heart. 2015;101(7):559–64. https://doi.org/10.1136/heartjnl-2014-306869.
• Kutty S, Bisselou Moukagna KS, Craft M, Shostrom V, Xie F, Porter TR. Clinical outcome of patients with inducible capillary blood flow abnormalities during demand stress in the presence or absence of angiographic coronary disease. Circ Cardiovasc Imaging. 2018;11(10):e007483. https://doi.org/10.1161/CIRCIMAGING.117.007483This study concludes that patients who have capillary blood flow abnormalities with non-obstructive CAD (majority women) have similar death/ nonfatal myocardial infarction rates compared to those with significant obstructive CAD.
From AM, Kane G, Bruce C, Pellikka PA, Scott C, McCully RB. Characteristics and outcomes of patients with abnormal stress echocardiograms and angiographically mild coronary artery disease (<50% stenoses) or normal coronary arteries. J Am Soc Echocardiogr. 2010;23(2):207–14. https://doi.org/10.1016/j.echo.2009.11.023.
•• Taqueti VR, Dorbala S, Wolinsky D, et al. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease—state-of-the-evidence and clinical recommendations. J Nucl Cardiol. 2017;24(4):1402–26. https://doi.org/10.1007/s12350-017-0926-8A consensus document by the American Society of Nuclear Cardiology on the evidence base of stress myocardial perfusion imaging, and addition of PET based coronary flow imaging for optimal risk stratification of women with stable ischemic heart disease.
Iskandar A, Limone B, Parker MW, Perugini A, Kim H, Jones C, et al. Gender differences in the diagnostic accuracy of SPECT myocardial perfusion imaging: a bivariate meta-analysis. J Nucl Cardiol. 2013;20(1):53–63. https://doi.org/10.1007/s12350-012-9646-2.
Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11(2):171–85. https://doi.org/10.1016/j.nuclcard.2003.12.004.
Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography. A Meta-Analysis. J Am Coll Cardiol. 2007;49(2):227–37. https://doi.org/10.1016/j.jacc.2006.08.048.
Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2(3):237–44. https://doi.org/10.1161/CIRCINTERVENTIONS.108.841056.
Shaw LJ, Mieres JH, Hendel RH, Boden WE, Gulati M, Veledar E, et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the what is the optimal method for ischemia evaluation. Circulation. 2011;124(11):1239–49. https://doi.org/10.1161/CIRCULATIONAHA.111.029660.
Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the clinical evaluation of magnetic resonance imaging in coronary heart disease (CE-MARC) trial. Circulation. 2014;129(10):1129–38. https://doi.org/10.1161/CIRCULATIONAHA.112.000071.
Phillips LM. CZT-SPECT: reaching its potential. JACC Cardiovasc Imaging. 2017;10(7):795–6. https://doi.org/10.1016/j.jcmg.2017.02.003.
Nudi F, Iskandrian AE, Schillaci O, Peruzzi M, Frati G, Biondi-Zoccai G. Diagnostic accuracy of myocardial perfusion imaging with CZT technology: systemic review and meta-analysis of comparison with invasive coronary angiography. JACC Cardiovasc Imaging. 2017;10(7):787–94. https://doi.org/10.1016/j.jcmg.2016.10.023.
Gimelli A, Bottai M, Quaranta A, Giorgetti A, Genovesi D, Marzullo P. Gender differences in the evaluation of coronary artery disease with a cadmium-zinc telluride camera. Eur J Nucl Med Mol Imaging. 2013;40(10):1542–8. https://doi.org/10.1007/s00259-013-2449-0.
Gulati M, Shaw LJ, Merz CNB. Myocardial ischemia in women: lessons from the NHLBI WISE study. Clin Cardiol. 2012;35(3):141–8. https://doi.org/10.1002/clc.21966.
DeKemp RA, Wells RG, Beanlands RS. Women image wisely the 3 mSv challenge for nuclear cardiology. JACC Cardiovasc Imaging. 2016;9(4):385–7. https://doi.org/10.1016/j.jcmg.2016.02.018.
•• Aggarwal NR, Bond RM, Mieres JH. The role of imaging in women with ischemic heart disease. Clin Cardiol. 2018;41(2):194–202. https://doi.org/10.1002/clc.22913. An excellent review that summarizes different imaging modalities across the full spectrum of ischemic heart disease in women, including coronary atherosclerosis and microvascular disease.
Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124(13):1414–25. https://doi.org/10.1161/CIRCULATIONAHA.111.026542.
Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51(4):466–72. https://doi.org/10.1016/j.jacc.2007.08.060.
Coelho-Filho OR, Seabra LF, Mongeon FP, Abdullah SM, Francis SA, Blankstein R, et al. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. JACC Cardiovasc Imaging. 2011;4(8):850–61. https://doi.org/10.1016/j.jcmg.2011.04.015.
Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored women’s ischemia syndrome evaluation (WISE). Circulation. 2004;109(24):2993–9. https://doi.org/10.1161/01.CIR.0000130642.79868.B2.
Morton G, Chiribiri A, Ishida M, Hussain ST, Schuster A, Indermuehle A, et al. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography. J Am Coll Cardiol. 2012;60(16):1546–55. https://doi.org/10.1016/j.jacc.2012.05.052.
Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2017;2(10):1100–7. https://doi.org/10.1001/jamacardio.2017.2471.
Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-sponsored women’s ischemia syndrome evaluation (WISE) study. J Am Coll Cardiol. 2006;47(3 SUPPL):S36–43. https://doi.org/10.1016/j.jacc.2005.03.080.
Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49(10):1052–8. https://doi.org/10.1016/j.jacc.2006.12.015.
Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96(12):974–8. https://doi.org/10.1136/hrt.2009.190066.
Xu R, Li C, Qian J, Ge J. Computed tomography-derived fractional flow reserve in the detection of lesion-specific ischemia an integrated analysis of 3 pivotal trials. Medicine (Baltimore). 2015;94(46):1–8. https://doi.org/10.1097/MD.0000000000001963.
Crea F, Camici PG, Noel C, Merz B. Clinical update coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11. https://doi.org/10.1093/eurheartj/eht513.
Michelsen MM, Mygind ND, Pena A, Aziz A, Frestad D, Høst N, et al. Peripheral reactive hyperemia index and coronary microvascular function in women with no obstructive CAD the iPOWER study. JACC Cardiovasc Imaging. 2016;9(4):411–7. https://doi.org/10.1016/j.jcmg.2016.02.005.
• Ciampi Q, Zagatina A, Cortigiani L, et al. Functional, anatomical, and prognostic correlates of coronary flow velocity reserve during stress echocardiography. J Am Coll Cardiol. 2019;74(18):2278–91. https://doi.org/10.1016/j.jacc.2019.08.1046A prospective, multi-center study of 3410 patients determines that coronary flow velocity reserve obtained from stress echo, shows independent value over regional wall motion abnormality when predicting adverse cardiovascular outcomes.
Mulvagh SL, Mokhtar AT. Coronary flow velocity reserve in stress echocardiography: time to go with the global flow? J Am Coll Cardiol. 2019;74(18):2292–4. https://doi.org/10.1016/j.jacc.2019.08.1042.
Wells RG, Marvin B, Poirier M, Renaud J, DeKemp RA, Ruddy TD. Optimization of SPECT measurement of myocardial blood flow with corrections for attenuation, motion, and blood binding compared with PET. J Nucl Med. 2017;58(12):2013–9. https://doi.org/10.2967/jnumed.117.191049.
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–24. https://doi.org/10.1161/CIRCULATIONAHA.111.050427.
Jerosch-Herold M, Vazquez G, Wang L, Jacobs DR, Folsom AR. Variability of myocardial blood flow measurements by magnetic resonance imaging in the multi-ethnic study of atherosclerosis. Investig Radiol. 2008;43(3):155–61. https://doi.org/10.1097/RLI.0b013e31815abebd.
Mauricio R, Srichai MB, Axel L, Hochman JS, Reynolds HR. Stress cardiac MRI in women with myocardial infarction and nonobstructive coronary artery disease. Clin Cardiol. 2016;39(10):596–602. https://doi.org/10.1002/clc.22571.
Buchthal SD, Den Hollander JA, Merz CNB, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342(12):829–35. https://doi.org/10.1056/NEJM200003233421201.
Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored women’s ischemia syndrome evaluation (WISE). Circulation. 2004;109(24):2993–9. https://doi.org/10.1161/01.CIR.0000130642.79868.B2.
Puchner DKK, Birch LL. High-risk plaque detected on coronary computed tomography angiography predicts acute coronary syndrome independent of significant stenosis in patients with acute chest pain – results from ROMICAT II trial. J Am Coll Cardiol. 2014;64(12):2391–404. https://doi.org/10.1038/jid.2014.371.
• Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the promise randomized clinical trial. JAMA Cardiol. 2018;3(2):144–52. https://doi.org/10.1001/jamacardio.2017.4973This study highlights that high risk plaque identified by coronary CTA is associated with greater MACE in patients with stable chest pain especially in those with nonobstructive coronary artery disease, younger patients, and women.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sharon L. Mulvagh reports personal fees from Lantheus Medical Imaging and Novo Nordisk outside the submitted work. Priya Koilpillai and Niti R. Aggarwal declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Koilpillai, P., Aggarwal, N.R. & Mulvagh, S.L. State of the Art in Noninvasive Imaging of Ischemic Heart Disease and Coronary Microvascular Dysfunction in Women: Indications, Performance, and Limitations. Curr Atheroscler Rep 22, 73 (2020). https://doi.org/10.1007/s11883-020-00894-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-020-00894-0