Abstract
Purpose of Review
Omega-3 fatty acid (O3FA) supplementation has shown conflicting evidence regarding its benefit in cardiovascular events. We performed a pairwise and network meta-analysis to elucidate the benefit of different doses of O3FA supplementation in cardiovascular prevention.
Recent Findings
Fourteen studies were identified providing data on 125,763 patients. A prespecified cut-off value of < 1 g per day was set for low-dose (LD) O3FA and > 1 g per day for high-dose (HD) O3FA. The efficacy outcomes of interest were total death, cardiac death, sudden cardiac death, myocardial infarction, stroke, coronary revascularization, unstable angina, and major vascular events. Safety outcomes of interest were bleeding, gastrointestinal disturbances, and atrial fibrillation events. HD treatment was associated with a lower risk of cardiac death (IRR 0.79, 95% CI [0.65–0.96], p = 0.03 versus control), myocardial infarction (0.71 [0.62–0.82], p < 0.0001 versus control and 0.79 [0.67–0.92], p = 0.003 versus LD), coronary revascularization (0.74 [0.66–0.83], p < 0.0001 versus control and 0.74 [0.66–0.84], p < 0.0001 versus LD), unstable angina (0.73 [0.62–0.86], p = 0.0001 versus control and 0.74 [0.62–0.89], p = 0.002 versus LD), and major vascular events (0.78 [0.71–0.85], p < 0.0001 versus control and 0.79 [0.72–0.88], p < 0.0001 versus LD). HD treatment was associated with increased risk for bleeding events (1.49 [1.2–1.84], p = 0.0002 versus control and 1.63 [1.16–2.3], p = 0.005 versus LD) and increased atrial fibrillation events compared to control (1.35 [1.1–1.66], p = 0.004).
Summary
HD O3FA treatment was associated with lower cardiovascular events compared to LD and to control, but increased risk for bleeding and atrial fibrillation events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) represents the leading cause of death worldwide, accounting for about one-third of all global deaths with significant healthcare-related costs [1]. Previous observational studies reported beneficial effects in lowering CVD risk with an adequate dietary intake or dietary supplementation of marine-derived omega 3 polyunsaturated fatty acids (O3FA), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [2].
The exact cardiovascular protective mechanism of O3FA remains largely unknown but may be related to their multiple favorable effects on triglycerides and blood pressure, as well as their anti-inflammatory, anti-arrhythmic, and anti-platelet effects [3,4,5].
Recent evidence from randomized controlled trials (RCTs) evaluating different doses and forms of O3FA supplementation have yielded conflicting results in lowering risk of adverse cardiovascular events across both primary and secondary prevention populations. It is, therefore, unclear whether the variable results of previous RCTs are due to the populations studied or differences in O3FA dosing and/or composition of DHA and EPA. Since the current evidence shows conflicting data supporting the systematic use of O3FA supplementation to reduce CVD risk, we sought to perform a systematic review and network meta-analysis to elucidate the benefit of different doses of O3FA supplementation therapy in the setting of primary and secondary cardiovascular prevention.
Materials and Methods
This systematic review and meta-analysis are in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) and were registered within the PROSPERO International Prospective Register of Systematic Reviews (CRD42019126434). Guidance for statistical method selection can be found in the network meta-analysis book [6]. The study design is published elsewhere [7•].
Data Sources and Searches
The literature search was conducted by a medical librarian for the concepts of omega 3 fatty acid and specific cardiovascular outcomes. Search strategies contained a combination of relevant controlled vocabulary and keywords and were executed in MEDLINE (PubMed, 1946–2019), Embase (Ovid, 1947–2019), the Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrials.gov. All searches were completed in February 2019 with results limited to the English language using database-supplied filters. A validated search hedge for randomized controlled trials was added to the MEDLINE and Embase search strategies [8]. After duplication removal, a search of the four databases returned a total of 6343 results.
All references were imported into Mendeley, the citation management program.
Studies that met inclusion criteria were selected for further appraisal.
Study Selection
Study abstracts were screened for established inclusion and exclusion criteria. Studies believed to be relevant to our search were downloaded and the full manuscripts reviewed. The cited articles of the reviewed manuscripts were assessed for studies not previously identified from the initial database search.
We included articles that satisfied the following inclusion criteria: RCTs comparing the use of O3FA supplementation and containing EPA alone, or EPA plus DHA, versus control; follow-up beyond 1 year of treatment; sample size of 500 participants or more; published results of cardiovascular outcomes; English language.
Outcomes and Definitions
Main efficacy outcomes of interest included total death, cardiac death, sudden cardiac death, myocardial infarction (fatal and non-fatal), stroke (ischemic and hemorrhagic), coronary revascularization (coronary artery bypass graft or percutaneous coronary intervention), unstable angina and major vascular events (a composite of first occurrence of nonfatal MI or cardiac death; nonfatal or fatal stroke; or any revascularization procedure). Safety outcomes of interest included bleeding events, gastrointestinal disturbances (i.e., diarrhea, constipation, nausea, and gastroesophageal reflux), and atrial fibrillation.
Data Extraction and Quality Assessment
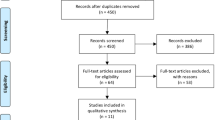
Two investigators (ML and JGC) independently reviewed study titles and abstracts, and only articles that satisfied the inclusion criteria were retrieved for full text evaluation. Discrepancies regarding data incorporation to the database were resolved through consensus among the authors. We extracted the following data from each selected study: number of participants, demographics, procedure strategies, dose of O3FA studied, and cardiovascular clinical outcomes of interest. Furthermore, we appraised the studies according to the Risk of Bias Assessment Tool version 2 (RoB 2) that was recommended by the Cochrane Collaboration [9]. See Fig. 1 for appraisal synthesis.
Data Synthesis and Analysis
For inferential purposes, frequentist fixed-effect network meta-analysis was used to estimate the incidence rate ratio (IRR) for incidence of cardiovascular clinical outcomes. A random-effect analysis was conducted when heterogeneity was detected between studies. Heterogeneity values are reported as a percentage in the online supplemental material.
Descriptive statistics on baseline characteristics of the patients in the studies are provided. Dichotomous variables were reported as counts and percentage, and continuous variables as mean ± standard deviation, or as median ± IQR (interquartile range) if the values were not normally distributed. All p values reported are two-sided and all confidence intervals are calculated at the 95% level. The pairwise and network meta-analysis was performed with the R statistical software (R project for statistical computing 4.0.0 version) using R packages “meta”, “netmeta” and “BUGSnet”.
Heterogeneity across studies was assessed with Cochran’s Q method. I2 testing was also performed to evaluate the magnitude of the heterogeneity between studies which was considered substantial when it was > 50%. The presence of publication bias for small study effect appraisal was assessed by visual examination of Funnel plots and was quantified by the Egger’s test.
Network Meta-analysis
A frequentist network meta-analysis was performed. This methodology is an extension of traditional pairwise meta-analysis and enables multiple comparisons with direct or indirect data, comparing the incidence of adverse outcomes between different types of bifurcation treatments.
Outcomes were pooled using the fixed effects model reporting incidence rate ratio (IRR) with corresponding 95% confidence interval (95% CI). Ranking probabilities with respect to each clinical outcome were obtained by using the surface under the cumulative ranking curve (SUCRA) and probability scores (P-scores) in order to identify the best-to-worst treatment, taking into account precision and accuracy of effect.
Results
Literature Search and Study Characteristics
Our initial search retrieved 6231 titles and after removal of duplicated abstracts, a total of 112 full-text articles were identified for detailed assessment (Online Fig. 1 and Online Table 1); 14 studies were ultimately included in our systematic review and meta-analysis. Our data summary and synthesis included 125,763 patients who were predominantly male (60%) with a mean age of 64.8 years. Characteristics of the included studies are presented in Table 1.
All studies were published between 1999 and 2019 [10•, 11,12,13,14,15, 16•, 17,18,19,20,21,22,23].
They were all randomized clinical trials, which evaluated marine-derived high dose (HD; > 1 g/day) or low dose (LD; ≤ 1 g/day) O3FA (EPA or EPA + DHA) supplementation vs control. The control comparison arms included mineral oil (n = 1), corn oil (n = 1), olive oil (n = 4), gelatin capsule (n = 2), or margarine (n = 1), unspecified placebo (n = 5). The median follow-up duration was 4.6 years. The majority of patients were receiving statins (78.6%) at baseline. Of the 14 included trials, three were in the setting of primary CVD prevention, eight secondary preventions, and three included both primary and secondary prevention.
Network Meta-analysis
Statistical inconsistency and heterogeneity were not significant among the main outcomes of interest (all I2 < 50% and p values > 0.05), except for gastrointestinal disturbance (I2 = 95.7%, p < 0.0001). The comparison-adjusted funnel plots, the ranking probabilities, SUCRA, and P-scores are shown in supplementary material (Online Tables 2–19 and Online Fig. 3–10).
Efficacy Endpoints
In the IRR analysis, HD O3FA supplementation yielded a significant reduction in cardiac death (IRR 0.79, 95% CI [0.65–0.96], p = 0.02 HD versus control; IRR 0.92, 95% CI [0.87–0.98], p = 0.009 LD versus control), myocardial infarction (IRR 0.71, 95% CI [0.62–0.82], p < 0.0001 HD versus control; IRR 0.79, 95% CI [0.67–0.92], p = 0.003 HD versus LD; and IRR 0.91, 95% CI [0.84–0.98], p = 0.01 LD versus control), coronary revascularization (IRR 0.74, 95% CI [0.66–0.83], p < 0.0001 versus control and IRR 0.74, 95% CI [0.66–0.84], p < 0.0001 HD versus LD), unstable angina (IRR 0.73, 95% CI [0.62–0.86], p = 0.0001 versus control and IRR 0.74, 95% CI [0.62–0.89], p = 0.002 HD versus LD), and major vascular events (IRR 0.78, 95% CI [0.71–0.85], p < 0.0001 versus control and IRR 0.79, 95% CI [0.72–0.88], p < 0.0001 HD versus LD).
This network meta-analysis also showed no associations with total death, sudden cardiac death, or stroke when compared to control (IRR 0.95, 95% CI [0.85–1.06], p = 0.38 regarding total death, IRR 0.83, 95% CI [0.67–1.02], p = 0.08 HD versus control regarding sudden cardiac death and IRR 0.89, 95% CI [0.76–1.05], p = 0.18 regarding stroke). Furthermore, we found no benefit of LD treatment compared to HD in any of the efficacy endpoints (Fig. 2).
Safety Endpoints
HD treatment was associated with increased risk for bleeding events (IRR 1.49, 95% CI [1.2–1.84], p = 0.0002 versus control and IRR 1.63, 95% CI [1.16–2.3], p = 0.005 versus LD). LD treatment was not statistically different compared to control regarding this endpoint. Furthermore, HD treatment was associated with increased risk of atrial fibrillation events compared to control (IRR 1.35, 95% CI [1.1–1.66], p = 0.004), but there were not any statistical difference compared to LD (IRR 1.23, 95% CI [0.97–1.56], p = 0.09). Due to the low number of events reported in the included trials, and with high inconsistency among them, gastrointestinal disturbances were not statistically different between O3FA and control (Fig. 3, Online Table 20–27 and Online Fig. 5–6).
Sensitivity Analysis
We explored whether the type of control could have influenced the analysis. We found no significant differences regarding cardiac death outcome between olive oil-based controls and other controls. We found, however, that the difference between omega-3 and controls was smaller, and not significant in those studies using an olive oil-based control, and larger, and significant, in those using other controls. This may suggest that olive oil is not an inert control (Online Table 28–29 and Online Fig. 7–12).
Pairwise Meta-analysis
We found no associations regarding total death, coronary revascularization, and risk of stroke between the O3FA group and control (IRR 0.98, 95% CI [0.94–1.02], p = 0.25; IRR 0.93, 95% CI [0.83–1.02], p = 0.13; and IRR 1.02, 95% CI [0.94–1.10], p = 0.69, respectively). In contrast, treatment with O3FA was associated with a lower risk of cardiac death (IRR 0.91, 95% CI [0.86–0.96], p = 0.001), sudden cardiac death (IRR 0.88, 95% CI [0.79–0.98], p = 0.02), and major vascular event (IRR 0.94, 95% CI [0.89–0.99], p = 0.02), myocardial infarction (IRR 0.86, 95% CI [0.77–0.95], p = 0.004), and unstable angina (IRR 0.84, 95% CI [0.71–0.99], p = 0.05) compared to control group.
Gastrointestinal disturbances and atrial fibrillation were associated with a higher risk in O3FA group compared with control (IRR 1.42, 95% CI [1.02–1.97], p = 0.04; IRR 1.28, 95% CI [1.12–1.46], p = 0.0003). On the contrary, no differences were found in terms of bleeding risk between omega 3 and control group (IRR 1.28, 95% CI [0.94–1.75], p = 0.12) (Online Fig. 13–16).
Discussion
Cardiovascular disease remains the most common cause of death worldwide despite improvements in preventive and therapeutic strategies. In recent decades, statins have become the mainstay of lipid-lowering therapy to reduce risk of cardiovascular events in patients with atherosclerotic CVD by primarily targeting low-density lipoprotein cholesterol (LDL-C), with a risk reduction effect proportional to LDL-C levels achieved [24, 25]. However, residual risk remains high in statin-treated patients [24,25,26,27]. One successful strategy is to further reduce LDL-C using select non-statins, such as ezetimibe and the proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors [28]. Another strategy is to reduce elevated triglyceride levels, which are frequently observed among patients who are obese, have metabolic syndrome, or diabetes, and are associated with increased CV risk [24, 25]. In this context, O3FA have emerged as an appealing treatment to improve cardiovascular outcomes since O3FA reduce triglyceride levels; yet, conflicting RCT data has constrained implementation. Of course, O3FA have other favorable CV effects (e.g., anti-arrhythmic, anti-platelet) that may support their use regardless of the patient’s TG levels [29].
Recently, ASCEND [12] and VITAL [13] evaluated the effects of LD O3FA (1 g/day) in primary prevention populations, but this strategy did not reduce major adverse cardiac events, possibly due to the LD and/or the formulation of EPA/DHA used. However, in the exploratory analysis of ASCEND trial and VITAL trial, there were, respectively, fewer vascular deaths and myocardial infarction events in the O3FA group than in the control group [12, 13]. These trials did not address the utility of LD O3FA in patients with established CV disease, patients were not required to be on statin therapy at baseline, and there were no TG level criteria for study entry. Contrarily, REDUCE-IT [10•, 26, 27] evaluated HD O3FA (4 g/day) in patients with established CV disease or diabetes mellitus and at least one additional CV risk factor and showed a significant benefit of HD O3FA supplementation on reducing major cardiovascular events and CV death. Notably, the trial investigators reported that these beneficial effects were independent of achieved TG levels at study end, providing further evidence that the biological effects of O3FA extend beyond TG lowering [10•].
We hypothesized that an insufficient dose of O3FA could have explained, at least in part, the conflicting results in the literature regarding the ischemic outcomes. For this reason, we used a prespecified cut-off value (i.e., > or < 1 g) to differentiate the minimal adequate O3FA supplementation for cardiovascular protection. Using the network meta-analysis frame, we compared different O3FA dosing versus control, to elucidate which dose may have yielded the best balance between ischemic and safety events. We found that a higher O3FA dose (> 1 g/day) was associated with a reduction in the ischemic events—cardiac death, myocardial infarction, coronary revascularization, unstable angina, and major vascular events—when compared with control, at the expense of an increased risk of bleeding and atrial fibrillation events. However, we found an increased bleeding risk for HD vs control and HD vs LD group with a lower IRR when compared HD to control group, and these observed disparities could be due to “play of chance” rather than real differences. While O3FA can certainly affect hemostatic pathways due to their antiplatelet effects, several studies published across many medical disciplines have yielded conflicting evidence on the risk of clinically significant bleeding events with O3FA supplementation and remains to be determined if their overall safety is affected by the concomitant use of antiplatelet and/or anticoagulation medications [30]. Furthermore, in our network meta-analysis, we found that the treatment with O3FA HD was associated with an increased risk of atrial fibrillation events. It is worth noting that these findings should be causally interpreted since that none of the included RCTs were designed to evaluate whether O3FA contributed to atrial fibrillation adverse events.
Reassuringly, in the O3FA groups, there was no increase in stroke development, which is the most important and debilitating complication of atrial fibrillation.
The omega-3 index (O3I) has been proposed as marker of erythrocyte membrane EPA plus DHA content, with a O3I of 8% considered to be protective against fatal CV events [5].
A predictive model was built by Walker et al. who found that approximately 2 g/daily of O3FA would likely be required in order to increase the O3I index to 8%. This data support our findings regarding the beneficial role on CV outcomes of a HD (> 1 g/day) of O3FA compared to LD and to control [31•]. Our results are also consistent with the results of a recent meta-analysis which found a linear dose relationship for total CV events and O3FA dosing. Particularly, the authors reported a stronger benefit after the inclusion in their analysis of the REDUCE-IT trial which evaluated higher doses of O3FA (4 g/day) for cardiovascular prevention [32].
Population selection is of critical importance when accounting for benefit from O3FA treatment. Even though outcome definition may differ among trials included in our analysis, we found no heterogeneity or inconsistency in our review—with the exception of gastrointestinal disturbances—supporting a beneficial role of O3FA in these ischemic outcomes. Furthermore, we included only large RCTs, thereby avoiding dispersion in the results due to the inclusion of small observational trials.
The variation in RCT results could be related to differences in DHA and EPA formulations. It is well demonstrated that both EPA and DHA are effective at lowering triglycerides; however, DHA raises high-density lipoprotein cholesterol (HDL-C) and increases LDL-C, while EPA does not [33]. While omega-3-acid ethyl esters and omega-3-carboxylic acids contain both EPA and DHA, icosapent ethyl—used in REDUCE-IT—is an EPA-only prescription product [34]. However, because the REDUCE-IT trial used an EPA-only product at a HD of 4 g/day, it remains unknown whether it was the dose or formulation that drove the observed CV benefit [10•].
The STRENGTH (STatin Residual risk reduction with EpaNova in hiGh CV risk patienTs with Hypertriglyceridemia) trial, which evaluated the cardioprotective effects of a O3FA 4 g/day supplementation using omega-3-carboxylic acids—which contains both EPA and DHA—has been stopped due to futility. Although the authors have not yet published the results, due to differences in study design and methodological differences compared to REDUCE-IT, we cannot exclude that a dosing or composition of O3FA may be responsible in obtaining benefit in cardiovascular events [35•]. Further trials are needed to settle this discussion.
Limitations
A limitation of the present study is the absence of a closed-loop between the network analysis in the mixed treatment comparison framework, as there is no direct comparison between HD and LD O3FA. Differences in the sub-groups of patients who may benefit most from O3FA treatment within the 14 included clinical trials (e.g., racial differences, age, primary versus secondary prevention settings) were not further investigated so the generalizability of our findings to these populations remains difficult. Furthermore, the different studies included various O3FA formulations that contained variable amounts of EPA and DHA, and various matching control formulations. Finally, an arbitrary cut-off of 1 g/day was used to differentiate the LD and HD of omega 3; however, we cannot exclude a different cut-off that would be able to further prove a better balance between ischemic and safety outcomes in cardiovascular patients.
Future Directions
For decades, there had been a gap in the development of novel lipid-lowering therapies with randomized controlled trial evidence for the primary and secondary prevention of CVD. Thus, the demonstrated cardiovascular benefit of HD O3FA is highly clinically relevant for medical and other professional societies, who play a significant role in informing and educating clinicians. It is also important for government agencies who play a significant role in advancing policy to educate and inform the public.
Recently, the US Food and Drug Administration (FDA) approved the O3FA, icosapent ethyl (Vascepa; Amarin Pharma), as an add-on to maximally tolerated statin therapy to reduce the risk of cardiovascular events in adults with TG levels ≥ 150 mg/dL and established CVD or diabetes and two or more additional risk factors for CVD. Furthermore, icosapent ethyl has been demonstrated to be cost effective in both primary and secondary prevention populations [36]. Yet, the adoption of novel therapies in real-world clinical practice settings is well known to lag behind the available evidence and will require systemic changes to adequately address [37].
Conclusion
In the present network meta-analysis, we found that HD O3FA (> 1 g/daily) reduces the risk of cardiac death, MI, coronary revascularization, unstable angina, and major vascular events compared to control. However, HD O3FA was associated with an increased risk of bleeding events and atrial fibrillation.
Abbreviations
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- 95% CI:
-
95% Confidence interval
- CVD:
-
Cardiovascular disease
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FDA:
-
Food and Drug Administration
- HD:
-
High dose
- HDL-C:
-
High-density lipoprotein cholesterol
- IQR:
-
Interquartile range
- IRR:
-
Incidence rate ratio
- LD:
-
Low dose
- LDL-C:
-
Low-density lipoprotein cholesterol
- O3FA:
-
Omega-3 fatty acids
- PCSK9:
-
Proprotein convertase subtilisin-kexin type 9
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- RCT:
-
Randomized clinical trial
- TG:
-
Triglyceride
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2017. Update. 135:2017. https://doi.org/10.1161/CIR.0000000000000485.Heart.
Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3). https://doi.org/10.1161/CIR.0000000000000510.
Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376(9740):540–50. https://doi.org/10.1016/S0140-6736(10)60445-X.
Chang CL, Deckelbaum RJ. Omega-3 fatty acids: mechanisms underlying ‘protective effects’ in atherosclerosis. Curr Opin Lipidol. 2013;24(4):345–50. https://doi.org/10.1097/MOL.0b013e3283616364.
Elagizi A, Lavie CJ, Marshall K, DiNicolantonio JJ, O'Keefe JH, Milani RV. Omega-3 polyunsaturated fatty acids and cardiovascular health : a comprehensive review. Prog Cardiovasc Dis. 2018;61(1):76–85. https://doi.org/10.1016/j.pcad.2018.03.006.
Biondi-Zoccai G. In: Biondi-Zoccai G, editor. Network meta-analysis: evidence synthesis with mixed treatment comparison. New York: Nova publishers; 2014.
• Lombardi M, Chiabrando JG, Vescovo GM, et al. Efficacy of different dose of omega 3 fatty acid on cardiovascular outcomes: rationale and design of a network meta-analysis. Minerva Cardioangiol. 2020;68:47–50. https://doi.org/10.23736/S0026-4725.19.05117-XRationale and design of the present meta-analysis.
Haynes RB, Wilczynski NL. Optimal search strategies for retrieving scientifically strong studies of diagnosis from Medline: analytical survey. Br Med J. 2004;328(7447):1040–2. https://doi.org/10.1136/bmj.38068.557998.ee.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8. https://doi.org/10.1136/bmj.l4898.
• Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. https://doi.org/10.1056/NEJMoa1812792In REDUCE-IT among patients with high trygliceride levels the risk of ischemic events was significantly lower among those who received 4 g/daily of O3FA.
Kromhout D, Giltay EJ, Geleijnse JM. For the AOTG. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26. https://doi.org/10.1056/nejmc1014112.
Bowman L, Mafham M, Wallendszus K, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540–50. https://doi.org/10.1056/NEJMoa1804989.
Manson JAE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23–32. https://doi.org/10.1056/NEJMoa1811403.
GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–30. https://doi.org/10.1016/S0140-6736(08)61239-8.
Marchioli R. Dietary supplementation with N-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354(9177):447–55. https://doi.org/10.1016/S0140-6736(99)07072-5.
• Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–8. https://doi.org/10.1016/S0140-6736(07)60527-3JELIS trial demonstrated tha 1.8 g/daily of EPA is protective for prevention of coronary events in Japanese hypercholesterolaemic patients.
Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–9. https://doi.org/10.1161/CIRCULATIONAHA.110.948562.
Einvik G, Ole Klemsdal T, Sandvik L, Hjerkinn EM. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur J Prev Cardiol. 2010;17(5):588–92. https://doi.org/10.1097/HJR.0b013e328339cc70.
Bonds DE, Harrington M, Worrall BB, et al. Effect of long-chain ω-3 fatty acids and lutein+zeaxanthin supplements on cardiovascular outcomes: results of the age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. 2014;174(5):763–71. https://doi.org/10.1001/jamainternmed.2014.328.
Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. Bmj. 2011;342(7787):36. https://doi.org/10.1136/bmj.c6273.
Roncaglioni MC, Tombesi M, Avanzini F, et al. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–8. https://doi.org/10.1056/NEJMoa1205409.
Bosch J, Gerstein HC, Dagenais GR, et al. N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18. https://doi.org/10.1056/NEJMoa1203859.
Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, Wever EF, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients. Jama. 2006;295(22):2613–9.
Wiggins BS, Dixon D, Bellone J, Gasbarro N, Marrs JC, Tran R. Key articles and guidelines in the management of dyslipidemia: 2019 update. J Pharm Pract. 2019:089719001986841. https://doi.org/10.1177/0897190019868413.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;2019:1–78. https://doi.org/10.1093/eurheartj/ehz455.
Harris WS. Understanding why REDUCE-IT was positive – mechanistic overview of eicosapentaenoic acid. Prog Cardiovasc Dis. 2019;62(5):401–5. https://doi.org/10.1016/j.pcad.2019.10.008.
Bittner V. Implications for REDUCE IT in clinical practice. Prog Cardiovasc Dis. 2019;62(5):395–400. https://doi.org/10.1016/j.pcad.2019.11.003.
Shapiro MD, Tavori H, Fazio S. PCSK9 from basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420–38. https://doi.org/10.1161/CIRCRESAHA.118.311227.
Sheikh O, Vande Hei AG, Battisha A, Al E. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: as it pertains to the recently published REDUCE-IT trial. Cardiovasc Diabetol. 2019;18(1):1–12. https://doi.org/10.1186/s12933-019-0887-0.
Wachira JK, Larson MK, Harris WS. N-3 fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights. Br J Nutr. 2014;111(9):1652–62. https://doi.org/10.1017/S000711451300425X.
• Walker RE, Jackson KH, Tintle NL, et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am J Clin Nutr. 2019;110(4):1034–40. https://doi.org/10.1093/ajcn/nqz161This predictive model demonstrated that a dose ≥ 2 g/daily of O3FA is protective against CV events.
Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8(19). https://doi.org/10.1161/jaha.119.013543.
Innes JK, Calder PC. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. 2018;19(2). https://doi.org/10.3390/ijms19020532.
Ito MK. A comparative overview of prescription omega-3 fatty acid products. P T. 2015;40(12):826–836.
• Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41(10):1281–8. https://doi.org/10.1002/clc.23055Ongoing trial regarding high dose O3FA supplementation has been stopped due to futility.
Weintraub WS, Bhatt D, Zhang Z, et al. Cost-effectiveness of icosapent ethyl in US REDUCE-IT patients. J Am Coll Cardiol. 2020;75(11, Suppl 1). https://doi.org/10.1016/S0735-1097(20)32541-9.
Dixon DL, Sharma G, Sandesara PB, Yang E, Braun LT, Mensah GA, et al. Therapeutic inertia in cardiovascular disease prevention: time to move the bar. J Am Coll Cardiol. 2019;74(13):1728–31.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception or design of the work. ML, JGC, GMV, EB, MDB contributed to the acquisition, analysis, or interpretation of data for the work; RK contributed to the acquisition of the data, CS, BVT, DLD contributed to interpretation of the data, AA and GBZ contributed to analysis and interpretation of the data. ML, JGC, GMV, EB, MDB drafted the manuscript. CS, BVT, DLD, AA, GBZ critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
All authors confirm that tables and figures were not published previously.
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any disclosure related to the contents of this paper. Salvatore Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association. Marco Lombardi, Juan G. Chiabrando, Giovanni M. Vescovo, Edoardo Bressi, Marco Giuseppe Del Buono, Salvatore Carbone, Rachel Koenig, Benjamin W. Van Tassell, Dave L. Dixon, Antonio Abbate, and Giuseppe Biondi-Zoccai declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Electronic supplementary material
ESM 1
(DOCX 6756 kb)
Rights and permissions
About this article
Cite this article
Lombardi, M., Chiabrando, J.G., Vescovo, G.M. et al. Impact of Different Doses of Omega-3 Fatty Acids on Cardiovascular Outcomes: a Pairwise and Network Meta-analysis. Curr Atheroscler Rep 22, 45 (2020). https://doi.org/10.1007/s11883-020-00865-5
Published:
DOI: https://doi.org/10.1007/s11883-020-00865-5