Abstract
A decade after our discovery of the involvement of proprotein convertase subtilisin/kexin type 9 (PCSK9) in cholesterol metabolism through the identification of the first mutations leading to hypercholesterolemia, PCSK9 has become one of the most promising targets in cholesterol and cardiovascular diseases. This challenging work in the genetics of hypercholesterolemia paved the way for a plethora of studies around the world allowing the characterization of PCSK9, its expression, its impact on reducing the abundance of LDL receptor, and the identification of loss-of-function mutations in hypocholesterolemia. We highlight the different steps of this adventure and review the published clinical trials especially those with the anti-PCSK9 antibodies evolocumab (AMG 145) and alirocumab (SAR236553/REGN727), which are in phase III trials. The promising results in lowering LDL cholesterol levels raise hope that the PCSK9 adventure will lead, after the large and long-term ongoing phase III studies evaluating efficacy and safety, to a new anticholesterol pharmacological class.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant hypercholesterolemia (ADH) is a heterogeneous genetic disorder characterized by a selective increase of LDL cholesterol (LDL-C) levels in plasma, giving rise to tendon and skin xanthomas, arcus cornea, and vascular deposits, leading to progressive and premature atherosclerosis, coronary heart disease (CHD), and death. The first two genes implicated in the disease are the gene that encodes the LDL receptor (LDLR at 19p13.3; OMIM 606945, 143890) [1] and the apolipoprotein B (apoB) gene (APOB at 2p23–p24; OMIM 107730, 144010), encoding the ligand of the LDL receptor [2]. The existence of a greater level of genetic heterogeneity in ADH and the involvement of a third locus named HCHOLA3 (formerly FH3; OMIM 603776) were reported by our team. In 2003, we discovered [3] that PCSK9 was the third gene implicated in ADH. This pioneering work revealed a new major player in cholesterol homeostasis and was the first step of the adventure involving proprotein convertase subtilisin/kexin type 9 (PCSK9) as a promising therapeutic target in lowering LDL-C levels and reducing the risk of cardiovascular diseases. In this review we will follow the PCSK9 adventure from the involvement of its mutations and variants in cholesterol disease and CHD to the several clinical trials that have been launched.
Discovery of the Involvement of PCSK9 in Cholesterol Metabolism
Through the French Research Network for ADH (Réseau National de Recherche sur les Hypercholestérolémies Familiales), families with hypercholesterolemia were recruited from several regions of France [3]. After the exclusion of the LDLR and APOB genes, a positional cloning strategy was used to identify the genetic region linked to the disease. Using this classic genetic approach, HCHOLA3 was mapped to 1p34.1–p32 in a French multiplex family (HC2) [4]. A year later Hunt et al. [5] confirmed this localization in an ADH family originating from Utah. Segregation analysis, genetic mapping, and sequencing studies performed helped in excluding several genes, and in refining the boundaries of the region through the identification, by Abifadel et al., of a new French multiplex family, HC92, linked to the same HCHOLA3 locus. Extensive sequencing studies of several candidate genes expressed in the liver allowed us the detection, on the 13 September 2002 in the HC2 and HC92 families, of a common mutation, p.S127R, in the PCSK9 gene and another mutation, p.F216L, in a third French family with ADH [3]. The PCSK9 gene, the ninth member of the proprotein convertase subfamily, had been characterized in 2003 by Seidah et al. [6], who identified it from a patented database in a BLAST search to find proteins related to a recently identified proprotein convertase called SKI-1 (site-1 protease). PCSK9 was formerly designated as neural apoptosis regulated convertase 1 (NARC1) as it was discovered in 2001 by Millenium Pharmaceuticals through the cloning of complementary DNA upregulated after apoptosis induced by serum deprivation in primary cerebellar neurons. It was also designated as LP251, which was identified by Eli Lilly and Co. in 2002 via the cloning of secretory proteins [6]. The mammalian serine proprotein convertase family is responsible for the proteolytic maturation of secretory proteins, including neuropeptides, prohormones, cytokines, growth factors, receptors, serum, and cell surface proteins [6, 7].
PCSK9 Protein: Structure and Function
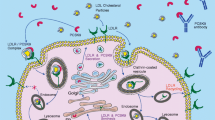
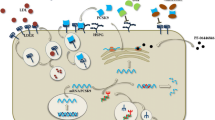
PCSK9 complementary DNA (NM_174936.2) spans 3,617 bp over 12 exons that encode the 692 amino acid protein PCSK9 (NP_777596.2). It is particularly expressed in the liver, gut, kidney, and nervous system [6, 8]. The detailed structure and processing of PCSK9 are given in Fig. 1 [6, 9–19]. The 60-kDa mature form and the furin-cleaved form of PCSK9 are present in the circulation [12, 17].
Structure, processing of proprotein convertase subtilisin/kexin type 9 (PCSK9), and impact of PCSK9 main variants and mutations. The PCSK9 structure is characterized by a signal sequence (amino acids 1–30), a prodomain (amino acids 31–152), and a catalytic domain, followed by a 243 amino acid cysteine-rich and histidine-rich C-terminal region. PCSK9 is synthesized as an inactive proenzyme and contains a triad of residues (Asp-186, His-226, and Ser-386) that are required for catalytic activity. The approximately 74-kDa precursor form of PCSK9 undergoes intramolecular autocatalytic cleavage in the endoplasmic reticulum (ER), which produces an approximately 60-kDa catalytic fragment. Autocatalytic cleavage of the zymogen in the ER is essential for transport from this compartment and for secretion. The PCSK9 crystal structure shows that the cleaved prodomain of approximately 14 kDa remains associated with the catalytic domain, blocking the PCSK9 active site, which could explain why no other proteolytic activity has been reported for PCSK9. The 60-kDa mature and secreted form is cleaved at the motif RFHR↓218 into an approximately 53-kDa inactivated or less efficient fragment by other proprotein convertases, particularly furin and/or proprotein convertase C5/6A (PC5/6A). PCSK9 degrades LDL receptor (LDLR) independently of its catalytic activity by involving mainly extracellular and possibly intracellular pathways. PCSK9 might work in a post-ER compartment, where it might target LDLR for degradation in lysosomes. The binding site for the LDLR EGF-A domain resides on the surface of PCSK9 that is formed primarily by residues 367–381. Key interactions with EGF-A are made by Arg-194 and Asp-238 of PCSK9. Several gain-of-function mutations are reported: The p.S127R variant interferes with autocatalytic cleavage, which is crucial for secretion from the cell. The p.D374Y variant binds LDLR 25 times more tightly than does wild-type PCSK9 at neutral pH, remains in a high-affinity complex at acidic pH, and is approximately tenfold more active in reducing LDLR levels than the wild-type protein. The p.R218S, p.F216L, and p.D374Y mutations result in total (p.R218S) or partial loss of the furin/PC5/6A processing of PCSK9, which increases the stability of PCSK9. Loss-of-function mutations are also represented: no protein was detected with the p.Y142X mutation, probably owing to nonsense-mediated messenger RNA decay. Some mutants associated with hypocholesterolemia either remain in the ER (p.C679X and the p.G106R mutations) or do not sort to endosomes (p.L253F and p.Q554E), resulting in loss of function (Benjannet et al. [9, 12], Lagace et al. [17], Cunningham et al. [10], McNutt et al. [11], Piper et al. [15], Nassoury et al. [19], Zhang et al. [14], Kwon et al. [16], Poirier et al. [13])
PCSK9 Mutation in Hypercholesterolemia
The p.S127R mutation in a highly conserved region between species in exon 2 was found in the first two French families studied: HC2 and HC92. The second mutation, p.F216L, in a conserved region in exon 4, was identified in a French family in which the proband died from myocardial infarction at the age of 49 years with a total cholesterol level of 441 mg/dl and an LDL-C level of 356 mg/dl [3, 20, 21]. These two mutations allowed us to identify for the first time the involvement of PCSK9 in ADH and cholesterol metabolism [3]. The third mutation, p.D374Y, was reported in 2004 in the hypercholesterolemic Utah kindred [22] previously linked to the 1p32 region [5]. The same mutation was found in three Norwegian families [23] and in three English families, with 12 affected patients having severe hypercholesterolemia and a family history of premature CHD [24].
Other mutations adjacent to these mutations were also reported: p.D374H in Portuguese patients with severe hypercholesterolemia [25]; p.R218S, which we identified in a French family whose proband at the age of 45 years had an LDL-C level of 293 mg/dl and presented with tendinous xanthoma and arcus corneae [26]; p.R215H in two families from Norway [27]; and p.D129G in a family originating from New Zealand [28]. A novel missense mutation of the PCSK9 gene, p.R306S, was found in a Chinese population [29]. More recently, we identified two gain-of-function mutations of PCSK9 in French families: (1) p.L108R, in a black family originating from Mauritius whose proband at the age of 41 years had an LDL-C level of 302 mg/dl and tendon xanthomas; (2) p.D35Y in a family’s proband who had an LDL-C level of 234 mg/dl at the age of 55 years [30]. The PCSK9 mutations inducing ADH are very rare, but well documented (familial segregation analysis, in vitro mutagenesis, etc.). The clinical findings that have been reported in PCSK9 heterozygote carriers are those related to hypercholesterolemia: tendon xanthomas, CHD, premature myocardial infarction, and stroke. Most enzymopathies are recessively inherited, and thus the dominance of the ADH trait associated with PCSK9 was in favor of a gain-of-function mechanism [3]. This was confirmed by cellular and animal models showing that these gain-of-function mutations decreased the number of LDL receptors at the cell surface, leading to hypercholesterolemia [17, 31, 32].
In vitro studies showed that the two gain-of-function mutations p.S127R and p.D374Y resulted in a 23 % decreased level of cell surface LDL receptors and a 38 % decreased level of internalization of LDL compared with wild-type PCSK9 [33]. It was shown more recently that the p.L108R mutant exhibited a marked approximately twofold enhanced degrading activity towards LDL receptor, resulting in a clear and significant gain-of-function in this assay [30]. The mechanisms of action of the gain-of-function mutations are depicted in Fig. 1.
PCSK9 and Hypocholesterolemia
Two years after our first report of the involvement of PCSK9 in cholesterol metabolism and disease, two nonsense mutations in PCSK9, p.Y142X and p.C679X, were identified in subjects with low plasma levels of LDL-C (below 58 mg/dl) from the Dallas Heart Study, a multiethnic population of Dallas County, Texas, USA [34]. Subjects with nonsense mutations had significantly lower plasma levels of total cholesterol and LDL-C, but not all of them were hypocholesterolemic [34]. In the USA, one in every 50 African Americans has a nonsense mutation in PCSK9. In the Atherosclerosis Risk in Communities (ARIC) study, comprising 3,363 black and 9,523 white participants aged 45–64 years from four American communities [35], the nonsense mutations occurred in 2.6 % of the black subjects examined and were associated with a 28 % reduction in mean LDL-C level and an 88 % reduction in the risk of CHD. These mutations were found at this same high frequency in a Nigerian population [36], in 3.7 % of African women from Zimbabwe and associated with a 27 % reduction in LDL-C levels [37], but were very rare in Americans of European origin (less than 0.1 %) [36]. However, another variant, p.R46L, was found in 3.2 % of the white subjects examined in the ARIC study and was associated with a 15 % reduction in LDL-C levels and a 47 % reduction in the risk of CHD [34, 35, 38]. The p.Q152H mutation of PCSK9 was identified in a French Canadian, with mean decreases in circulating PCSK9 and LDL-C concentrations of 79 % and 48 %, respectively, compared with unrelated noncarriers [39]. The p.G106R mutation segregated with low LDL-C levels in a Norwegian family [18]. The impacts of these variants on CHD have been studied and are reported in Fig. 1.
A woman originating from Zimbabwe, homozygous for p.C679X, was reported [37] with a very low LDL-C level (15 mg/dl). Furthermore, Zhao et al. [40] reported a compound heterozygote for the p.Y142X mutation and an in-frame 3-bp deletion (c.290_292delGCC) that deletes an arginine at codon 97. She had no immunodetectable circulating PCSK9. This 32-year-old African American woman with an LDL-C level of only 14 mg/dl was apparently healthy, fertile, and normotensive, with grossly normal hepatic, neuronal, and renal function test results [40]. A 49-year-old Caucasian man with a heterozygous double PCSK9 mutation, undetectable circulating PCSK9, and profound familial hypobetalipoproteinemia (FHBL) (LDL-C level 16 mg/dl) was also reported. A monoallelic PCSK9 double-mutant R104C/V114A cosegregated with FHBL, with no mutation found at other FHBL-causing loci [41]. Two nonsense mutants, p.A68fsL82X and p.W428X, have been identified in Sicilian and Japanese hypocholesterolemic patients [42, 43], respectively. One proband heterozygous for a novel single nucleotide deletion in exon 1 (c.202delG), which causes a frameshift in messenger RNA (mRNA), leading to a premature stop codon (A68fsL82X), was a 34-year-old white overweight male (body mass index 30 kg/m2) who had been referred to the clinic for fatty liver. This loss-of-function mutation was also identified in two healthy blood donors who had no clinical or laboratory signs of liver disease; the results of other routine laboratory tests were normal [42]. In the Dallas Heart Study, no significant difference in the median content of hepatic triglycerides or in the prevalence of hepatic steatosis between the subjects with and without an LDL-lowering mutation in PCSK9 was observed in either ethnic group [36]. Hypocholesterolemia due to a deficiency of PCSK9 appears to be benign, in contrast to other Mendelian forms of severe hypocholesterolemia such as abetalipoproteinemia (OMIM 200100) and homozygous hypobetalipoproteinemia (OMIM 107730), which are both associated with malnutrition, hepatic steatosis, steatorrhea, and manifestation of fat-soluble vitamin deficiency [40].
PCSK9 in CHD and Large Population Studies
PCSK9 variants have variable frequencies in different populations, and their impact on cholesterol levels and CHD was analyzed in African [37], American [35], and European [18, 44] populations and in different studies (ARIC [35], PROSPER [45], LCAS [46], TEXGEN [46], PLIC [47]) by evaluating either the protection of the loss-of-functions variants or the severity of coronary atherosclerosis associated with gain-of-functions polymorphisms (mainly p.E670G). These studies, their objectives, their results, and their conclusions are summarized in Table 1 [35, 45–59]. They showed that genotype is a better predictor of lifelong exposure to LDL-C than LDL-C measured in adult life. But the impact on LDL may not be the only effect of PCSK9 on atherogenesis [60]. It is noteworthy that several genome-wide association studies identified an association of the PCSK9 locus and of some PCSK9 variants with the variability of LDL-C levels or early-onset myocardial infarction [61].
Genotype–Phenotype Correlation
PCSK9 polymorphisms account for cholesterol variability not only in normolipemic subjects but also among familial hypercholesterolemia (FH) patients sharing the same mutation of LDLR [62]. We showed that PCSK9 might constitute a modifier gene in FH: in Lebanese FH patients sharing the LDLR p.C681X mutation, p.Leu21dup, in exon 1 of PCSK9, known to be associated with lower LDL-C levels in general populations, is also associated with a reduction of LDL-C levels in FH [62]. Furthermore, additive effects of mutations of LDLR and gain-of-function mutations of PCSK9 on the phenotype of FH have been reported in several studies [26, 63] and might be associated with a severe phenotype. It is noteworthy that the p.R496Q variant in PCSK9 was identified [33] in a subject homozygous for apolipoprotein E2 who presented with type III hyperlipoproteinemia.
We identified PCSK9 p.L21tri (p.L15_L16ins2L) mutation in two French-Canadian families with familial combined hypercholesterolemia (FCHL) and in one French-Canadian woman and her father with hypercholesterolemia [64]. Our report of the involvement of the L11 variant of PCSK9 in FCHL was the first report of the involvement of PCSK9 in this disease. This was confirmed by Brouwers et al. [65], who showed that PCSK9 levels were higher in FCHL patients than in normolipidemic relatives and spouses. They also reported that PCSK9 levels were related to markers of cholesterol synthesis in FCHL [66].
PCSK9 and ApoB
In vivo kinetics of apoB100-containing lipoproteins studied in two subjects carrying the p.S127R mutation in PCSK9 showed that PCSK9 mutation increased the production rate of apoB100 by threefold compared with controls or LDLR-mutated subjects, which is related to direct overproduction of VLDL (threefold), intermediate-density lipoprotein (threefold), and LDL (fivefold) [67]. Expression of the PCSK9 p.D374Y variant increases secretion of apoB100-containing lipoproteins from the cells by twofold to fourfold probably by reducing the degradation of nascent protein [24]. This also suggests that the variants of PCSK9 found in FH influence the secretion of apoB-containing lipoproteins. The same team produced transgenic mice expressing the p.D374Y variant of the human PCSK9 gene at physiological levels and showed that the phenotype closely matched that found in heterozygous p.D374Y patients and that reduced LDL receptor activity is not the sole cause of their hypercholesterolemia. The p.D374Y mice secreted more triglyceride-rich lipoproteins into the circulation than did wild-type mice [68]. Recently Sun et al. [69] studied the impact of PCSK9 overexpression (approximately 400-fold above the baseline) on apoB synthesis and secretion in mouse models. They demonstrated that endogenous PCSK9 interacted with apoB in hepatocytes. The physical interaction of PCSK9 with apoB acts to shunt apoB away from autophagosomes and degradation. In turn, most of the apoB would be destined for assembly and secretion as VLDL from hepatocytes. This observation is consistent with increased apoB production on overexpression of PCSK9. They thus proposed a new role for PCSK9 that involves shuttling between apoB and LDL receptor.
PCSK9 Expression
PCSK9 expression seems regulated by nutritional and hormonal status. PCSK9 is upregulated and increased by overexpression of sterol responsive element binding protein 2 (SREBP-2), cholesterol depletion [70], inflammation, administration of insulin, and statin therapy [71]. PCSK9 is downregulated by the suppression of SREBP-2, cholesterol feeding, and berberine but also by glucagon [72], ethinylestradiol [72], chenodeoxycholic acid, and farnesoid X receptor agonist [73]. It is now established that several antihyperlipidemic drugs such as statins, fibrates, and ezitimibe induce an increase of PCSK9 levels. This might attenuate their cholesterol-lowering effect by reducing LDL receptor abundance at the cell surface. In 2004 Dubuc et al. [71] showed for the first time that the expression of PCSK9 mRNA was strongly induced by statins in a dose-dependent manner and that human, mouse, and rat PCSK9 promoters contain two typical conserved motifs for cholesterol regulation: a sterol regulatory element and an Sp1 site. Cellular and animal studies by several teams showed that statins increase SREBP-2 levels and lead to an increase of LDL receptor levels but also of the levels of PCSK9, which decreases the abundance of LDL receptor on the cell surface, limiting the hypocholesterolemic action of statins. Several studies in humans showed that different statin (atorvastatin, simvastatin, rosuvastatin, etc.) treatments caused an increase in serum PCSK9 levels. The increase of PCSK9 levels caused by atorvastatin was 47 % for 80 mg versus 14 % for 10 mg. These data suggest that the explanation for why increasing doses of statins fail to achieve proportional LDL-C lowering is that statins increase PCSK9 levels in a dose-dependent fashion, and that the increased PCSK9 levels largely negate further statin-induced increases in hepatic LDL receptor levels [74]. Thus, it was suggested that a combination of a statin with a PCSK9 inhibitor could overcome this effect and enhance reduction of cholesterol levels. An initial proof-of-concept was provided by statin administration to Pcsk9 −/− mice that produced an exaggerated increase in LDL receptors levels in liver and enhanced LDL clearance from plasma [75]. This has been confirmed in nonhuman primate models and humans.
Furthermore, when added to statin therapy, ezetimibe leads to a further increase of PCSK9 levels (77 % vs 45 % with statins alone) [76, 77]. Several studies have investigated the impact of fibrates on the circulating levels of PCSK9, but the results are conflicting [78–80]. This might be due to the use of different analytical techniques to measure circulating PCSK9 levels. However, there is more evidence currently that fibrates increases serum PCSK9 levels and that these increases are highly correlated with fenofibrate-induced changes in LDL-C levels [81].
PCSK9 Levels in Blood
PCSK9 is present in human plasma, but the factors that contribute to differences in plasma concentrations are not very well known. Several teams have developed an enzyme-linked immunosorbent assay (ELISA) to measure PCSK9 in plasma. Plasma levels of PCSK9 vary at least 100-fold [82]. Serum PCSK9 levels measured by ELISA seem to be directly correlated with serum LDL-C and total cholesterol levels [83]. In hypercholesterolemic subjects, PCSK9 levels were higher than in control subjects, and increased in proportion to the dose of statin, combined or not combined with ezetimibe [71]. Plasma PCSK9 levels are positively associated with LDL-C levels in FH patients, and might contribute to the phenotypic severity in this disorder [84]. Serum PCSK9 levels display a diurnal rhythm that closely parallels that of cholesterol synthesis [85]. PCSK9 concentrations were lower with a polyunsaturated fatty acid diet [86], a Mediterranean diet [87], administration of estrogens [88], and administration of growth hormone [88]. The PCSK9 level was found to be associated with the γ-glutamyl transferase level in diabetic patients [89] and with carotid intima–media thickness in hypertensive patients [90]. The plasma level of lipoprotein-associated phospholipase A2 is inversely correlated with PCSK9 levels [91]. The plasma level of PCSK9 was increased at the baseline in proteinuric subjects, predicted lipoprotein responses to proteinuria reduction, but remained unchanged after proteinuria reduction [92]. At physiological levels observed in human obesity, it was shown that resistin increases cellular expression of PCSK9, which enhances intracellular LDL receptor lysosomal degradation [93]. Nevertheless, no positive association of plasma PCSK9 with resistin was found in lean and moderately obese individuals [94].
Therapeutic Strategies to Reduce PCSK9 Levels or Inhibit PCSK9
Several strategies to inhibit PCSK9 or lower PCSK9 levels have been investigated. Specific inhibition of PCSK9 via a classic pharmaceutical approach such as orally active molecules targeting PCSK9 seems difficult. Strategies known to target proteins not accessible to small molecules have been tested. Gene silencing by RNA interference and specific antibodies or competing peptides targeting PCSK9 have been developed. The details of these molecules or antibodies, and the results obtained in cellular models or animal models (mice or monkeys) and the related patents were reviewed in a previous article [95]. Clinical studies have been launched by several pharmaceutical companies. The details of these studies, their results, and the adverse reactions are given in Table 2 [96, 97•, 98–108, 109••, 110, 111, 112••, 113–115]. The first strategies based on gene silencing that targets PCSK9 intracellular and extracellular functions consisted in a subcutaneous administration of antisense oligonucleotide (ASO) targeting PCSK9 or small interfering RNA (siRNA). ASO studies have been conducted mainly with a second-generation ASO produced by Isis Pharmaceuticals, or with a 13-mer locked nucleic acid (LNA) ASO or a 14-mer LNA-ASO specific for a human PCSK9 sequence from Santaris Pharma. They showed in cellular, mouse, and monkey models a significant reduction of hepatic Pcsk9 mRNA expression and of total cholesterol and LDL-C levels. These ASOs were well tolerated in animals. The most frequent adverse event with this approach was injection-site erythema that seems to resolve spontaneously. To determine whether injection of these compounds results in toxic effects in humans, a clinical trial has been launched by Bristol-Myers Squibb using BMS-844421 (BMS-PCSK9Rx), which is an ASO developed by Isis. Nevertheless, the clinical study has been discontinued and no data are available. The clinical trial launched by Santaris Pharma to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of SPC5001 (a 14-mer LNA-ASO specific for a human PCSK9) has also been discontinued and no data are available either (Table 2). PCSK9 gene silencing in mice and monkeys has also been achieved using siRNA. Active, cross-species siRNAs capable of targeting murine, rat, nonhuman primate, and human PCSK9 have been developed by Frank-Kamenetsky and coworkers [97•, 116]. Delivery of the PCSK9 siRNA to the liver was facilitated by a lipidoid nanoparticle, minimizing toxicity. A phase I clinical trial was conducted by Alnylam Pharmaceuticals to determine the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single dose of ALN-PCS02. The results are given in Table 2.
Other molecules that are currently being studied are adnectins (BMS-962476) [96], which are in phase I trials (Table 2), and small molecule inhibitors (SX-PCSK9, detailed at http://www.serometrix.com/pipeline.html).
Several antibodies or competing peptides targeting PCSK9 have been developed and studied in cellular and animal models (mice and monkeys). Clinical studies are being performed by pharmaceutical companies: LGT209 by Novartis is in a phase II study, LY3015014 by Eli Lilly is in a phase II study, RG7652 (MPSK3169A) [98] by Genentech (Roche) is in a phase II study, and RN316 (bococizumab) by Pfizer has undergone phase I studies and is now in phase II [99] and phase III studies. Available published results of these studies are reported in Table 2.
Many phase I and phase II studies have been published recently in several interesting articles for two antibodies targeting and inhibiting PCSK9 interaction with LDL receptor: AMG 145 (evolocumab) developed by Amgen (Thousand Oaks, CA, USA), and SAR236553/REGN727 (alirocumab) developed by Regeneron Pharmaceuticals (Tarrytown, NY, USA) and Sanofi-Aventis (Paris, France). These antibodies and related patents were given in our previously published review on PCSK9 patents [95], but the details of the clinical trials, the doses given every 2 or 4 weeks subcutaneously, the results, and the adverse events are given in Table 2 [100–108, 109••, 110, 111, 112••, 113–115].
Phase III studies have been initiated by Amgen and Sanofi and Regeneron. The results of two of these phase III studies with evolocumab have been published and are detailed in Table 2, and several other phase III studies have been launched but have not been published yet. For alirocumab, an important program (ODYSSEY) concerning a large number of patients in short-term or long-term trials and targeting several populations has also been initiated. The design of these studies is summarized in Table 2 as well. Long-term studies that will involve 20,000 patients for both evolocumab and alirocumab will provide results regarding the long-term efficacy, safety, and tolerability of these anti-PCSK9 antibodies that are eagerly awaited.
Other PCSK9 Interactions and Studies in Other Diseases
PCSK9 interactions and the possibility of the involvement of PCSK9 in several diseases such as liver diseases, obesity, Alzheimer disease, cognitive performance [58] and cancer [59] have been studied (Table 1). Jonas et al. [117] showed that overexpression of PCSK9 in cells decreased cellular levels of BACE1, a membrane protease responsible for the production of toxic β-amyloid peptides that accumulate in neuritic plaques of Alzheimer disease brains. However, Liu et al. [118] found that PCSK9 does not have a role in regulating LDL receptor family members or BACE1 protein levels in the adult mouse brain and that the development of PCSK9 therapies for CHD is probably not to be hampered by potential CNS adverse effects. Devay et al. [119] discovered recently that PCSK9 interacts via its C-terminal domain directly and in a pH-dependent manner with amyloid precursor protein as well as amyloid-precursor-protein-like protein 2. It is notable that no genetic association was found between PCSK9 polymorphisms and Alzheimer disease and plasma cholesterol level in Japanese patients studied by Shibata et al. [120]. PCSK9 reduces the protein levels of LDL receptor in mouse brain during development and after ischemic stroke [121]. In vivo, endogenous PCSK9 regulates VLDL receptor protein and triglyceride accumulation in visceral adipose tissue. In a clinical perspective, because Pcsk9 −/− mice do not develop liver steatosis and are not prone to obesity, the administration of a PCSK9 inhibitor developed for hypercholesterolemia treatment should not result in adverse effects [122]. A potential role of PCSK9 in the pancreas is also controversial. PCSK9 deficiency reduces liver metastasis by its ability to lower cholesterol levels and by possibly enhancing TNFα-mediated apoptosis [123]. Furthermore studies in Xenopus oocytes and in epithelia showed that PCSK9 noncatalytically reduced the abundance of the epithelial Na+ channel, a major contributor to blood pressure control [124]. PCSK9 interacts with annexin A2 [125]. Possible other unknown functions of PCSK9 and unidentified binding partners could exist; thus, it is important for the safety of new cholesterol-lowering therapy to target specifically PCSK9 action on the LDL receptor. An antiviral effect of circulating liver PCSK9 on hepatitis C virus in cells has recently been shown, and PCSK9 downregulates in vitro the level of expression of mouse liver CD81, a major hepatitis C virus receptor [126]. Conditional knockout mice lacking PCSK9 in hepatocytes have impaired liver regeneration after a partial hepatectomy, suggesting that on hepatic damage, patients lacking PCSK9 could be at risk [127].Thus, liver problems, hepatitis, or muscle problems are taken into consideration before inclusion or exclusion and are closely monitored during clinical trials. In clinical trials, anti-PCSK9 antibodies seem well tolerated, with no clinically significant safety findings in phase I and phase II/III studies, the most commonly reported adverse events being nasopharyngitis, injection-site pain, headache, skin burning sensation, upper respiratory tract infection, influenza, and back pain [100–108, 109••, 110, 111, 112••, 113–115]. Longer-term studies will provide the highly awaited long-term efficacy, safety, and tolerability of these anti-PCSK9 antibodies.
Conclusions
Reduction of PCSK9 levels or inhibition of PCSK9 is especially interesting in patients with hypercholesterolemia or an atherogenic lipid profile who fail to reach their individual cholesterol goal from classic lipid-lowering treatment, patients at high risk of developing side effects from statins, poor responders to statin therapy alone, and patients with severe hypercholesterolemia, particularly some carriers of a mutation of the LDLR, APOB, or PCSK9 gene. The tremendous commitment from all the centers of the French Research Network for Hypercholesterolemia that helped us in recruiting French patients and the enormous amount of genetic and molecular work we performed were very important in our pioneering step linking PCSK9 to LDL-C metabolism and paving the way for the work of several other teams. Finally, the PCSK9 story is a wonderful example of how collaboration between teams (Boileau’s and Seidah’s teams) conducting research in completely different fields can be initiated and prove to be highly successful. It is also a fine example of the power of genetic research strategies in revealing new therapeutic targets.
The results of the phase III studies using the anti-PCSK9 antibodies with or without statins or other hypocholesterolemic drugs are highly awaited, with the hope that this new class of blockbuster candidates will keep its promises in helping lowering cholesterol levels and fighting against cardiovascular disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Goldstein JL, Brown MS. Familial hypercholesterolemia: pathogenesis of a receptor disease. Johns Hopkins Med J. 1978;143:8–16.
Innerarity TL, Weisgraber KH, Arnold KS, Mahley RW, Krauss RM, Vega GL, et al. Familial defective apolipoprotein B-100: low density lipoproteins with abnormal receptor binding. Proc Natl Acad Sci U S A. 1987;84:6919–23.
Abifadel M, Varret M, Rabès J-P, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
Varret M, Rabès JP, Saint-Jore B, Cenarro A, Marinoni JC, Civeira F, et al. A third major locus for autosomal dominant hypercholesterolemia maps to 1p34.1-p32. Am J Hum Genet. 1999;64:1378–87.
Hunt SC, Hopkins PN, Bulka K, McDermott MT, Thorne TL, Wardell BB, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089–93.
Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–33.
Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94.
Naureckiene S, Ma L, Sreekumar K, Purandare U, Lo CF, Huang Y, et al. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch Biochem Biophys. 2003;420:55–67.
Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–75.
Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol. 2007;14:413–9.
McNutt MC, Lagace TA, Horton JD. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem. 2007;282:20799–803.
Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J Biol Chem. 2006;281:30561–72.
Poirier S, Mayer G, Poupon V, McPherson PS, Desjardins R, Ly K, et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J Biol Chem. 2009;284:28856–64.
Zhang D-W, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–12.
Piper DE, Jackson S, Liu Q, Romanow WG, Shetterly S, Thibault ST, et al. The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure. 2007;15:545–52.
Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci U S A. 2008;105:1820–5.
Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995–3005.
Berge KE, Ose L, Leren TP. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler Thromb Vasc Biol. 2006;26:1094–100.
Nassoury N, Blasiole DA, Tebon Oler A, Benjannet S, Hamelin J, Poupon V, et al. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic. 2007;8:718–32.
Abifadel M, Rabès J-P, Boileau C, Varret M. PCSK9, du gène à la protéine: un nouvel acteur dans l’homéostasie du cholestérol (PCSK9, from gene to protein: a new actor involved in cholesterol homeostasis). Med Sci. 2006;22:916–8.
Abifadel M, Rabès J-P, Boileau C, Varret M. Après le récepteur des LDL et l'apolipoprotéine B, l'hypercholestérolémie familiale révèle son troisième protagoniste : PCSK9 (After the LDL receptor and apolipoprotein B, autosomal dominant hypercholesterolemia reveals its third protagonist: PCSK9). Ann Endocrinol. 2007;68:138–46.
Timms KM, Wagner S, Samuels ME, Forbey K, Goldfine H, Jammulapati S, et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum Genet. 2004;114:349–53.
Leren TP. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet. 2004;65:419–22.
Sun X-M, Eden ER, Tosi I, Neuwirth CK, Wile D, Naoumova RP, et al. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum Mol Genet. 2005;14:1161–9.
Bourbon M, Alves AC, Medeiros AM, Silva S, Soutar AK. Familial hypercholesterolaemia in Portugal. Atherosclerosis. 2008;196:633–42.
Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat. 2005;26:497.
Cameron J, Holla OL, Laerdahl JK, Kulseth MA, Ranheim T, Rognes T, et al. Characterization of novel mutations in the catalytic domain of the PCSK9 gene. J Intern Med. 2008;263:420–31.
Homer VM, Marais AD, Charlton F, Laurie AD, Hurndell N, Scott R, et al. Identification and characterization of two non-secreted PCSK9 mutants associated with familial hypercholesterolemia in cohorts from New Zealand and South Africa. Atherosclerosis. 2008;196:659–66.
Lin J, Wang L, Liu S, Wang X, Yong Q, Yang Y, et al. A novel mutation in proprotein convertase subtilisin/kexin type 9 gene leads to familial hypercholesterolemia in a Chinese family. Chin Med J (Engl). 2010;123:1133–8.
Abifadel M, Guerin M, Benjannet S, Rabès J-P, Le Goff W, Julia Z, et al. Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis. 2012;223:394–400.
Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 2004;101:7100–5.
Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci U S A. 2005;102:2069–74.
Cameron J, Holla ØL, Ranheim T, Kulseth MA, Berge KE, Leren TP. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum Mol Genet. 2006;15:1551–8.
Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–5.
Cohen JC, Boerwinkle E, Mosley Jr TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC, et al. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am J Hum Genet. 2006;78:410–22.
Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a southern African population. Atherosclerosis. 2007;193:445–8.
Humphries SE, Neely RDG, Whittall RA, Troutt JS, Konrad RJ, Scartezini M, et al. Healthy individuals carrying the PCSK9 p.R46L variant and familial hypercholesterolemia patients carrying PCSK9 p.D374Y exhibit lower plasma concentrations of PCSK9. Clin Chem. 2009;55:2153–61.
Mayne J, Dewpura T, Raymond A, Bernier L, Cousins M, Ooi TC, et al. Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French-Canadian family and with impaired processing and secretion in cell culture. Clin Chem. 2011;57:1415–23.
Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23.
Cariou B, Ouguerram K, Zaïr Y, Guerois R, Langhi C, Kourimate S, et al. PCSK9 dominant negative mutant results in increased LDL catabolic rate and familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol. 2009;29:2191–7.
Fasano T, Cefalù AB, Di Leo E, Noto D, Pollaccia D, Bocchi L, et al. A novel loss of function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2007;27:677–81.
Miyake Y, Kimura R, Kokubo Y, Okayama A, Tomoike H, Yamamura T, et al. Genetic variants in PCSK9 in the Japanese population: rare genetic variants in PCSK9 might collectively contribute to plasma LDL cholesterol levels in the general population. Atherosclerosis. 2008;196:29–36.
Abifadel M, Rabès J-P, Devillers M, Munnich A, Erlich D, Junien C, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat. 2009;30:520–9.
Polisecki E, Peter I, Robertson M, McMahon AD, Ford I, Packard C, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis. 2008;200:95–101.
Chen SN, Ballantyne CM, Gotto Jr AM, Tan Y, Willerson JT, Marian AJ. A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J Am Coll Cardiol. 2005;45:1611–9.
Norata GD, Garlaschelli K, Grigore L, Raselli S, Tramontana S, Meneghetti F, et al. Effects of PCSK9 variants on common carotid artery intima media thickness and relation to ApoE alleles. Atherosclerosis. 2010;208:177–82.
Evans D, Beil FU. The E670G SNP in the PCSK9 gene is associated with polygenic hypercholesterolemia in men but not in women. BMC Med Genet. 2006;7:66.
Hallman DM, Srinivasan SR, Chen W, Boerwinkle E, Berenson GS. Relation of PCSK9 mutations to serum low-density lipoprotein cholesterol in childhood and adulthood (from the Bogalusa Heart Study). Am J Cardiol. 2007;100:69–72.
Abboud S, Karhunen PJ, Lütjohann D, Goebeler S, Luoto T, Friedrichs S, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) gene is a risk factor of large-vessel atherosclerosis stroke. PLoS One. 2007;2:e1043.
Scartezini M, Hubbart C, Whittall RA, Cooper JA, Neil AHW, Humphries SE. The PCSK9 gene R46L variant is associated with lower plasma lipid levels and cardiovascular risk in healthy U.K. men. Clin Sci Lond Engl 1979. 2007;113:435–41.
Kathiresan S. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med. 2008;358:2299–300.
Folsom AR, Peacock JM, Boerwinkle E. Variation in PCSK9, low LDL cholesterol, and risk of peripheral arterial disease. Atherosclerosis. 2009;202:211–5.
Huang C-C, Fornage M, Lloyd-Jones DM, Wei GS, Boerwinkle E, Liu K. Longitudinal association of PCSK9 sequence variations with low-density lipoprotein cholesterol levels: the Coronary Artery Risk Development in Young Adults Study. Circ Cardiovasc Genet. 2009;2:354–61.
Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–42.
Guella I, Asselta R, Ardissino D, Merlini PA, Peyvandi F, Kathiresan S, et al. Effects of PCSK9 genetic variants on plasma LDL cholesterol levels and risk of premature myocardial infarction in the Italian population. J Lipid Res. 2010;51:3342–9.
Chernogubova E, Strawbridge R, Mahdessian H, Mälarstig A, Krapivner S, Gigante B, et al. Common and low-frequency genetic variants in the PCSK9 locus influence circulating PCSK9 levels. Arterioscler Thromb Vasc Biol. 2012;32:1526–34.
Postmus I, Trompet S, de Craen AJM, Buckley BM, Ford I, Stott DJ, et al. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res. 2013;54:561–6.
Folsom AR, Peacock JM, Boerwinkle E. Sequence variation in proprotein convertase subtilisin/kexin type 9 serine protease gene, low LDL cholesterol, and cancer incidence. Cancer Epidemiol Biomark Prev. 2007;16:2455–8.
Brown MS, Goldstein JL. Biomedicine. Lowering LDL—not only how low, but how long? Science. 2006;311:1721–3.
Myocardial Infarction Genetics Consortium. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41.
Abifadel M, Rabès J-P, Jambart S, Halaby G, Gannagé-Yared M-H, Sarkis A, et al. The molecular basis of familial hypercholesterolemia in Lebanon: spectrum of LDLR mutations and role of PCSK9 as a modifier gene. Hum Mutat. 2009;30:E682–91.
Pisciotta L, Priore Oliva C, Cefalù AB, Noto D, Bellocchio A, Fresa R, et al. Additive effect of mutations in LDLR and PCSK9 genes on the phenotype of familial hypercholesterolemia. Atherosclerosis. 2006;186:433–40.
Abifadel M, Bernier L, Dubuc G, Nuel G, Rabès J-P, Bonneau J, et al. A PCSK9 variant and familial combined hyperlipidaemia. J Med Genet. 2008;45:780–6.
Brouwers MCGJ, van Greevenbroek MMJ, Konrad RJ, Troutt JS, Schaper NC, Stehouwer CDA. Circulating PCSK9 is a strong determinant of plasma triacylglycerols and total cholesterol in homozygous carriers of apolipoprotein ε2. Clin Sci Lond Engl 1979. 2014;126:679–84.
Brouwers MCGJ, Konrad RJ, van Himbergen TM, Isaacs A, Otokozawa S, Troutt JS, et al. Plasma proprotein convertase subtilisin kexin type 9 levels are related to markers of cholesterol synthesis in familial combined hyperlipidemia. Nutr Metab Cardiovasc Dis. 2013;23:1115–21.
Ouguerram K, Chetiveaux M, Zair Y, Costet P, Abifadel M, Varret M, et al. Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler Thromb Vasc Biol. 2004;24:1448–53.
Herbert B, Patel D, Waddington SN, Eden ER, McAleenan A, Sun X-M, et al. Increased secretion of lipoproteins in transgenic mice expressing human D374Y PCSK9 under physiological genetic control. Arterioscler Thromb Vasc Biol. 2010;30:1333–9.
Sun H, Samarghandi A, Zhang N, Yao Z, Xiong M, Teng B-B. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler Thromb Vasc Biol. 2012;32:1585–95.
Maxwell KN, Soccio RE, Duncan EM, Sehayek E, Breslow JL. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J Lipid Res. 2003;44:2109–19.
Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–9.
Persson L, Gälman C, Angelin B, Rudling M. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology. 2009;150:1140–6.
Langhi C, Le May C, Kourimate S, Caron S, Staels B, Krempf M, et al. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 2008;582:949–55.
Konrad RJ, Troutt JS, Cao G. Effects of currently prescribed LDL-C-lowering drugs on PCSK9 and implications for the next generation of LDL-C-lowering agents. Lipids Health Dis. 2011;10:38.
Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–9.
Davignon J, Dubuc G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans Am Clin Climatol Assoc. 2009;120:163–73.
Dubuc G, Tremblay M, Paré G, Jacques H, Hamelin J, Benjannet S, et al. A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res. 2010;51:140–9.
Kourimate S, Le May C, Langhi C, Jarnoux AL, Ouguerram K, Zaïr Y, et al. Dual mechanisms for the fibrate-mediated repression of proprotein convertase subtilisin/kexin type 9. J Biol Chem. 2008;283:9666–73.
Lambert G, Ancellin N, Charlton F, Comas D, Pilot J, Keech A, et al. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem. 2008;54:1038–45.
Noguchi T, Kobayashi J, Yagi K, Nohara A, Yamaaki N, Sugihara M, et al. Comparison of effects of bezafibrate and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 and adipocytokine levels in dyslipidemic subjects with impaired glucose tolerance or type 2 diabetes mellitus: results from a crossover study. Atherosclerosis. 2011;217:165–70.
Troutt JS, Alborn WE, Cao G, Konrad RJ. Fenofibrate treatment increases human serum proprotein convertase subtilisin kexin type 9 levels. J Lipid Res. 2010;51:345–51.
Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–43.
Alborn WE, Cao G, Careskey HE, Qian Y-W, Subramaniam DR, Davies J, et al. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin Chem. 2007;53:1814–9.
Huijgen R, Fouchier SW, Denoun M, Hutten BA, Vissers MN, Lambert G, et al. Plasma levels of PCSK9 and phenotypic variability in familial hypercholesterolemia. J Lipid Res. 2012;53:979–83.
Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci U S A. 2009;106:9546–7.
Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–12.
Richard C, Couture P, Desroches S, Benjannet S, Seidah NG, Lichtenstein AH, et al. Effect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndrome. Br J Nutr. 2012;107:705–11.
Persson L, Henriksson P, Westerlund E, Hovatta O, Angelin B, Rudling M. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler Thromb Vasc Biol. 2012;32:810–4.
Cariou B, Le Bras M, Langhi C, Le May C, Guyomarc’h-Delasalle B, Krempf M, et al. Association between plasma PCSK9 and gamma-glutamyl transferase levels in diabetic patients. Atherosclerosis. 2010;211:700–2.
Lee CJ, Lee Y-H, Park SW, Kim KJ, Park S, Youn J-C, et al. Association of serum proprotein convertase subtilisin/kexin type 9 with carotid intima media thickness in hypertensive subjects. Metabolism. 2013;62:845–50.
Constantinides A, Kappelle PJWH, Lambert G, Dullaart RPF. Plasma lipoprotein-associated phospholipase A2 is inversely correlated with proprotein convertase subtilisin-kexin type 9. Arch Med Res. 2012;43:11–4.
Kwakernaak AJ, Lambert G, Slagman MCJ, Waanders F, Laverman GD, Petrides F, et al. Proprotein convertase subtilisin-kexin type 9 is elevated in proteinuric subjects: relationship with lipoprotein response to antiproteinuric treatment. Atherosclerosis. 2013;226:459–65.
Melone M, Wilsie L, Palyha O, Strack A, Rashid S. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J Am Coll Cardiol. 2012;59:1697–705.
Kwakernaak AJ, Lambert G, Dullaart RPF. Relationship of proprotein convertase subtilisin-kexin type 9 levels with resistin in lean and obese subjects. Clin Biochem. 2012;45:1522–4.
Abifadel M, Pakradouni J, Collin M, Samson-Bouma M-E, Varret M, Rabès J-P, et al. Strategies for proprotein convertase subtilisin kexin 9 modulation: a perspective on recent patents. Expert Opin Ther Pat. 2010;20:1547–71.
Stein EA, Kasichayanula S, Turner T, Kranz T, Arumugam U, Biernat L, et al. LDL Cholesterol reduction with BMS-962476, an adnectin inhibitor of PCSK9: results of a single ascending dose study. J Am Coll Cardiol. 2014;63(12 Suppl):A172. doi:10.1016/S0735-1097(14)61372-3.
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8. This is a report of a phase I trial using RNA interference to reduce PCSK9 levels.
Tingley W, Luca D, Leabman M, Budha N, Kahn R, Baruch A, et al. Effects of RG7652, a fully human mAb against proprotein convertase subtilisin/kexin type 9, on LDL-c: a Phase I, randomised, double-blind, placebo-controlled, single- and multiple-dose study. Eur Heart J. 2013;34:P4183.
Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang E, Plowchalk D, et al. Efficacy and safety of bococizumab (RN316/PF-04950615), a monoclonal antibody against proprotein convertase subtilisin/kexin type 9 in statin-treated hypercholesterolemic subjects: results from a randomized, placebo-controlled, dose-ranging study (NCT: 01592240). J Am Coll Cardiol. 2014;63(12 Suppl):A1374. doi:10.1016/S0735-1097(14)61374-7.
Dias CS, Shaywitz AJ, Wasserman SM, Smith BP, Gao B, Stolman DS, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98.
Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17.
Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006.
Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–506.
Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–17.
Kohli P, Desai NR, Giugliano RP, Kim JB, Somaratne R, Huang F, et al. Design and rationale of the LAPLACE-TIMI 57 trial: a phase II, double-blind, placebo-controlled study of the efficacy and tolerability of a monoclonal antibody inhibitor of PCSK9 in subjects with hypercholesterolemia on background statin therapy. Clin Cardiol. 2012;35:385–91.
Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129:234–43.
Mearns BM. Dyslipidaemia: 1-year results from OSLER trial of anti-PCSK9 monoclonal antibody evolocumab. Nat Rev Cardiol. 2014;11:63.
Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–20.
Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–19. This article reviews the DESCARTES phase III trial investigating the effect of AMG 145 (evolocumab) given for 52 weeks in patients with hyperlipidemia.
Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–40.
Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541–8.
Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–18. This article describes the results of three phase I trials using REGN727 (alirocumab) in single-dose and multiple-doses studies.
Crunkhorn S. Trial watch: PCSK9 antibody reduces LDL cholesterol. Nat Rev Drug Discov. 2012;11:11.
Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–900.
McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand A-C, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53.
Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–20.
Jonas MC, Costantini C, Puglielli L. PCSK9 is required for the disposal of non-acetylated intermediates of the nascent membrane protein BACE1. EMBO Rep. 2008;9:916–22.
Liu M, Wu G, Baysarowich J, Kavana M, Addona GH, Bierilo KK, et al. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J Lipid Res. 2010;51:2611–8.
DeVay RM, Shelton DL, Liang H. Characterization of proprotein convertase subtilisin/kexin type 9 (PCSK9) trafficking reveals a novel lysosomal targeting mechanism via amyloid precursor-like protein 2 (APLP2). J Biol Chem. 2013;288:10805–18.
Shibata N, Ohnuma T, Higashi S, Higashi M, Usui C, Ohkubo T, et al. No genetic association between PCSK9 polymorphisms and Alzheimer’s disease and plasma cholesterol level in Japanese patients. Psychiatr Genet. 2005;15:239.
Rousselet E, Marcinkiewicz J, Kriz J, Zhou A, Hatten ME, Prat A, et al. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J Lipid Res. 2011;52:1383–91.
Roubtsova A, Munkonda MN, Awan Z, Marcinkiewicz J, Chamberland A, Lazure C, et al. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol. 2011;31:785–91.
Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012;14:1122–31.
Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. 2012;287:19266–74.
Seidah NG, Poirier S, Denis M, Parker R, Miao B, Mapelli C, et al. Annexin A2 is a natural extrahepatic inhibitor of the PCSK9-induced LDL receptor degradation. PLoS One. 2012;7:e41865.
Labonté P, Begley S, Guévin C, Asselin M-C, Nassoury N, Mayer G, et al. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology. 2009;50:17–24.
Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–54.
Acknowledgments
This work was supported by a grant from Fondation-Leducq (FLQ # 13 CVD 03) through the Transatlantic Networks of Excellence in Cardiovascular Research program (“The function and regulation of PCSK9: a novel modulator of LDLR activity”); Institut National de la Santé et de la Recherche Médicale (INSERM); Conseil de la Recherche de l’Université Saint-Joseph (Beirut, Lebanon), and Conseil National de la Recherche Scientifique Libanais.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Marianne Abifadel is member of the advisory board of Amgen and is involved in anti-PCSK9 studies and trials with Amgen and with Regeneron and Sanofi. Jean-Pierre Rabès and Catherine Boileau are involoved in anti-PCSK9 studies with Regeneron and Sanofi.
Sandy Elbitar, Petra El Khoury, Youmna Ghaleb, Mélody Chémaly, Marie-Line Moussalli, and Mathilde Varret declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Rare Diseases and Lipid Metabolism
Rights and permissions
About this article
Cite this article
Abifadel, M., Elbitar, S., El Khoury, P. et al. Living the PCSK9 Adventure: from the Identification of a New Gene in Familial Hypercholesterolemia Towards a Potential New Class of Anticholesterol Drugs. Curr Atheroscler Rep 16, 439 (2014). https://doi.org/10.1007/s11883-014-0439-8
Published:
DOI: https://doi.org/10.1007/s11883-014-0439-8