Abstract
Purpose of Review
The adaptive immune response orchestrated by type 2 T helper (Th2) lymphocytes, strictly cooperates with the innate response of group 2 innate lymphoid cells (ILC2), in the protection from helminths infection, as well as in the pathogenesis of allergic disease. The aim of this review is to explore the pathogenic role of ILC2 in different type 2-mediated disorders.
Recent Findings
Recent studies have shown that epithelial cell-derived cytokines and their responding cells, ILC2, play a pathogenic role in bronchial asthma, chronic rhinosinusitis, and atopic dermatitis.
Summary
The growing evidences of the contribution of ILC2 in the induction and maintenance of allergic inflammation in such disease suggest the possibility to target them in therapy. Biological therapies blocking ILC2 activation or neutralizing their effector cytokines are currently under evaluation to be used in patients with type 2-dominated diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
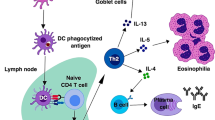
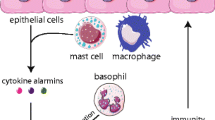
Group 2 innate lymphoid cells (ILC2), recently described in both mice and humans, belong to the innate immunity and are specialized in the induction and maintenance of type 2 inflammation [1]. ILC2 share a lot of functional similarities with Th2 lymphocytes, and these two subsets collaborate during type 2 immune responses, both the protective ones, such as the response to parasites and also the pathologic ones towards allergens, that are responsible for the allergic inflammation [2]. Differently from Th2 lymphocytes that preferentially localize in peripheral blood or lymphoid tissues, ILC2 mainly reside at the interface between the host and the environment, in the sub-mucosa of the lung and intestine as well as in the derma, and this strategical localization allows them to behave as initiators of type 2 immunity [3]. Indeed, ILC2 can be easily activated by epithelial cell which not only operate as a physical barrier, but also secrete damage-associated molecular patterns (DAMPs), cytokines, and chemokines, in response to pathogens, in this way, playing an important role in the beginning of the immune response [4].
ILC2 are part of a larger innate lymphoid cell (ILC) family, which also includes ILC1, ILC3, and NK cells, and differentiate from a common ILC precursor found in the bone marrow. Human ILC2 were first identified in the fetal and adult gut and lung but also in peripheral blood (PB) and nasal polyps of patients with chronic rhinosinusitis (CRS), and were found to express a panel of surface markers that can be used for both identification and enrichment [5•, 6, 7]. Human ILC2s lack the expression of classical lineage defining markers, but express the leucocyte marker CD45 and the “chemokine receptor homologous molecule expressed on TH2 cells” (CRTH2 = CD294), a molecule previously described to be expressed on human CD4+ Th2 cells [8]. In addition, ILC2 express the NK cell receptor CD161 [9] and ST2, a member of the IL-1 family receptors, which is part of the IL-33 receptor complex [10]. Among cytokine receptors, the IL-2Rα (CD25) and IL-7R are indispensable for the development, homoeostasis, and activation of ILC2s. The IL-7Rα chain forms a heterodimer with the “thymic stromal lymphopoietin” (TSLP) receptor. TSLP is able to activate cytokine production by ILC2s, but works more efficiently in combination with IL-2. A combination of these markers is frequently used for identifying human ILC2s (Table 1).
Unlike adaptive lymphocytes, ILCs do not express rearranged antigen receptors but they can be activated by several factors, and their knowledge is of help in understanding their physiologic and pathophysiologic activity. The just cited epithelial cytokines able to activate ILC2 are IL-33, IL-25, and TSLP; ILC2s are activated also by cytokines produced by T lymphocytes (IL-2, IL-4, IL-7, and IL-9) and lipid mediators (PGD2, cysteinyl leukotriens). Inhibitory signals comprehend soluble factors, such as IL-27, IFNγ, and IFN-α, and also cell-to-cell interaction (KLRG1/E-cadherin) [11]. The integration of activating and inhibitory signals determines their activity. Once activated, ILC2 secrete IL-4, IL-13, the epidermal-growth-factor-like molecule amphiregulin, and large amounts of IL-5, whose production is considered to be a hallmark of their function [12] (Table 1). Production of type 2 cytokines is not the unique function of ILC2, that have been described to be able to express CD154 and stimulate the production of IgE by B lymphocytes, independently by T helper cells, through IL-25/IL-33 stimulation or TLR triggering [5•]. Moreover, It has been suggested that they can directly activate CD4 + T cells, thanks to the expression of MHC class II, OX40L, CD80, and CD86 [13].
ILC2s have been shown to accumulate at the site of allergic inflammation, including the lungs of patients with bronchial asthma, the nasal polyps and sinus mucosa of subjects with chronic rhinosinusitis, and the skin lesions in atopic dermatitis. This review has the purpose to discuss recent findings about the pathogenic role of ILC2 in allergic diseases, such as bronchial asthma, chronic rhinosinusitis, and atopic dermatitis.
ILC2 and Bronchial Asthma
Asthma is a serious global health problem affecting all age group, whose prevalence is increasing in many countries, especially among children. It is characterized by several symptoms such as wheeze, shortness of breath, chest tightness, and cough associated with variable expiratory airflow limitation. Both symptoms and airflow limitation in bronchial asthma are the consequence of an inflammation of the airways. The inflammatory scenario in asthma varies among the different “asthma phenotypes,” for the different underlying disease processes. The most common phenotype of asthma is the allergic one, in the pathogenesis of which both Th2 cells and ILC2, surely play a pivotal role [2]. The functional importance of ILC2s in asthma inflammation has been firstly demonstrated in ILC2-deficient mice that exhibited less-severe lung inflammation and reduced eosinophils migration, and IL-13 production in comparison with wild-type mice, in response to intranasal administration with allergens, such as papain and house dust mites [14]. In particular, ILC2 seem to be involved in the process of airway remodeling, thanks to their constitutive expression of the epidermal-growth factor family member amphiregulin [15].
Epithelial cells of the airways are the main source of ILC2 activation. As airways are in continuous contact with the external environment, which results in exposure to an enormous array of allergens, microbes, and noxious particulate matter, airways epithelium responds to external environmental factors through activating sensory receptors, that allow detection of pathogen-associated molecular patterns and damage-associated molecular patterns, by releasing cytokines that activate neighboring cells [16]. These cytokines IL-25, IL-33, and thymic stromal lymphopoietin play a pivotal role in ILC2 activation and in the beginning of allergic inflammation [17]. In particular, the ability of IL-33 in driving the early type 2 response to allergens has been shown in a mouse model of acute airway inflammation that was induced by exposure to the fungus Alternaria alternata. Alternaria, but not other aeroallergens, possessed intrinsic serine protease activity that elicited the rapid release of IL-33 into the airways of mice through a mechanism that was dependent upon the activation of protease-activated receptor-2 and adenosine triphosphate signaling [18]. This peculiar ability of A. alternata to stimulate a massive release of IL-33 has important consequences in pathophysiology. It is indeed well known that fungal allergy is, among the sensitization to aeroallergens, the one most frequently associated with asthma [19], and in particular with the more severe forms of the disease, with acute exacerbations, and sometimes with fatal events [20]. The strong activation of ILC2 induced by the mold plays a crucial role in this “dangerous liaison,” between fungal sensitization and asthma.
Significantly greater numbers of total and type 2 cytokine-producing ILC2s were detected in blood and sputum of patients with severe asthma compared to mild asthmatics, and these cells could promote the persistence of airway eosinophilia [21•]. Recently, it has been reported that frequencies of ILC2 are similar in PBMCs of allergic subjects when compared with those of non-allergic control subjects [5•]. However, higher numbers of circulating ILC2s have been found in patients with allergic asthma when compared with allergic patients that do not display asthma [22]. The higher frequencies of ILC2s in the blood of asthmatic patients relative to non-asthmatic subjects might reflect the expansion of those cells before homing to the inflamed lower airways. Notably, also a strong linkage between activated ILC2s in the periphery and asthma control status has been described. In particular, uncontrolled asthma and partly controlled asthma patients had significantly higher percentages of IL-13 producing ILC2s compared to well-controlled asthma and healthy control groups [23]. The observation that these high frequencies of IL-13 producing ILC2, dramatically decreased when patients are treated and their symptoms become controlled, suggests the possibility that ILC2s might serve as a reliable predictor of asthma control status [23].
The growing evidences on the importance of ILC2 in asthma pathogenesis and the association with asthma control status have prompted several authors to hypothesize to target them in asthma therapy. It is still controversial whether ILC2 cells are corticosteroid resistant. The observation that ILC2 are increased in the airways of severe asthmatics despite high dose oral corticosteroid therapy compared to mild asthmatics has suggested that they may be steroid insensitive. Moreover, it has been reported that TSLP induced by allergen exposure makes ILC2s resistant to steroid-induced cell death through STAT5 activation [24]. On the other hand, in the presence of dexamethasone, both ILC2s and CD4+ T cells from mild asthmatics attenuate the IL-33-stimulated generation of type 2 cytokines, supporting the concept of their susceptibility to steroids [25]. Anyway, ILC2’s potential role as early source of type 2 cytokines and involvement in persistent asthma makes them an attractive cell type for therapeutic intervention, beyond their susceptibility to steroids. In this view, new therapeutic strategies targeting molecules involved in ILC2 activation are developing, particularly in eosinophilic asthma. The production of toxic mediators by eosinophils in the lungs of patients with asthma is responsible for tissue damage and remodeling. This is the reason why, in the last years, several inhibitors of IL-5 have been proposed to treat severe eosinophilic asthma [26]. The possibility to act on ILC2, the earliest source of eosinophil activation, through neutralization of TSLP has been investigated and proven to be effective in mild asthma [27], whereas the blocking of IL-25 and IL-33 pathways in humans is currently in course of evaluation in clinical trials. Since Th2 and ILC2 cooperate in the induction and maintenance of allergic inflammation, the possibility to use a drug that contemporary inhibits both the subsets may represent an intriguing and novel approach for asthma treatment, independently by its severity. In this direction, the DP2 receptor (CRTH2) inhibitor, fevipiprant, ables to reduce eosinophilic airway inflammation in patients with persistent moderate-to-severe asthma, represent a favorable tool in this direction [28]. Indeed, CRTH2 is expressed not only on ILC2 and Th2 lymphocytes, but also on eosinophils themselves, and in each of these subsets, induces cell-activating signals.
Collectively, several ports from recent literature agree on the opportunity to consider ILC2 as a potential target of treatment not only in allergic asthma, but also in those forms of non-allergic eosinophilic asthma that are supported by a type 2 inflammation, such as the aspirin-exacerbated asthma.
ILC2 and Chronic Rhinosinusitis
In addition to their presence in the lungs and peripheral blood, ILC2s may also be found in human nasal tissues and are found in increased numbers in patients with chronic rhinosinusitis (CRS) [5•, 29]. CRS is a heterogeneous disease characterized by local inflammation of the upper airways that affects 10% of the population in Europe and the USA. Based on histology and physical examination, CRS exist in two distinct forms, with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP). CRSwNP patients exhibit a greater infiltration of inflammatory cells, particularly ILC2s, and sustain higher levels of IL-5 and IL-13, when compared to CRSsNP patients without polyps, and to healthy controls. In this context, the finding that TSLP activity is increased in nasal polyps of patients with chronic rhinosinusitis is unsurprising [29]. Moreover, it has been reported an accumulation of ILC2 in nasal polyps of CRSwNP patients and in allergic CRS. In these patients, ILC2 numbers significantly correlated with Th2 cell frequencies, suggesting that, besides nasal polyps, allergy also has an independent positive association with the percentage of ILC2s [30].
All ILC subsets are present in nasal polyps, but ILC2 were dominant and significantly elevated compared to PBMC, tonsil, CRSsNP, and normal sinus tissue. In addition, ILC2 from nasal polyps express higher levels of inducible T cell co-stimulator (ICOS), whereas CD127 was downregulated respect to blood or tonsil ILC2. Furthermore, sorted ILC2, from nasal polyps, differently from the ones isolated from PB, spontaneously released type 2 cytokines including IL-5 and IL-13 [31]. Beyond the well-known role in promoting eosinophilic inflammation through IL-5 secretion, ILC2 in nasal polyps also induced Epstein–Barr virus-induced protein 2 (EBI2) expression on B cells, a marker of extrafollicular plasmablasts, which are B cells that are activated outside of the germinal center [32]. This observation suggests that, within NP, there is a unique B cell activation environment and that ILC2s may play an important role in B cell activation, as also reported elsewhere [5•].
These evidences, in their complex, indicate that ILC2 may play a significant pathogenic role in CRSwNP and suggest the need to develop new therapeutic strategies that interfere with the pathways of their recruitment and activation. This prospective would be of help particularly in those patients in which CRS and asthma are associated. It is indeed well known the frequent coexistence of CRS with asthma, as well as the impairment of asthma control in these subjects. Severe asthma is associated with high ILC2 cell counts in nasal tissues, and nasal surgery significantly improved asthma control and lung function in these patients [33]. The rapid decline in lung function associated with poor asthma control, observed in patients with severe asthma and CRS could at least in part explained by the pro-inflammatory activity played by ILC2.
ILC2 and Atopic Dermatitis
Atopic dermatitis (AD) is an inflammatory skin disease characterized by epidermal barrier dysfunction, due to complex genetic and environmental susceptibility factors, such as the filaggrin-null mutations [34]. This inherited epidermal abnormality is often associated with a type 2 immune responses to common environmental allergens, as shown by the high levels of IL-13 and IL-4 found in lesions of AD patients [35].
All three ILC subsets have been identified in healthy human adult skin, being ILC2 about 25% of the total ILC pool [36]. In AD patients, ILC2 infiltrate the skin, where they produce the type 2 cytokines IL-5 and IL-13, in addition to amphiregulin. Furthermore, it has been discovered that E-cadherin ligation on human ILC2, through interaction with KLRG1 (killer cell lectin-like receptor G1), results in a clear reduction of IL-5 and IL-13 production [37•]. The fact that downregulation of E-cadherin is characteristic of filaggrin insufficiency, suggests that the two mechanisms may cooperate in AD pathogenesis. On one hand, the absence of the suppressive activity on ILC2 caused by the absence of E-cadherin ligation favor a type 2 inflammation, on the other hand, the filaggrin deficiency is involved in the epidermal barrier dysfunction. Even if the increased numbers of ILC2 cells in AD lesions is not necessarily indicative of their contribution to inflammation, particularly in light of their additional role in restoring tissue homeostasis, some evidences suggest that excessive production of type 2 cytokines is associated with increased susceptibility to infections and inflammatory skin lesions [38•]. Recently, it has been shown in mice that the expression of IL-33 in the skin activates an immune response involving ILC2 and that this process might play a crucial role in the pathogenesis of allergic inflammation that is characteristic of atopic dermatitis. Indeed, transgenic mice with IL-33 expressed under the keratin 14 promoter developed a spontaneous AD-like inflammation of the skin which associated with ILC2 infiltration [39]. Another pathogenic cytokine in AD is IL-13, as shown by the finding that elevated IL-13 levels in the skin predisposes mice to inflammatory skin lesions. Of note, it has been demonstrated that ILC2 are the predominant IL-13-producing population in the skin [40]. More importantly, in humans, the blocking of IL-13 activity trough dupilumab, a mAb specific for IL4R-α chain, improved the signs and symptoms of atopic dermatitis [41]. The robust effects of dupilumab on skin inflammation confirm the pathogenic role of IL-4 and IL-13 signaling in AD, and further support the application of Th2 cytokine antagonists in the treatment of this disease. Also, TSLP has been proven to be involved in the activation of a population of skin-resident group 2 ILCs present in healthy human skin and enriched in lesional human skin from AD patients [42].
Collectively, these results demonstrate that ILC2 are present within the skin and suggest that they may play a role in AD pathogenesis.
Conclusions
ILC2 are essential in the initiation of allergic inflammation and are involved in the maintenance and propagation of inflammation in the airways and in the skin (Fig. 1). Therefore, therapeutic strategies for the treatment of type 2-mediated diseases should focus on ILC2 responses. Among such diseases, bronchial asthma probably represent the most rapid opportunity to evaluate the impact of ILC2 blocking in clinical manifestations and disease control. This is mainly due to the fact that several drugs blocking the activity of type 2 cytokines are developed in the recent years and approved or in course of validation, for asthma treatment. Most of these drugs, such as the IL-5 inhibitors, the IL-4/IL-13 inhibitor dupilumab and the CRTH2 inhibitor fevipiprant contemporary act on both Th2 and ILC2 subsets. This approach, that seems to be so promising in dampening type 2 inflammation, will be evaluated in the next years in terms of clinical efficacy. Other developing drugs that prevalently act on ILC2 will be more useful in the comprehension of the relative contribution of ILC2 and Th2 subsets in asthma pathogenesis. Anti-TSLP specific mAb has been proven to be efficacy in mild asthma, whereas IL-25 and IL-33 neutralization are currently under evaluation. Once established the efficacy of these drugs in asthma, it will be possible to eventually extend their usage to chronic sinusitis and atopic dermatitis.
ILC2 work like a bridge between epithelial cells and the immune system. ILC2 reside in human respiratory and gastrointestinal submucosal tissues as well as in derma. ILC2 can be promptly activated by epithelial release of alarmins (IL-25, IL-33, and TSLP) and also directly by PAMPs recognition. Once activated, ILC2 orchestrate the protective type 2 response that provokes helminth expulsion and tissue repair. When ILC2 are activated by environmental allergens, they work as initiators of allergic inflammation by stimulating both the innate and the adaptive immune response
In any case, it is generally accepted that ILC2 are involved in type 2-mediated inflammation, and, as consequence, interference with their activity represent a promising tool for the treatment of allergic diseases.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Karta MR, Broide DH, Doherty TA. Insights into group 2 innate lymphoid cells in human airway disease. Curr Allergy Asthma Rep. 2016;16:8. doi:10.1007/s11882-015-0581-6.
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56.
Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42.
Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92.
• Maggi L, Montaini G, Mazzoni A, Rossettini B, Capone M, Rossi MC, et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J Allergy Clin Immunol. 2017;139:964–76. ILC2s, once activated, express CD154 and produce type 2 cytokines, becoming able to induce IgE production in autologous B cells. It is also reported that ILC2 can be activated by ligands of Toll-like receptors 1, 4, and 6.
Halim TY. Group 2 innate lymphoid cells in disease. Int Immunol. 2016;28:13–22.
Nausch N, Mutapi F. Group 2 ILCs: a way of enhancing immune protection against human helminths? Parasite Immunol. 2017; doi:10.1111/pim.12450.
Cosmi L, Annunziato F, Galli G, Iwasaki M, Maggi E, Manetti R, et al. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9.
Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–35.
Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL- 33 receptor complex. J Immunol. 2007;179:2551–5.
Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–74.
Cosmi L, Annunziato F. ILC2 are the earliest recruiters of eosinophils in lungs of allergic asthmatic patients. Am J Respir Crit Care Med. 2017; doi:10.1164/rccm.201704-0799ED.
Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283.
Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133:1142–8.
Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746.
Mitchell PD, O'Byrne PM. Epithelial-derived cytokines in asthma. Chest. 2017;151:1338–44.
Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017;278:162–17.
Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–92.
Valero A, Quirce S, Dávila I, Delgado J, Domínguez-Ortega J. Allergic respiratory disease: different allergens, different symptoms. Allergy. 2017; doi:10.1111/all.13141.
Vianello A, Caminati M, Crivellaro M, El Mazloum R, Snenghi R, Schiappoli M, et al. Fatal asthma; is it still an epidemic? World Allergy Organ J. 2016;9:42.
• Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86. Authors found greater numbers of total and type 2 cytokine-producing ILC2s in blood and sputum of patients with severe asthma compared to mild asthmatics, suggesting a possible pathogenic correlation between ILC2 and asthma.
Lombardi V, Beuraud C, Neukirch C, Moussu H, Morizur L, Horiot S, et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol. 2016;138:305–8.
Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, et al. IL-13+type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am J Respir Cell Mol Biol. 2016;55:675–83.
Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675.
Chen R, Smith SG, Salter B, El-Gammal A, Oliveria JP, Obminski C, et al. Allergen-induced increases in sputum levels of group 2 innate lymphoid cells in asthmatic subjects. Am J Respir Crit Care Med. 2017; doi:10.1164/rccm.201612-2427OC.
Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16:186–200.
Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014 May 29;370:2102–10.
Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MF, Bacher G, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016:699–707.
Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.
Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–61.
Poposki JA, Klingler AI, Tan BK, Soroosh P, Banie H, Lewis G, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017; doi:10.1002/iid3.161.
Feldman S, Kasjanski R, Poposki J, Hernandez D, Chen JN, Norton JE, et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin Exp Allergy. 2017;47:457–66.
Lee TJ, Fu CH, Wang CH, Huang CC, Huang CC, Chang PH, et al. Impact of chronic rhinosinusitis on severe asthma patients. PLoS One. 2017;12:e0171047.
Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. NatGenet. 2006;38:441–6.
Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–7.
Teunissen MB, Munneke JM, Bernink JH, Spuls PI, Res PC, Te Velde A, et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR ILC3 in lesional skin and blood of psoriasis patients. J Investig Dermatol. 2014;134:2351–60.
• Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–50. Authors show that E-cadherin ligation on human ILC2 inhibits IL-5 and IL-13 production. Being the down-regulation of E-cadherin characteristic of filaggrin insufficiency, both these features may concur to atopic dermatitis pathogenesis.
• Roediger B, Kyle R, Le Gros G, Weninger W. Dermal group 2 innate lymphoid cells in atopic dermatitis and allergy. Curr Opin Immunol. 2014;31:108–14. Exhaustive review on the possible role played by ILC2 in the physiology and pathology of mouse and human skin.
Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–6.
Roediger R, Kyle KH, Yip N, Sumaria TV, Guy BS, Kim AJ, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–73.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16.
Acknowledgments
We thank Dr. Beatrice Rossettini and Dr. Gianni Montaini for their support in the preparation of the figure
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Immune Deficiency and Dysregulation
Rights and permissions
About this article
Cite this article
Cosmi, L., Liotta, F., Maggi, L. et al. Role of Type 2 Innate Lymphoid Cells in Allergic Diseases. Curr Allergy Asthma Rep 17, 66 (2017). https://doi.org/10.1007/s11882-017-0735-9
Published:
DOI: https://doi.org/10.1007/s11882-017-0735-9