Abstract
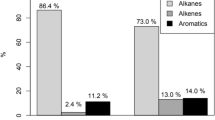
High-ozone concentrations currently represent the main air pollution problem in the city of São Paulo, Brazil. To elucidate the main volatile organic compounds (VOCs), which act as ozone precursors, samples from air quality monitoring stations were evaluated. Thirty-five samples were collected in August–September of 2006 and 43 in July–August of 2008, when the consumption of ethanol was about 50 % of the total fuel used in the São Paulo Metropolitan Area. Samples were collected using electropolished stainless canisters. Chemical analyses were performed on pre-concentrated samples followed by gas chromatograph with flame ionization and mass spectrometry detection. The incremental reactivity scale was used to rank the ozone precursors using the Ozone Isopleth Package for Research (OZIPR) trajectory model coupled with chemical mechanism Statewide Air Pollution Research Center (SAPRC). Sixty-nine species of VOCs were quantified, and the ten main ozone precursors identified in 2008 were as follows: formaldehyde (42.8 %), acetaldehyde (13.9 %), ethene (12.2 %), propene (5.1 %), 1-methylcyclopentene (3.0 %), p-xylene (2.4 %), 1-butene (2.1 %), trans-2-pentene (1.9 %), 2-methyl 2-butene (1.7 %) and trans-2-butene (1.6 %). Volatile organic compound mass distribution showed that in 2008 alkanes represented 46 % of the total VOCs, alkenes 27 %, aromatics 14 %, alkadienes 1 % and aldehydes 12 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric pollution in urban areas has been steadily increasing in developing countries due to the intensive growth of vehicular fleets and industrial activities. Urban air quality degradation has a negative impact in population health, animals and plants (WHO 2006).
In Brazil, most of the population live in urbanized spaces. The city of São Paulo, located in the southeast region of the country, accounts for 11 million inhabitants (IBGE 2013). This city and the areas surrounding it are part of an expanded metropolitan complex known as the São Paulo Metropolitan Area (or SPMA). The SPMA is formed by 39 municipalities and is inhabited by more than 20 million people. Those numbers make it the sixth largest metropolitan area of the world. Air quality standards are often exceeded in this area, mostly due to vehicular emissions, which has drawn the attention of public authorities in order to control vehicular pollution (CETESB 2015a, b, c).
Currently, ozone (O3) levels represent the main air pollution problem in the SPMA. In 2014, the national hourly air quality standard (160 μg m−3) was exceeded on 43 days as presented in Fig. 1, and even more in previous years. Studies conducted on tropospheric ozone demonstrate a complex temporal and spatial variability in this area. In order to decrease O3 concentrations, it is necessary to reduce emissions of ozone precursors, such as volatile organic compounds (VOCs), which play an important role in its formation through photochemical processes (CETESB 2015a, b, c; Orlando et al. 2010; Martins et al. 2015).
The effect of regional photochemistry on O3 levels is defined by the production and loss rates of the odd oxygen family (Ox = O3 + O(1D) + O(3P) + NO2 + 2 × NO3 + 3 × N2O5 + HNO3 + HNO4 + PAN), as defined by the Intergovernmental Panel on Climate Change (IPCC) (2007). The production and loss of odd oxygen species (Ox) is considered being equal to the production and loss of O3. This definition accounts for the rapid cycling between O3 and other forms of odd oxygen species.
The main urban air pollution source in the SPMA is the transportation sector, using gasoline, diesel and biofuels. Buses and trucks use diesel with 7 % of biodiesel (B7); automobiles use hydrated ethanol (gasoline with 25 % of anhydrous ethanol known as gasohol or Gasoline C), flex-fuel cars and compressed natural gas (CNG); and more recently is the intensive use of motorcycles (including flex-fuel motorcycles). Light vehicles, even though equipped with emission control technologies, tend to increase the problem due to the fleet quantity and age. Around 1.7 million of those vehicles are older than 15 years. They are also characterized by low efficiency, transporting only 1.2 people on average. PROCONVE (a national air pollution control program for automobiles) managed to decrease some of those emissions through regulations during its 20 years of operation. Nevertheless, air pollution problems still persist (Fig. 1). Although the state air quality standard value has dropped from 160 to 140 μg m−3 in 2012, the calculation is now based on an 8-h window.
Currently, the SPMA concentrates one of the world’s largest fleets, with more than 7 million vehicles (CETESB 2015a, b, c). Stationary and mobile sources combined account for approximately 132 kt/year of carbon monoxide (CO), 42 kt/year of hydrocarbons (VOCs), 77 kt/year of nitrogen oxides (NOx), 4.5 kt/year of particulate matter (PM) and 11 kt/year of sulphur oxides (SOx) emissions. However, the proportion of emissions related to motor vehicles are 97 % of CO, 81 % of VOCs, 80 % of NOx, 48 % of SOx and 40 % of PM. Light-duty vehicles are the main sources of CO and VOC emissions. Among them, gasohol vehicles are responsible for 50.7 % of CO emissions, ethanol vehicles for 6.1 %, flex vehicles for 8.0 % (both gasohol and ethanol emissions) and gasohol and flex motorcycles for 20.1 and 0.2 % of emissions, respectively. Despite flex-fuel and gasohol vehicle emissions showing similar figures, gasohol vehicles might have a greater emission factor due to their older and more polluting engines. In the last decade, the majority of light vehicles sold in Brazil were flex-fuel vehicles. Even though motorcycles represent a smaller fleet, their emission factor is relatively high, e.g. 20.3 % for CO and 9.7 % for VOCs (Garcia et al. 2013). Finally, heavy-duty vehicles are also important due to their NOx emissions, which account for 60.9 % of the total emissions in this area. Currently, in the SPMA fleet, gasoline corresponds to 45 % of the total fuel consumed, followed by 31 % of diesel and 24 % of ethanol (CETESB 2015a, b, c). Martin et al. (2002) suggested that 12 % of medical appointments in the Institute for the Heart of São Paulo are related to air pollution. Moreover, they also concluded that risk factors for cancer in São Paulo is 10 % higher compared to less polluted areas and 5–10 % of mortality (excluding accidents) in São Paulo could be associated to air pollutants.
Although studies on VOC composition, concentration, fluxes and emissions have been carried out in some Brazilian urban areas, those are scarce and focused mainly in the cities of São Paulo, Rio de Janeiro, Salvador, Porto Alegre and Curitiba (Grosjean 1997; Martins et al. 2015; Vasconcellos et al. 2005; Orlando et al. 2010).
Ethanol vehicles emit four times less CO than do gasoline vehicles. However, the incomplete combustion of ethanol produces more formaldehyde and acetaldehyde than gasoline (CETESB 2015a, b, c; Salvo and Geiger 2014). In Brazil, studies on the aldehydes in the atmosphere started in the 1980s, when ethanol was introduced as vehicular fuel. Nevertheless, the state environmental agency has not yet implemented a regulatory, temporally and spatially representative monitoring of VOCs. Aldehydes are more reactive in the atmosphere than alcohols (Grosjean 1997).
In 1975, the Brazilian National Bio-Fuel Program (Proálcool) was implemented. Currently, gasoline contains up to 25 % of anhydrous ethanol on a volumetric basis. Due to ethanol’s increasing popularity and usage, understanding the atmospheric chemistry of oxygenated VOCs is essential. Alcohols are generally emitted to the atmosphere through vegetation, solvent evaporation, fuels, fuel additives and fuel burning. Brazil is currently the leading country in use of ethanol as fuel (Grosjean 1997; Salvo and Geiger 2014).
Many important questions arise on the basis of the scenario presented above at the SPMA, such as the following: which are the most abundant VOCs? What are the main ozone precursors? How does ethanol use affect the atmospheric composition and ozone formation?
The main objective of this study is to determine the main VOCs that act as ozone precursors (using the incremental reactivity scale) and therefore elaborate strategies for ozone reduction in the singular scenario presented by the SPMA. Policymakers can use our results in order to determine which compounds are more important for continuous monitoring and what actions are needed to realistically lower emission limits.
Methodology
Sampling site
Samples were collected at CETESB station Cerqueira César, located at Avenida Dr. Arnaldo (23° 33′ 12″ S, 46° 40′ 23″ W), during August–September of 2006 (35 samples) and July–August of 2008 (43 samples), from 6:00 to 18:00 h every 2 h.
The sampling site is located 10 m from a very busy road close to an important financial centre, characterized by frequent, daily traffic jams with light-duty vehicles and buses, with an average of 43,000 vehicles/day on rush hours (according to CET 2014—Traffic Engineering Company of São Paulo). Besides the proximity to commercial and residential two- or three-storey buildings, there are also large green areas nearby (e.g. a university campus and cemeteries). There are also other busy roads in a 500-m radius around the site.
VOC chemical analyses
Hydrocarbons
The hydrocarbon (HC) sampling was based on the TO-14 and TO-15 methodologies (US EPA 1999a; 1999b). A Varian 3800 gas chromatograph (GC), with simultaneous detection by mass spectrometry (MS) (Saturn 2000) and flame ionization detection (FID), was used for chemical analyses. MS was used for the HC identification, and FID was used for quantification. This GC-MS-FID was used for the determination of HC with more than four carbon atoms.
Samples with 200 mL volume were analysed directly in the gas phase, using the cryogenic pre-concentration technique at −180 °C using a 1/8″ tube filled with a silanized glass bead. A DB-1 capillary column (60 m, 1.0 μm and 0.32 mm) was used, and a temperature program started at −50 °C and heated up to 200 °C at 6 °C min−1.
At the end of the chromatographic column, the gas sample was split in half using a Valco T-connector of low dead volume. One aliquot was carried into the MS, while the other half was quantified in the FID. An evacuated fused silica tube of 0.1 mm (ID) and 50 cm was used for MS and 0.32 mm (ID) and 25 cm for FID. The dimensions of these tubes were adjusted to compensate the high vacuum conditions required by the MS and atmospheric pressure in the FID.
For the identification and quantification of the light HC (C2 to C3), a Varian 3800 GC with FID was used, with a fused silica PLOT column of Al2O3/Na2SO4 (50 m, 0.53 mm and 10 μm). The pre-concentration step was similar to the one described previously, except for the temperature range (−50 to 180 °C at 10 °C min−1). For instrument calibration and to help on HC quantifications, we made use of a 10-ppbv standard gas mixture with 30 ozone precursors from the National Physical Laboratory (NPL). This standard gas contains alkanes, alkenes, alkynes, alkadienes and aromatic compounds diluted in nitrogen. Analytical curves were made by injecting the standard in different volumes, using a mass flow controller (Sierra Instrument 902C). Samples and standards were analysed in triplicates, following standard protocols (ABNT/INMETRO 2003; US EPA 1999c).
Aldehydes
The aldehydes were measured by CETESB using the TO-11A US EPA methodology (1999b). A sampling train composed of a critical orifice vacuum pump calibrated for 0.7 L min−1 was used for sample collection. To avoid condensation, the air sample was heated to 60 °C. A potassium iodide scrubber for O3 removal was used, connected prior to the silica cartridge coated with 2,4-dinitrophenylhydrazine (DNPH) solution (available commercially by several chemical suppliers). DNPH reacts with carbonyl compounds and produces hydrazone derivatives. After the sampling, the cartridges were sealed, packed and kept under refrigeration until the extraction procedure. In the laboratory, cartridges were extracted slowly with acetonitrile-grade HPLC in the opposite direction of the sampling, using a glass syringe to a 5-mL volumetric flask. The same procedure was performed for a field blank cartridge.
The identification and quantification of hydrazones were performed by HPLC, in a Shimadzu-10A instrument, using a Zorbax ODS (4.6 mm × 250 mm × 5 μm) analytic column, UV detection at 365 nm, acetonitrile/water (65:35) in a mobile phase at 1.0 mL min−1 and injection volume of 10 μL. Quantification was performed using the external standard method, using an analytical curve elaborated with formaldehyde and acetaldehyde standards, from Sigma-Aldrich.
OZIPR trajectory model
In this study, we used the Ozone Isopleth Package for Research (OZIPR) trajectory model coupled with the Statewide Air Pollution Research Center (SAPRC) chemical model. Both models are widely accepted by the international research community in atmospheric chemistry and are publicly available to users.
The OZIPR trajectory model (Tonnessen 2000) is a one-dimensional model (without spatial resolution) used to simulate ozone episodes common in urban areas. It requires hourly emission information and initial concentration data of CO, NOx and VOCs and its speciation and meteorological data. This model allows multiple simulations using several VOCs and NOx concentrations, providing an O3 isopleth graph. Finally, it is a useful tool for estimating VOC reactivity scales and has been largely used by the international community to help policymakers on reducing ozone-associated primary emissions aiming to improve air quality (Park and Kim 2002; Hong et al. 2005; Orlando et al. 2010; Teixeira et al. 2012; Garcia et al. 2013).
The SAPRC chemical model (Carter 1990, 2000) resolves thermal and photochemical reactions, with about 214 reactions and 83 species. Not all species are explicitly treated in the mechanism, so a grouping methodology was used. There were two grouping criteria: structure and reactivity similarities. In this work, we used five groups of alkanes, two of alkenes and two of aromatics.
The incremental reactivity (IR) was used for calculating the main ozone precursors emitted in the SPMA. This calculation was performed as detailed in Orlando et al. (2010). The IR of each VOC was multiplied by its respective concentration (μg m−3). The resulting IR is the average of positive (IR+) and negative (IR−) values. These are calculated by the increase or decrease respectively of 0.2 % of total VOC concentration (ppbC) for each VOC. The O3 concentration change is calculated using the maximum hourly O3 concentration after the increase or decrease of 0.2 % of VOCs and subtracted from the base value of O3.
where
- [O3]+ :
-
Ozone concentration after the increase in VOCs
- [VOC]+ :
-
VOC concentration after the increase
where
- [O3]− :
-
Ozone concentration after the decrease in VOCs
- [VOC]− :
-
VOC concentration after the decrease
The average between the IR+ and IR− values provided the maximum incremental reactivity IR for each VOC.
Averages of CO, NOx and VOC concentrations were measured during July and August of 2008. The NOx and CO concentration values were obtained by automatic analysers. The aldehydes were measured by CETESB using the TO-11A methodology by US EPA (1999b). For ethanol and methanol, we used the Sao Paulo city average concentrations obtained by Colón et al. (2001).
The hourly emissions of CO, VOCs and NOx were based on the emission inventory from CETESB, considering the time distribution of traffic jam (CETESB 2009). Because they represent the majority of the emissions, only the mobile source methodology is mentioned. For the CO, VOC and NOx emission calculation, a bottom-up approach was used, which considered the total fleet size and composition by each different type of vehicle, the annual distance travelled for each vehicle type, their respective emission factors and fuel efficiency and use. The annual distance travelled was based on a CETESB report on the intensity of vehicle usage by type (CETESB 2015b). The intensity of use was obtained from the fuel consumption observed in the SPMA, from the Brazilian Oil, Gas and Biofuel National Agency (ANP), and adjusted according to Eq. 4:
where
- AIadjusted :
-
Adjusted annual intensity of use per vehicle type (km year−1)
- AIreference :
-
Annual intensity of use per vehicle type (km year−1)
- Consumeobserved :
-
Total annual fuel consumption presented by ANP (L year−1)
- Consumeestimated :
-
Total annual fuel consumption (from all vehicle types), estimated from the reference values of intensity of use (L year−1)
Equation 5 describes the exhaust emission calculation.
where
- E:
-
Pollutant mass emitted during the considered period (g year−1)
- EF:
-
Emission factor, which varies according to the type of vehicle, pollutant and fuel consumed (g km−1)
- AI:
-
Intensity of use, or annual average distance travelled by the vehicle (km year−1)
- N:
-
Total vehicle fleet, by the type of vehicle and year (number of vehicles)
Emission factors vary according to vehicle age of manufacture, usage (total distance travelled), maintenance conditions and driving patterns. Regarding the increase in emissions due to usage, the National Inventory defines average emission increments per kilometer travelled for light-duty vehicles running on gasohol and ethanol based on governmental programs aimed to decrease emission factors, such as PROCONVE. These values are determined for the pollutants CO, NOx, VOCs and RCHO (aldehydes) and should be added to the emission factors every 80,000 km. Evaporative emissions were calculated based on the methodology presented by Vicentini (2010) and adapted to local conditions from the European Emission Guide (Vicentini 2010; CETESB 2015a, b, c). Temperature and relative humidity data were obtained at the CETESB station Pinheiros, located 4 km from Cerqueira Cesar station and with similar land use characteristics, and the mixing layer height from the Laboratory of Environmental and Laser Applications from IPEN, using a Light Detection and Ranging (LIDAR) remote sensing system.
Results and discussion
VOC study in the CETESB station in 2006 and 2008
Figure 2 shows the correlation between average CO concentrations and total VOCs for the samples collected in September 2006 and August 2008. The average total VOC concentration ranged from 57.1 ppbv (minimum 12:00 to 14:00 h) to 96.7 ppbv (maximum 6:00 to 8:00 h), presenting a 41 % decrease from morning to noon in 2006. In 2008, the average of the VOC maximum concentrations was 126.7 ppbv (6:00 to 8:00 h) and that of the average minimum 53.7 ppbv (14:00 to 16:00 h), corresponding to a 58 % decrease between the morning and afternoon. In 2006, CO concentrations ranged from 1.14 ppmv (12:00 to 14:00 h) to 1.78 ppmv (8:00 to 10:00 h), and in 2008 from 1.12 ppmv (14:00 to 16:00 h) to 2.53 ppmv (8:00 to 10:00 h). In 2006, there were 48 days with unfavourable atmospheric conditions for pollutant dispersion, while in 2008 there were 60 days. During autumn and winter (April to September), greater atmospheric stability leads to clear skies associated to atmospheric inversion layers very close to the surface in this region (below 200 m), with unfavourable conditions for the dispersion of pollutants in the SPMA (CETESB 2009). Wind speeds are usually low, with daily averages <1.5 m s−1, or even <0.5 m s−1 for many hours.
Figure 2 shows that during the consecutive sampling times of 6:00 to 8:00 h and 8:00 to 10:00 h (e.g. morning hours), the total VOC concentration decreased while the CO concentration increased. In this period, the average vehicle speed decreases due to increased traffic, triggering a difference in the engine speed. As CO is a primary pollutant, its increase is somewhat expected. Since VOCs are sensitive to photochemical reactions, its decrease is expected to occur mainly after 8:00 h.
The correlation coefficients between VOC and CO concentrations were 0.67 in 2006 and 0.87 in 2008, strongly suggesting that the main VOC source in the SPMA is also vehicular (Fig. 2). According to the CETESB emission inventory of 2011, the main VOC source from vehicles is the exhaust pipe, accounting for 48.7 % of emissions, followed by 28.4 % from crankcase (internal combustion engine) and evaporative emissions, 9.6 % from storage tank emissions and 13.3 % from industrial processes.
Figure 3 shows average concentrations for the five main VOCs (ppbv) and CO (ppmv), from 6:00 to 18:00 h, along with the average traffic congestion length of west São Paulo (where the monitoring sites stations are located), obtained from the local Traffic Engineering Company (CET).
In 2006, traffic length was 11 % higher in the overall studied period compared to 2008. Within the same time range (e.g. 6:00 to 12:00 h), traffic was 27 % more congested in 2006 than 2008. Nevertheless, within the same time interval VOC and CO concentrations were 22 and 27 % higher respectively in 2008 than in 2006. Higher pollutant concentrations in 2008 could be associated to factors such as boundary layer height, meteorological parameters and increase of the vehicular fleet. For instance, in the 2006 campaign the average wind speed was 1.63 m s−1, accumulated precipitation on the order of 60 mm (sampling days) and 15 thermal inversion episodes below 200 m high from August to September, and 14.6 % of days were considered calm or with no winds (surface wind speed <0.5 m s−1). In contrast, in 2008, the average wind speed was 0.85 m s−1, precipitation was of 41 mm, thermal inversion episodes (July–August) were recorded during 20 days and 21.5 % of the days were with calm or no winds.
Table 1 shows the 69 VOCs determined from the 43 samples collected in 2008 and the 70 VOCs determined from the 35 samples collected in 2006, in decreasing concentration order (ppbv) and their associated uncertainties. The rate coefficient for the compound-OH reaction is also shown (Carter 2010).
In 2006, the 10 most abundant VOCs represented 55 % of the total (during the 6:00 to 18:00-h interval), while in 2008 they were 63 %. Total concentrations were 11 % higher in 2008 than in 2006 (e.g. 82 versus 91 ppbv). While in 2006 the 10 most abundant VOCs added up to 45 ppbv, in 2008 the increase was 57 ppbv. In both years, VOC mass distributions per class of compound were very similar.
In this work, mean formaldehyde/acetaldehyde ratios of 1.1 and 1.0 were found for 2006 and 2008 respectively. The lowest ratios found were of 0.8 (2006) and 0.9 (2008) between 6:00 and 8:00 h. In the morning hours, solar radiation incidence is lower, and therefore, it is assumed that formaldehyde cannot be formed by photochemistry. Consequently, it must be originated from vehicular emissions. In 2006, the highest formaldehyde/acetaldehyde ratio of 1.4 was found at the sampling time 14:00 to 16:00 h. In 2008, a ratio of 1.1 was observed during 10:00 to 12:00 h, and 14:00 to 16:00 h sampling times. At these hours, both primary (vehicular emission) and secondary (photochemical) contributions are relevant.
In the work of Parrish et al. (2012) in the Houston-Galveston-Brazoria (HGB), it was estimated that about 92 % of the formaldehyde formation could be attributed to secondary reactions from compounds originated from stationary sources. However, in areas with prevailing vehicular sources of VOCs, the proportion of secondary and primary formaldehyde could be near 3:1. According to CETESB (2015a, b, c) for the SPMA, 81 % of the VOCs are emitted from vehicular sources, which use a blend of 75 % gasoline and 25 % of ethanol (Gasoline C—the official gasoline type in all Brazilian territory). In a previous work on aldehyde emissions from car exhausts using chassis dynamometers (Alvim et al. 2011), ethanol vehicles emitted on average 2.0 mg km−1 of formaldehyde and 10.7 mg km−1 of acetaldehyde, whereas Gasoline C vehicles emitted average values of 1.5 mg km−1 of formaldehyde and 4.8 mg km−1 of acetaldehyde. Light-duty vehicles emitted average values of 43 mg km−1 of formaldehyde and 15 mg km−1 of acetaldehyde. Given these figures, it is reasonable to conclude that the ratio between secondary and primary acetaldehyde in the SPMA is probably even lower than 3:1.
In Brazil, the formaldehyde/acetaldehyde ratios are generally low, because during the combustion of ethanol, acetaldehyde emissions are higher than formaldehyde (CETESB 2009). For instance, Vasconcellos et al. (2005) reported ratios of 0.8 to 2.8 at Juscelino Kubitschek Avenue (located in the western side of São Paulo) and 0.2 to 0.9 in samples collected near Complexo viário Maria Maluf, a tunnel in the southeast area of São Paulo. On both sites, measurements were taken every 2 h between 8:00 and 16:00 h in 2001. In contrast, during the combustion of ethanol-free gasoline, more formaldehyde is emitted than acetaldehyde. Consequently, in other cities worldwide, formaldehyde/acetaldehyde ratios are typically high, e.g. 7.2 in Osaka (Japan), 6.2 in Burbank and 32.2 in Lenox (USA).
In 2006, vehicular fleet ethanol usage represented 47.5 % of total fuels in the SPMA (considering the amounts of ethanol used by vehicles that run on ethanol and also the 25 % of ethanol which is added to Gasoline C). In 2008, this usage increased to 56.9 % in this area (CETESB 2009).
Table 2 shows the comparison of concentrations of the 15 most abundant compounds, higher than C2, in seven world megacities. The differences between these cities occur due to the difference in the fuel composition and engine technology as well as geographical and climatic differences. The main VOCs identified have two to nine carbon atoms. Toluene is one of the main eight compounds in all cities presented. M + p-xylenes are among the 15 most abundant VOCs in São Paulo, Dallas, Jinan and Yokohama. In all cities, alkenes were the most emitted VOCs. The main sources for those are gasoline evaporation, liquefied petroleum gas (LPG), natural gas emissions and incomplete fuel combustion. Aromatic hydrocarbons are emitted both from vehicle tailpipes and from solvent use.
In São Paulo, the vehicular fleet accounts for 97 % of VOC emissions. Mexico City, located in a valley surrounded by higher mountains and with poor ventilation, presented VOC concentrations 22 times higher than those found in Jinan, 13 times higher than what was found in Yokohama and Houston, 8 times higher compared to Dallas and 5 times higher compared to Taiwan. Lower VOCs such as propane, i-butane and n-butane in Mexico City were associated to the use of LPG (Blake and Rowland 1995; Hsieh and Tsai 2003; Qin et al. 2007; Wang et al. 2015; Tiwari et al. 2010; Johansson et al. 2014).
Main ozone-forming VOCs in 2008
The modelling procedure used is detailed in the work of Orlando et al. (2010). Hourly emissions of CO, NOx and VOCs were adjusted to fit ozone experimental and modelled values. The average initial concentrations of NOx, CO and VOCs for the studied period are presented in Table 3. Parameters such as mixing layer height and NOx and O3 dry deposition rates were adjusted to fit experimental CO, NOx and O3 concentrations and modelled values. CO was chosen for this first adjustment due to its low reactivity and for being emitted directly from the sources. It is important to point out that emission data obtained for the model are averaged values. Real emission values vary considerably during the day, mainly according to the number of vehicles in the SPMA, which is affected by rush hours, driving conditions and meteorological conditions. Figure 4 shows the results of simulated and experimental CO values after the aforementioned adjustments were performed.
After the CO adjustment, NOx was also adjusted using hourly emission values. At this step, an adjustment in its hourly concentration had to be made in order to reproduce observed measurements (daily average emission value was kept constant). Moreover, a small adjustment in the deposition rates of this pollutant was also necessary. Figure 4 shows the comparison between simulated and experimental NOx and O3 values. The adjustment of ozone experimental and modelled values was performed using VOC hourly emission values.
Ozone concentrations are not measured at Cerqueira César monitoring station. We used the CETESB stations Pinheiros and Parque D. Pedro II was used for our comparisons. Pinheiros station, located 4 km from Cerqueira César station, was chosen because it is also located in west Sao Paulo, in an area characterized by similar land use (two- to three-storey commercial and residential buildings and green areas), and also directly under the influence of emissions form a busy road nearby, although mainly by light-duty vehicles. Parque Pedro II station (5 km from Cerqueira César station) was used because it is located in a commercial area close to a bus terminal, also exposed to buses as Cerqueira César station.
After the adjustments of CO, NOx and O3 profiles, simulations were performed decreasing hourly emissions of VOCs and NOx in 5, 10, 20 and 30 %, independently and simultaneously, which are shown in Table 4. The results showed that a decrease in the emission rates of NOx would not be efficient for decreasing ozone concentration levels. Instead, a 30 % decrease would result in an actual increase in ozone levels by 20 %. This find is similar to what has been reported by Chiquetto et al. (2015), using the WRF/Chem model for the same study area. Results from Table 4 also indicate that the simultaneous decrease in NOx and VOCs also would not be very efficient, although some decrease in ozone levels may occur. On the other hand, a decrease of 5 % in VOC emission, keeping the current NOx levels, would lead to a decrease of about 6 % on ozone concentrations. In order to decrease ozone concentrations in 35 %, a decrease of 30 % in VOC emission would be necessary.
With the output data from the model, an isopleth graph for ozone was built (Fig. 5). This plot shows that, in the region corresponding to the SPMA conditions (the dot in the top area), an increase in VOCs leads to an increase in ozone, and a decrease in VOCs, a decrease in ozone. In Andrade et al. (2006), the Eulerian air quality model California Institute of Technology (CIT) was used to simulate decrease in VOC concentration, which also indicated a decrease in ozone concentration.
These results are significant due to the general lack of consistent VOC monitoring for this area. Currently, in the SPMA, there are 28 air quality monitoring stations, in a 7946.84-km2 area, with a population of more than 20 million inhabitants. However, none of the monitoring stations perform VOC monitoring in a regular basis. VOC regulation and monitoring (with the appropriate spatial and temporal representativeness) should be given more attention from policymakers both in the national and state levels.
In Fig. 6, the positive values of incremental reactivity are shown for the main 20 VOCs, which are ozone precursors. Formaldehyde presents an ozone formation potential about three times higher than the second compound studied.
Considering the ozone formation potential (IR) calculated, the five most important compounds for the winter of 2008 were formaldehyde, cis-2-butene, trans-2-butene, acetaldehyde and cis-2-pentene. In this work, only two species of aldehydes were measured (formaldehyde and acetaldehyde). However, the actual role of each species in ozone formation is shown in Table 5, obtained by multiplying its ozone formation capacity by its concentration in the atmosphere. In this scenario, formaldehyde was responsible for 43 % of the ozone in the atmosphere, while acetaldehyde accounts for 14 %. Together, they summed 57 % of ozone in the São Paulo atmosphere. The next class of compounds were the alkenes, which were responsible for 32 % of ozone in the atmosphere, followed by the aromatics with 10 %, and finally the alkadienes with 1 %. The alkanes, which represented 46 % in mass in the atmosphere, were responsible for only 0.03 % of the ozone formation in the atmosphere, due to their low reactivity. Considering the OH reaction rate with VOC species from the SAPRC mechanism and their concentration, the main species for ozone formation were 1-methylcyclopentene (11 %), ethane (8 %), acetaldehyde (7 %), trans-3-methyl-2-pentene (7 %) and 2-methyl-2-butene (6 %).
While urban atmospheres vary considerably in composition, aldehydes are important in the SPMA (3rd and 4th in concentration in this study). Moreover, according to the International Agency for Research on Cancer (IARC) (2015), formaldehyde is classified as a group 1 compound (carcinogenic for humans) and acetaldehyde as 2B (possibly carcinogenic). They are emitted directly to the atmosphere by biogenic and anthropogenic sources and can also be formed via photochemical reactions. Harrison (1998) reported that, in a population of one million people, six could develop leukaemia when exposed to a benzene concentration of 0.31 ppbv during their lives, and at SPMA benzene levels found were 1.09 ppbv in 2006 and 0.81 ppbv in 2008. Benzene is considered a group 1 compound and therefore carcinogenic to humans (see IARC 2015). The other monoaromatics are classified as probably carcinogenic or mutagenic. From the vehicular sources, toluene is the 8th and 7th compound with higher concentration found in this study in 2006 and 2008 respectively while benzene is the 22nd and 26th.
Conclusions
The 10 most abundant organic compounds measured at the CETESB station Cerqueira César in 2006 were (in order of abundance) isopentane, ethene, formaldehyde, ethane, acetaldehyde, butane, propane, toluene, 1-butene and pentane. In 2008 were ethene, propane, ethane, formaldehyde, acetaldehyde, butane, toluene, isopentane, pentane and propene. Considering the ozone formation potential using the OZIPR model for the study performed at this site during the winter of 2008, the five most reactive compounds for ozone formation were formaldehyde, cis-2-butene, trans-2-butene, acetaldehyde and cis-2-pentene. However, a clear scenario of each VOC contribution to ozone formation is attained when the IR, calculated by the OZIPR model, is multiplied by the compound concentration in the atmosphere. Formaldehyde is responsible for the formation of 43 % of ozone and 14 % of acetaldehyde. Other species found in this study that were also important for ozone formation are ethene, propene and 1-methylcyclopentene. The next class in importance were the alkenes, which accounted for 32 % of ozone formation, followed by the aromatics, responsible for 10 %, and lastly the alkadiene class with 1 %. Alkanes, which represented 46 % in mass in the atmosphere, were responsible for only 0.03 % for the tropospheric ozone formed, due to their low reactivity.
These results are important due to the scarcity of VOC monitoring in this area. They can be used to point out which measures could be more efficient when designing air quality management plans aimed at tropospheric ozone concentration control, in light of the unique fleet and fuel usage characteristics of the SPMA. Finally, these results also provide an idea of what kind of impact would be expected in the atmosphere of other large cities in the world, should they choose to adopt intensive ethanol fuel usage.
References
Alvim DS, Gatti LV, Côrrea SM, GarciaL FA, Vilarrubia ACF, Odriozola RR (2011) Determination of trace pollutants gases emission factors and the relative contribution of each type of vehicle/fuel on ozone formation in the SPMA. 51° Brazilian Congress of Chemistry, São Luiz, Brazil—(Document in Portuguese)
Andrade MF, Sánchez-Ccoyllo OR, Ynoue RY, Martins LD (2006) Impacts of ozone precursor limitation and meteorological variables on ozone concentration in São Paulo, Brazil. Atmos Environ 40:S552–S562
Blake DR, Rowland FS (1995) Urban leakage of liquefied petroleum gas and its impact on Mexico City air quality. Science 269:953–956
Carter WPL (1990) A detailed mechanism for the gas-phases atmospheric reactions of organic compounds. Atmos Environ 24A:481–518
Carter WPL (2000) Documentation of the SAPRC-99 Chemical Mechanism for VOC Reactivity Assessment. Final report to California Air Resources Board, Contract 92-329 and Contract 95-308
Carter WPL (2010) Development of the SAPRC-07 chemical mechanism. Atmos Environ 44:5324–5335
CET Companhia de Engenharia de Tráfego (2014) Traffic speed and figures research for the main road systems in São Paulo, 2013—(Document in Portuguese), Diretoria Adjunta de Planejamento, Projetos e Educação de Trânsito—DP
CETESB (2009) Air quality report for the Sao Paulo State 2008—(Document in Portuguese), ISSN 0103-4103
CETESB (2015a) Air quality report for the Sao Paulo State 2014—(Document in Portuguese), ISSN 0103-4103
CETESB (2015b) Use intensity curves by type of motor vehicle fleet of the city of São Paulo. São Paulo, 2013. 67 p. (Reporting Series)—(Document in Portuguese)
CETESB (2015c) Fuel evaporation inventory methodology in the supply of light vehicles of the Otto cycle. Sao Paulo-SP (Document in Portuguese)
Chiquetto JB, Silva MES, Ynoue RY, Cabral-Miranda W (2015) Changes in the spatial distribution of ozone, NO and CO in different vehicular emission scenarios in Sao Paulo. American Geophysical Union Fall Meeting 2015, San Francisco, U.S., 2015, doi 10.13140/RG.2.1.2688.1048
Colón M, Pleil JD, Hartlage TA, Guardani ML, Martins MH (2001) Survey of volatile organic compounds associated with automotive emissions in the urban airshed of São Paulo. Brazil Atmos Environ 35:4017–4031
Garcia LFA, Corrêa SM, Penteado R, Daemme LC, Gatti LV, Alvim DS (2013) Measurements of emissions from motorcycles and modeling its impact on air quality. J Braz Chem Soc 24(3):375–384
Grosjean D (1997) Atmospheric chemistry of alcohols. J Braz Chem Soc 8(4):433–442
Harrison RM (1998) In air pollution and health, issues in environmental science and technology. The Royal Soc of Chem 57:73
Hong YD, Lee SU, Han JS, Lee SJ, Kim SD, Kim YS (2005) A study on the reduction of photochemical ozone concentration using OZIPR in Seoul area. Korean J Environ Impact Assess 14:117–126
Hsieh CC, Tsai JH (2003) VOC concentration characteristics in Southern Taiwan. Chemosphere 50:545–556
IBGE (2013) 2012 Brazilian census—(Document in Portuguese), Instituto Brasileiro de Geografia e Estatística
INMETRO (2003) Rio de Janeiro: ABNT, INMETRO, 120 (Guidelines for expressing uncertainty in measurements —document in Portuguese)
Intergovernmental Panel on Climate Change (IPCC) (2007) Climate change 2007—the physical science basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC, vol 4. Cambridge University Press, New York, p. 549
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (IARC) (2015) Monographs on the evaluation of carcinogenic risk to humans. Outdoor Air Pollution. France, v. 109
Johansson JKE, Mellqvist J, Samuelsson J, Offerle B, Lefer B, Rappenglück B, Flynn J, Yarwood G (2014) Emission measurements of alkenes, alkanes, SO2, and NO2 from stationary sources in Southeast Texas over a 5-year period using SOF and mobile DOAS. JGR: Atmospheres 119:1973–1991
Martins LC, Latorre MRDO, Cardoso MRA, Gonçalves FLT, Saldiva PHN, Braga ALF (2002) Air pollution and emergency room visits due to pneumonia and influenza in São Paulo, Brazil (document in Portuguese). Rev Saude Publica 36(1):88–94
Martins EM, Nunes ACL, Corrêa SM (2015) Understanding ozone concentrations during weekdays and weekends in the urban area of the City of Rio de Janeiro. J Braz Chem Soc 26:1967–1975
Orlando JP, Alvim DS, Yamazaki A, Corrêa SM, Gatti LV (2010) Ozone precursors for the São Paulo metropolitan area. Sci Total Environ 408:1612–1620
Park JY, Kim YP (2002) On the optimum ozone control strategy in Seoul: case studies using OZIPR. J Korean Soc Atmos Environ 5:427–433
Parrish DD, Ryerson TB, Mellqvist J, Johansson J, Fried A, Richter D, Walega JG, Washenfelder RA, de Gouw JA, Peischl J, Aikin KC, McKeen SA, Frost GJ, Fehsenfeld FC, Herndon SC (2012) Primary and secondary sources of formaldehyde in urban atmospheres: Houston Texas region. Atmos Chem Phys 12:3273–3288
Qin Y, Walk T, Gary R, Yao X, Elles S (2007) C2-C10 non-methane hydrocarbons measured in Dallas, USA—seasonal trends and diurnal characteristics. Atmos Environ 41:6018–6032
Salvo A, Geiger FM (2014) Reduction in local ozone levels in urban São Paulo due to a shift from ethanol to gasoline use, Nat. Geoscience 7:450–458
Teixeira JR, Souza CV, Sodré ED, Corrêa SM (2012) Volatile organic compound emissions from a landfill, plume dispersion and the tropospheric ozone modeling. J Braz Chem Soc 23:496–504
Tiwari V, Hanai Y, Masunaga S (2010) Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Quality Atmos Health 3:65–75
Tonnessen GS (2000) User’s guide for executing OZIPR Version 2.0. USEPA, North Caroline
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY (1999a) Compendium method TO-11. Determination of formaldehyde in ambient air using adsorbent cartridge followed by high performance liquid chromatography (HPLC) [Active Sampling Methodology]. Jan., 1999. (EPA/625/R-96/010b)
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY COMPENDIUMMETHOD TO-14A (1999b) Determination of volatile organic compounds (VOCs) in ambient air using specially prepared canisters with subsequent analysis by Gas. Jan., 1999 (EPA/625/R-96/010b)
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY COMPENDIUMMETHOD TO-15 (1999c) Determination of volatile organic compounds (VOCs) in air collected in specially-prepared canisters and analyzed by gas chromatography/mass spectrometry (GC/MS). Jan., 1999. (EPA/625/R-96/010b)
Vasconcellos PC, Carvalho LRF, Pool CS (2005) Volatile organic compounds inside urban tunnels of São Paulo City, Brazil. J Brazil Chem Soc 16:1210–1216
Vicentini PC (2010) Methodology for the inventory of evaporative emissions from the fuel Otto cycle vehicle power system: product performance engines. PETROBRAS, Rio de Janeiro Document in Portuguese
Wang N, Li Na, Liu Z, Evans E (2015) Investigation of chemical reactivity and active components of ambient VOCs in Jinan, China. Air Quality Atmos Health 1–9
WORLD HEALTH ORGANIZATION (WHO) (2006) Air Quality Guidelines Global Update 2005: particulate matter, ozone, nitrogen dioxide and sulphur dioxide. Druckpartner Moser, Germany
Acknowledgments
This work was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (Sao Paulo State Research Foundation) (No. 03/14125-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPQ (National Counsel of Technological and Scientific Development) (No. 142777/2007-2). We are grateful for CETESB for the invitation to join the campaign for hydrocarbon collection and for providing data on other pollutants in this work. We also thank the Companhia de Engenharia de Tráfego—CET (Traffic Engineering Company) for providing us with the traffic data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvim, D.S., Gatti, L.V., Corrêa, S.M. et al. Main ozone-forming VOCs in the city of Sao Paulo: observations, modelling and impacts. Air Qual Atmos Health 10, 421–435 (2017). https://doi.org/10.1007/s11869-016-0429-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-016-0429-9