Opinion Statement
Hematopoietic stem cell transplantation (HSCT) is considered, since 1957, a potentially curative therapeutic option for many hemopathies. Although it is an aggressive procedure, improvements in transplantation techniques and supportive strategies have markedly decreased treatment-related mortality, and the prevalence of HSCT survivors is expected to exceed half a million by 2030. At the same time, there is a growing awareness of the potentially negative effects of HSCT-related therapies on the cardiovascular (CV) system, and HSCT survivors constitute a population at high cardiovascular (CV) risk. Cardio-oncology has been proposed as a new approach to prevent cardiovascular toxicity during and after HSCT. The present article attempts to provide a multidisciplinary and practical approach to the prevention, monitoring, and management of the most common cardiovascular complications in patients undergoing hematopoietic stem cell transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematopoietic stem cell transplantation (HSCT) is considered the only potentially curative therapeutic option for many hemopathies since 1957 [1]. Today more than 50,000 patients are expected to undergo HSCT annually worldwide, and the numbers are increasing each year [2, 3].
This upward trend is due to a better understanding of the procedure, improvements in the management of HSCT-associated complications, the possibility of transplanting older patients, and the increasing use of alternative hematopoietic sources like umbilical cord or haploidentical donors [4, 5]. Advances in transplantation techniques and supportive strategies have markedly decreased treatment-related mortality, and the prevalence of HSCT survivors is expected to exceed half a million by 2030 [4,5,6]. However, cardiovascular diseases (CVDs) are an important competing risk for morbidity and mortality, for up to 10 years, after transplantation [7, 8••, 9]. Thereby, cardio-oncology (CO), the multidisciplinary cardiovascular (CV) care of cancer patients, has been proposed as a new approach to improve prevention, early identification, and management of cardiotoxicity [10••, 11••, 12].
The present article attempts to provide a multidisciplinary and practical approach to the prevention, monitoring, and treatment of CV toxicities in patients referred for HSCT.
Hematopoietic stem cell transplantation overview
Depending on the origin of the infused stem cells, HSCT can be considered autologous (infusion of the patient’s own stem cell) or allogeneic (infusion of stem cell from a healthy compatible donor). Currently the most common indication for allogeneic HSCT in adults is acute myeloid leukemia (followed by acute lymphocytic leukemia, myelodysplastic/myeloproliferative syndromes, Hodgkin lymphoma and non-Hodgkin lymphomas) and for autologous HSCT multiple myeloma and other plasma cell dyscrasias [3,4,5].
HSCT is a long process that starts with several baseline tests and examinations to confirm HSCT indication and to assess patient’s general health. Once a patient is deemed fit for HSCT, key steps to move toward this therapy are collecting the stem cells (either from the own patient or a donor) and conditioning treatments before transplantation. The objective of conditioning protocols is both to destroy the existing bone marrow cells and eradicate cancer and, in allogeneic HSCT, to induce the immunosuppression that permits engraftment and prevents both rejection and graft versus host disease (GVHD) [13]. Subsequently, induced aplasia is reversed by reinfusion of hematopoietic progenitors.
Conditioning regimen protocols may differ according to baseline patient’s conditions and hematologic HSCT indications. In myeloablative conditioning regimens (MAC), high-intensity chemotherapy is administrated to completely eliminate the recipient’s bone marrow. Non-myeloablative (NMA) or reduced intensity (RIC) protocols are less toxic, but receptor’s hematopoiesis is not completely eliminated. NMA protocols are generally recommended in aged patients or those in whom MAC-induced toxicity is expected to be unacceptable due to concomitant comorbidities [14,15,16]. Overall survival is similar in NMA and MAC-HSCT based on a balance between treatment-efficacy and treatment-related mortality [17].
Direct HSCT-induced cardiovascular toxicity risk
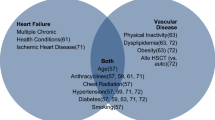
To define a global patient’s CV toxicity risk, we need to consider several factors: cardiotoxic effects of anticancer therapies received prior HSCT, the HSCT type, HSCT-related complications, and preexisting comorbidities (Table 1) [8••]. High doses of anthracyclines (cumulative doxorubicin or equivalent ≥ 250 mg/m2) or radiotherapy to a volume including the heart prior to HSCT increase the risk of CV toxicity [8••]. In addition, a higher cumulative incidence of CV events (CVEs) after allogeneic HSCT (7.5% incidence at 15 years F/U vs 2.3% after autologous HSCT) is expected, directly related with the presence of at least two cardiovascular risk factors (CVRFs) [18]. The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) is developed to predict non-relapse mortality risk in HSCT patients. HCT-CI score considers multiple variables including cardiovascular risk factors, cardiovascular diseases, cerebrovascular diseases, gastrointestinal diseases, renal failure, psychiatric disturbances, infections, rheumatologic diseases, and pulmonary diseases [19].
Although adverse CVEs are not frequent during the first days after HSCT, some patients may experience supraventricular arrhythmias, congestive heart failure, hypotension, pericardial effusion, and thromboembolic complications. In the early phase of HSCT, the most frequent CVEs are cardiac arrhythmias (atrial fibrillation and atrial flutter) with an incidence that may increase up to 27% [20]. CV toxicity in MAC protocols is mostly associated with the use of the alkylating agent cyclophosphamide. High doses of cyclophosphamide before HSCT are associated with a wide range of CVEs, being miopericarditis (7–26%), supraventricular tachyarrhythmias, and heart failure the most frequent [21]. NMA protocols have resulted in a lower incidence of CV toxicity. Melphalan, commonly used before autologous HSCT, is the most arrhythmogenic agent increasing the risk of atrial fibrillation up to 11% in patients receiving > 140 mg/m2 [22]. Fludarabine may induce cardiac dysfunction and heart failure in 8% of patients. The incidence of CVEs increases up to 14% in patients treated with both melphalan and fludarabine (2.5% severe CVEs) [23]. Carmustine can cause hypotension and tachyarrhythmias, and pericarditis has been described under cytarabine [8••].
The administration of pre-HSCT total body irradiation (TBI) further increases the vulnerability of the CV system. TBI may induce diabetes mellitus, dyslipidemia, metabolic syndrome, or hypertension and increases the risk of heart failure, coronary artery disease, conductions disorders, and pericardial effusion [24]. Moreover, acute GVHD is associated with thrombosis and inflammatory myocardial damage (myocarditis, heart failure, conduction abnormalities, arrhythmias, and pericardial effusions), and chronic GVHD (grades II–IV) has been related with the increasing risk of hypertension, diabetes, and dyslipidemia [25].
Cardio-oncology team approach
A standardized cardio-oncology approach is recommended in patients referred to HSCT [7, 8••, 10••, 11••, 12]. Multiple patient-related and treatment-related risk factors may co-exist, and a multidisciplinary team is required to stratify CV toxicity risk and implement preventive strategies (Figure 1).
Cardio-oncology team approach for HSCT: risk stratification and cardiovascular monitoring. ^Risk score for CV events in > 12 m HSCT survivors: age (< 30: 0 point; 30 ≤ 50: 2 points, ≥ 50: 3 points), anthracycline dose (> 250 mg/m2 1 point), chest radiation (1 point), hypertension (2 points), diabetes (2 points), and smoking (1 point) (Fig. 1). Patients are classifies in low (≤ 3 points), medium (4–5) and high (≥ 6) risk. Echo is recommended every 1-2 years in patients at high risk and every 5 years in low and medium risk population (reference (26): Armenian SH et a. Blood Adv 2018;2:1756–64). * Baseline computed tomography calcium score should be considered to optimize CV risk stratification in low and moderate risk patients; **Echocardiography should be performed at 3 and 12 months after HSCT, and in the long-term follow-up, recommendations are based on the presence of symptoms and CV toxicity risk. Abbreviations: BP Blood pressure; CV: cardiovascular; CVRF: cardiovascular risk factors; ECG: electrocardiogram; ECHO: echocardiography; Hb A1c: glycated hemoglobin; NPs: natriuretic peptides; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; DDI. Drug-drug interactions; Rt radiotherapy
Baseline cardiovascular toxicity risk stratification
Beyond the direct adverse effect of cancer therapies, preexisting modifiable and non-modifiable CVRF and CVDs are strong predictors of post-HSCT CV toxicity risk. Among non-modifiable risk factors, female sex and age (younger and older age at the time of administration) are the most well describe, although a genetic predisposition has been also suggested [10••, 11••, 12]. CVRF incidence is reported to be very high in patients referred for HSCT: 25% patients suffer hypertension, 5% diabetes, and 32% dyslipidemia [27, 28]. Indeed, these CVRF and obesity are associated with a 5.2-fold increased risk of vascular events (coronary artery disease or cerebrovascular disease) in HSCT survivors [29]. Preexisting CVDs, especially heart failure and coronary artery disease, are also common in patients with hematologic malignancies and increase CV toxicity risk [30] (Table 1). The HCT-CI score considers several CV risk factors and CV diseases to predict non-relapsed mortality: diabetes, obesity (BMI > 35 kg/m2), atrial fibrillation or flutter, sick sinus syndrome, ventricular arrhythmias, coronary artery disease, myocardial infarction, moderate to severe valvular heart diseases, and congestive heart failure or EF < 50%. HCT-CI categorizes with 1 point each cardiovascular condition except moderate to severe valvular heart diseases which is categorized with 3 points [19].
Baseline CV assessment goal is to detect and treat subclinical diseases and stratify CV toxicity risk. In patients without overt CVDs or previous cardiotoxicity, it can be considered a primary prevention strategy; whereas interventions in patients with known CVD or evidence of CV toxicity fall into the category of secondary prevention [31]. CV evaluation should include a comprehensive medical history and physical exam, body mass index calculation, heart rate and blood pressure measurement and fasting blood glucose, lipid profile, and HbA1c determination. Baseline computed tomography calcium score should be considered to optimize CV risk stratification in low and moderate risk patients [32, 33].
Baseline electrocardiography is required to rule out significant tachyarrhythmias and conduction abnormalities and to measure QT interval (using the Fridericia correction) [11••, 34].
The Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology, in collaboration with the ESC Council of Cardio-Oncology Council, has recently published a position statement on the use of biomarkers in cancer patients [35]. Although definite conclusions regarding the routine baseline determination of natriuretic peptides (NPs: NT-proBNP or BNP) and cardiac troponins (cTn) cannot be taken in HSCT patients, a comprehensive review of the literature suggests that baseline NPs and cTn may detect subclinical myocardial injury from previous anticancer treatments and help to identify patients at high risk of CV toxicity independent of changes in left ventricular ejection fraction (LVEF) [31, 36,37,38••].
Cardiac imaging plays a critical role, both from the diagnostic and the prognostic point of view [39]. We use cardiac imaging to stratify patients’ risk before HSCT (particularly low normal EF and moderate to severe valvular heart diseases), to identify early and late cancer therapy related cardiac dysfunction (CTRCD), and to detect cardiac damage in long-term HSCT survivors. Currently, surveillance and diagnosis of CTRCD is performed by using biplane echocardiography-derived LVEF (2D-LVEF) [11••, 39]. However, 2D-LVEF intratechnique variability exceeds 10% in some patients, making challenge early myocardial dysfunction detection and surveillance [39]. To identify early asymptomatic myocardial damage, we need to use more sensitive and reproducible parameters. From the echo perspective, if available, three-dimensional echocardiography LVEF (3D-LVEF) is the best method [11••, 39,40,41]. 3D-LVEF correlates well with cardiac magnetic resonance, which is currently considered the goal standard. It provides lower temporal variability than 2DE during cancer therapy [42] and, therefore, increases the ability to detect earlier and smaller changes in LVEF (average inter-observer variability 5%) [43]. Another critical question is if cardiac monitoring just based on EF is enough. In a recent series of patients treated with anthracyclines, early heart failure treatment based on 2D-LVEF drop allows for full recovery in only 11% patients [44]. At present, we can identify CTRCD at a preclinical phase, before symptoms onset and LVEF decreases, using myocardial deformation techniques. Technologies such as speckle tracking have demonstrated, in different oncologic or non-oncologic heart failure scenarios, that global longitudinal deformation (GLS) is a robust and sensitive marker to detect the presence of preclinical myocardial damage (stage B heart failure: asymptomatic structural heart disease) improving prognostic stratification in patients at risk of heart failure [11••, 12, 39, 45••].
In patients referred to HSCT a baseline echocardiography is the preferred imaging modality to assess cardiac function and detect valvular or pericardium diseases. If echo is unavailable or non-diagnostic, a cardiac magnetic resonance is preferred over nuclear techniques to minimize radiation exposure and provide a complete cardiac evaluation not just based on LVEF [11••, 12, 39]. Assessment of baseline LVEF and GLS is needed to rule out baseline left ventricular subclinical dysfunction and serves a control to interpret potential changes during treatment. A baseline low normal baseline LVEF (50–54%) or a reduced LVEF (< 50%) increase the risk of CTRCD [11••, 12, 39]. In fact, severe post-transplant cardiac dysfunction develops in up to 43% of patients with baseline reduced LVEF [8••]. Patients with moderate or severe valvular heart disease experience significantly higher non-relapse mortality after HSCT due to pulmonary complications, renal injury, and heart failure [46]. Further studies are needed to determine if cardioprotection or valvular intervention prior to HSCT may reduce this risk.
GLS is also an important diagnostic tool in patients with light-chain cardiac amyloidosis (AL) to detect cardiac involvement. Longitudinal left ventricular function can be severely depressed in AL cardiac amyloidosis despite a normal EF with a typically preserved apical GLS pattern [47]. GLS correlates well with late gadolinium enhancement in cardiac magnetic resonance (a marker of interstitial expansion due to amyloid deposits), and both parameters showed a base to apex gradient [47]. Additionally, GLS is a prognostic marker in patients with AL cardiac amyloidosis undergoing hematopoietic stem cell transplantation [48].
Early CV toxicity prevention, diagnosis, and management
Early detection and optimization of CVRF and subclinical CVDs would result in interventions that may delay or prevent the onset of clinically apparent CVDs. An aggressive management of CVRF according to general practice guidelines is recommended during the HSCT process [33, 49, 10••, 11••, 12]. (Table 2)
A close CV monitoring, including clinical, cardiovascular imaging, and biomarker evaluation, is recommended during the first year after HSCT. It is necessary to maintain a high clinical suspicion, and if symptoms occur, the patient should be referred to the cardio-oncology clinic [10••, 11••, 12]. Biomarkers are particularly useful given their high negative predictive value for future CVEs [38••]. Figure 1 summarizes HSCT patient’s follow-up during the early and chronic phase after HSCT and general CV preventive strategies. At 3 months after HSCT a comprehensive CV evaluation is recommended, including echocardiography. Although studies regarding prevention of CV toxicity after HSCT are relatively limited, early findings like an increase in NPs, a decrease in GLS > 15% form baseline or a reduced LVEF, even asymptomatic, may lead to considered the initiation of heart failure therapies (ACE inhibitors or angiotensin receptor blocker and beta-blockers) to prevent further left ventricular remodeling [49, 10••, 11••, 12].
Among patients undergoing HSCT, the risk of developing supraventricular arrhythmias, especially atrial fibrillation (AF), is high in the peri-transplant period [21, 22]. Although no association with increase short-term mortality has been shown, these arrhythmias increase the risk of heart failure and left ventricular dysfunction, the time to discharge, and the cost of care. The risk is higher among patients treated with melphalan and those with preexisting AF risk factors (older age, infections, dilated left atrium in baseline echocardiography, cardiac amyloidosis, asymptomatic conduction blocks and premature atrial contractions on baseline EKG, preexisting cardiac arrhythmias, and history of hypertension, heart failure, or coronary artery disease). Identification of these high-risk patients before HSCT may allow development of specific EKG monitoring (telemetry monitoring) for early diagnosis and management.
Peri-HSCT atrial fibrillation management requires weight and fluid management, especially for patients undergoing autologous HSCT and those with predisposing conditions for heart failure (low normal EF and preexisting cardiovascular diseases). Although a rate control strategy with beta-blockers is the most widely used during the first days after HSCT, this strategy is often challenging for patients with concurrent symptomatic heart failure, sepsis, or hypotension. A rhythm control strategy with electrical or pharmacological cardioversion might be considered in young patients and those with heart failure or beta-blocker contraindications. Amiodarone is the anti-arrhythmic agent generally considered for pharmacological cardioversion in HSCT patient. Recently, ibutilide was also found to be safe and effective in patients with cancer; however more studies are needed to determine the best strategy. Drug-drug interactions with antiarrhythmic drugs, chemotherapy and supportive medications (antifungal, antibiotics, antiemetic, etc.), and QT interval monitoring is required when amiodarone or ibutilide are used. Anticoagulation with therapeutic doses of LMWH is indicated if platelet count > 50,000 and CHA2DS2-VASc score ≥ 2. No guidelines are available to address the use of anticoagulant therapy in patients with a CHA2DS2-VASc score 0-1, and a high prothrombotic state because of the underlying hematologic malignancy and anticoagulation in these patients should be decided on a case-by-case basis after a multidisciplinary discussion [50]
Long-term CV toxicity after HSCT: prevalence and management
Although chronic GVHD and disease recurrence remain the principal cause of mortality in long-term HSCT survivors [51, 52], in the last few years, progresses in HSCT strategies, supportive care, and older HSCT candidates have led to a growing population at high CV risk [8••]. Compared to non-transplant population, the risk of late death due to CV complications is 4-fold higher after autologous HSCT in females and 2.3-fold higher after allogeneic HSCT [51, 52]. The risk of CV complications is also higher in older patients and in those patients with HSCT-specific complications such as chronic GVHD. Additionally, long-term cardiovascular complications often occur earlier than might be expected in the general population [8••, 18].
Compared to the general population, survivors have 7.0- to 15.9-fold increased risk of cardiovascular risk factors (CVRFs) at a much younger age [53, 54]. The prevalence of hypertension, diabetes, and dyslipidemia was significantly higher in HCST recipients (n = 1087; median age 44 years) compared to the general population (hypertension 43 vs 34.6%, diabetes 18.7 vs 8.5%, dyslipidemia 43 vs 40%) [55]. In addition, there is also a significant increase in the prescription of CVRF treatments at 1 year after HSCT [56].
HSCT survivors experience a premature risk of CVDs (RR: 0.6–5.6) compared with age-sex-matched controls without cancer, and the incidence of late CVEs increases over time [18, 58]. The pathogenesis of accelerated CVDs includes both the direct cardiotoxic effect of anticancer medications (cardiotoxic chemotherapy and/or radiotherapy prior to HSCT and the specific conditioning schemes pre-HSCT) and an indirect injury due to preexisting CVRF/CVD and CV lifestyle modifications (i.e., deconditioning, weight gain) during and following HSCT [54, 58]. Armenian et al. have recently proposed a risk model for the prediction of CVD (heart failure (HF) and coronary artery disease (CAD)) in 1-year HSCT survivors [26••]. They prospectively observed a population of 1828 patients (median age 45) who underwent HSCT and survived 1-year free of clinically evident CVD. CVDs occurred in 7.4% of individuals at 10-year follow-up (92 patients HF, 43 patients CAD). Risk score was based on age, anthracycline dose (> 250 mg/m2), chest radiation, hypertension, diabetes, and smoking (Fig. 1). A case cohort (n = 580) was used to validate the risk model. The 10-year cumulative incidences of CVDs in the low-, medium-, and high-risk groups were 3.7, 9.9, and 26.2%, respectively. Individuals in the high- and medium-risk groups were at 7.8-fold (95% confidence interval, 5.0–12.2) and 2.9-fold (95% confidence interval, 1.9–4.6) risk of developing CVD (referent group: low risk).
Among long-term HSCT survivors, CVD presents more frequently as arterial vascular disease (cerebrovascular disease and coronary artery disease) and congestive heart failure.
Vascular disease
Arterial disease involves inflammatory endothelial changes that are related to an accelerated and premature atherosclerotic process, attributed to pre-HSCT and conditioning-related radiotherapy, and compounded by cardiotoxic drugs and the development of hypertension, diabetes, and dyslipidemia in the early post-HSCT period [59, 60]. The appearance of CVRF is more common among patients treated with allogeneic than autologous HSCT [17, 18, 55]. Metabolic syndrome (overweight or obesity, hypertension, hypertriglyceridemia, low high-density lipoprotein cholesterol levels, and glucose intolerance) and support therapies that worsen glucose control (e.g., corticoids) also contribute to long-term CV morbidity-mortality, particularly after allogeneic HSCT [61]. Conditioning with total body irradiation (TBI) has been associated with an increased risk of dyslipidemia and diabetes (OR 3.4) [55]. However, the mechanism by which TBI increase these risk factors is not clear.
Graft versus host disease is a condition where donor T lymphocytes attack healthy tissue of the recipient by recognizing those cells as being “non-self.” Treatment used to manage this condition, such as corticoides and calcineurin inhibitors, can also increase risk of CVRF. A history of chronic GVHD has been associated with an increased risk of hypertension (RR of 3.2) and dyslipidemia (RR for 3.2) [25]. Acute GVHD is independently associated with both hypercholesterolemia and hypertriglyceridemia after allogeneic transplant [25].
The cumulative incidence of arterial events, such as clinically overt coronary artery disease or cerebrovascular disease, after allogeneic HSCT is 10% at 15 years and > 20% at 20 years [62]. The median age of the first CVD event was 40–49 years in different series [18], which is much earlier than would be expected for the general population (67 years) [63].
As a general rule, CVRF and CAD should be managed according to general guidelines, taking into account some peculiarities of the transplant management, such as multiple statin interactions with CYP 450 pathway transplant drugs (calcineurin inhibitors and antifungals). However, since statin treatment is related to a decrease in rate relapse and GVHD, its interruption before HSCT should be avoided [64]. In patients with metabolic syndrome and high hypertriglyceridemia levels, fibrates should be considered (Table 2).
Heart failure
The cumulative anthracycline dose (> 250 mg/m2) and radiation therapy (chest radiotherapy ≥ 35 Gy to a volume including the heart) are predictive of cancer therapy-related cardiac dysfunction. Armenian et al. evaluated the incidence of congestive heart failure in a case-control study in at least 1-year HSCT survivors, reporting an incidence of congestive heart failure of 11.7% with a median onset of symptoms 3 years post-transplant [65]. Currently, early treatment of symptomatic and asymptomatic left ventricular dysfunction, according to general HF guidelines, is of primary importance to prevent further clinical events. [66, 67]
Cardiac rehabilitation (CR) has shown to be safe and feasible in HSCT survivors. Current evidence indicates that exercise may attenuate the cancer treatment-induced declines in cardiorespiratory fitness and prevent CVEs. Although confirmation in larger studies is needed, exercise programs are particularly useful in patients with low functional capacity and those with or at risk of heart failure [69,68,69,71].
Follow-up protocols
Given the risks of late complications, an appropriate systematic long-term follow-up (LTFU) is critical for HSCT survivors. It is crucial to optimize CVRF and promote healthy lifestyle for all HSCT survivors and establish early screening and preventive practice in this population [8••, 72, 73] (Table 2). Health-care providers should increase awareness in patients and their families about the potential late effects of cancer therapies to increase their adherence to preventive strategies [7].
Lifestyle modifications (smoking cessation, healthy diet, weight reduction in the obese, avoidance of excess alcohol intake, and regular aerobic exercise) represent a crucial step of the survivorship care for the treatment and prevention of CVRFs and CVDs. No prospective, randomized studies are available in HSCT survivors to define optimal CVRF goals and treatment strategies. As a consequence, drug categories recommended in general population should be also used in this specific context, taking into consideration the presence of comorbidities and drug-drug interactions [8••, 10,11,••, 11••, 12, 73). Optimization of lips profile is one of the more complex tools because risk scores to decide LDL goals are not standardized for cancer patients [74, 75]. Based on previous consensus documents and general lipid guidelines, author’s protocol is summarized in Table 2. The very high-risk category includes patients with clinical or unequivocal imaging documented CVD (ischemic heart disease or cerebrovascular disease), diabetes mellitus with target organ damage (i.e., proteinuria), or a major risk factor such as smoking or marked hypercholesterolemia or marked hypertension, severe chronic kidney disease, or a calculated score ≥ 10%. High-risk patients are those with diabetes mellitus, moderate chronic kidney disease, a markedly elevated single risk factors, total cholesterol > 310 mg/dL, blood pressure ≥ 180/110 mmHg, or a calculated score ≥ 5% and < 10%.
A comprehensive CV evaluation is recommended at 3 months (± 100 days), and 12-month post-HSCT (Fig. 1, Table 2) [8••, 73] HSCT-specific recommendations for long-term surveillance include yearly physical exam including blood pressure and body mass index assessment, fasting lipid panel, blood sugar, and HbA1c measurement as well as ECG. Additionally NP determination is recommended in high-risk patients. An evaluation of global cardiac function echocardiography should be performed at 3 and 12 months after HSCT, and in the long-term follow-up, recommendations are based on the presence of symptoms and CV toxicity risk. Currently, echo is recommended every 1–2 years in asymptomatic high-risk patients (≥ 2CVRF, CVDs, or cardiotoxicity, high-dose doxorubicin ±radiotherapy, allogeneic HSCT and HSCT-related complications) and every 5 years in asymptomatic low–medium-risk patients. However these recommendations are based con expert consensus rather than on evidence-based trials. The use of GLS to diagnose early asymptomatic CTRCD in patients after HSCT remains to be determined; however, preliminary studies confirm that an early decrease in GLS after HSCT had the strongest predictive value for abnormal LVEF at 12 months (area under the curve 0.86; 95% CI, 0.76–0.96) [76].
Conclusions
Given the increasing number of HSCT survivors and their increased risk for premature CVRFs and CVDs, these patients may benefit from dedicated cardio-oncology programs that may help to minimize CV toxicity risk, to validate risk prediction models, and to organize evidence-based long-term follow-up protocols. Patient’s involvement in their own CV care is critical to minimize long term CVEs.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Little MT, Storb R. History of haematopoietic stem-cell transplantation. Nat Rev Cancer. 2002 Mar;2(3):231–8. https://doi.org/10.1038/nrc748.
World Health Organization (WHO). Hematopoietic stem cell transplantation. Available at: http://www.who.int/transplantation/hsctx/en/. Accessed 2 Jan 2021.
Worldwide Network for Blood & Marrow Transplantation. https://www.wbmt.org/wp-content/uploads/2020/07/2016-survey-slides-for-the-website_4_2020-Read-Only.pdf. Accessed 2 Jan 2021.
Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019, Bone Marrow Transplant. 2019;54(10):1525–52. https://doi.org/10.1038/s41409-019-0516-2.
Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T's come into focus. Bone Marrow Transplant. 2020;55(8):1604–13. https://doi.org/10.1038/s41409-020-0826-4.
Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–501. https://doi.org/10.1016/j.bbmt.2013.07.020.
Giaccone L, Felicetti F, Butera S, Faraci D, Cerrano M, Dionisi Vici M, et al. Optimal delivery of follow-up care after allogeneic hematopoietic stem-cell transplant: improving patient outcomes with a multidisciplinary approach. J Blood Med. 2020;11:141–62. https://doi.org/10.2147/JBM.S206027.
•• Oliveira GH, Al-Kindi SG, Guha A, Dey AK, Rhea IB, De Lima MJ. Cardiovascular risk assessment and management of patients undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2020. https://doi.org/10.1038/s41409-020-01080-1 Updated review on the topic.
Armenian SH, Horak D, Scott JM, Mills G, Siyahian A, Berano The J, et al. Cardiovascular function in long-term hematopoietic cell transplantation survivors. Biology of blood and marrow transplantation. J Am Soc Blood Marrow Transplant. 2017;23:700–5. https://doi.org/10.1016/j.bbmt.2017.01.006.
•• Lancellotti P, Suter TM, Lopez-Fernandez T, Galderisi M, Lyon AR, Van der Meer P, et al. Cardio-oncology services: rationale, organization, and implementation: a report from the ESC cardio-oncology council. Eur Heart J. 2019;40(22):1756–63. https://doi.org/10.1093/eurheartj/ehy453 This article summarizes tips and tricks to organize a cardio oncology service with practical recommendation regarding human and technical resources needed as well as standards for cardio-oncology team practices.
•• Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–801. https://doi.org/10.5603/KP.2016.0156 ESC consensus document highlighting main CV side effects of anticancer therapies and CV toxicity management.
López-Fernández T, Martín García A, Santaballa Beltrán A, Montero Luis A, García Sanz R, Mazón Ramos P, et al. Cardio-onco-hematology in clinical practice. Position Paper and Recommendations. Rev Esp Cardiol (Engl Ed). 2017;70(6):474–86. https://doi.org/10.1016/j.rec.2016.12.041.
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26. https://doi.org/10.1056/NEJMra052638.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. https://doi.org/10.1016/j.bbmt.2009.07.004.
Shimoni A, Hardan I, Shem-Tov N, Rand A, Herscovici C, Yerushalmi R, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21(10):2109–16. https://doi.org/10.1038/sj.leu.2404886.
Rubio MT, Labopin M, Blaise D, Socié G, Contreras RR, Chevallier P, et al. The impact of graft-versus-host disease prophylaxis in reduced-intensity conditioning allogeneic stem cell transplant in acute myeloid leukemia: a study from the acute leukemia working party of the European Group for Blood and Marrow Transplantation. Haematologica. 2015;100(5):683–9. https://doi.org/10.3324/haematol.2014.119339.
Shimoni A, Labopin M, Savani B, Volin L, Ehninger G, Kuball J, et al. Long-term survival and late events after allogeneic stem cell transplantation from HLA-matched siblings for acute myeloid leukemia with myeloablative compared to reduced-intensity conditioning: a report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J Hematol Oncol. 2016;9(1):118. https://doi.org/10.1186/s13045-016-0347-1.
Tichelli A, Bucher C, Rovó A, Stussi G, Stern M, Paulussen M, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–71. https://doi.org/10.1182/blood-2006-10-054080.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. https://doi.org/10.1182/blood-2005-05-2004.
Baker JK, Shank-Coviello J, Zhou B, Dixon J, McCorkle R, Sarpong D, et al. Cardiotoxicity in hematopoietic stem cell transplant: keeping the beat. Clin Lymphoma Myeloma Leuk. 2020;20(4):244–251.e4. https://doi.org/10.1016/j.clml.2019.12.027.
Dhesi S, Chu MP, Blevins G, Paterson I, Larratt L, Oudit GY, et al. Cyclophosphamide-induced cardiomyopathy: a case report, review, and recommendations for management. J Investig Med High Impact Case Rep. 2013;1(1):2324709613480346. https://doi.org/10.1177/2324709613480346.
Feliz V, Saiyad S, Ramarao S, Hammad K, Leonelli F, Guglin M. Melphalan induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol. 2011;34:356–9. https://doi.org/10.1002/clc.20904.
Peres E, Levine JE, Khaled YA, Ibrahim RB, Braun TM, Krijanovski OI, et al. Cardiac complications in patients undergoing a reduced intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(1):149–52. https://doi.org/10.1038/bmt.2009.97.
Ratosa I, Ivanetic PM. Cardiotoxicity of mediastinal radiotherapy. Rep Pract Oncol Radiother. 2019;24(6):629–64. https://doi.org/10.1016/j.rpor.2019.09.002.
Rackley C, Schultz KR, Goldman FD, Chan KW, Serrano A, Hulse JE, et al. Cardiac manifestations of graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:773–80. https://doi.org/10.1016/j.bbmt.2005.07.002.
•• Armenian SH, Yang D, Teh JB, Atencio LC, Gonzales A, Wong FL, et al. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018;2:1756–64. https://doi.org/10.1182/bloodadvances.2018019117 Risk score for CVD in HSCT survivors.
Blaser BW, Kim HT, Alyea EP 3rd, Ho VT, Cutler C, Armand P, et al. Hyperlipidemia and statin use after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2012;18(4):575–83. https://doi.org/10.1016/j.bbmt.2011.08.003.
Griffith ML, Savani BN, Boord J. B, Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2010;116(8):1197–204.
Armenian SH, Sun CL, Mills G, Berano The J, Francisco L, Durand JB, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(8):1138–44. https://doi.org/10.1016/j.bbmt.2010.02.021.
Al-Kindi SG, Oliveira GH. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for Onco-Cardiology. Mayo Clin Proc. 2016;91(1):81–3. https://doi.org/10.1016/j.mayocp.2015.09.009.
Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society. Eur J Heart Fail. 2020;22:1945–60. https://doi.org/10.1002/ejhf.1920.
Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging. 2018;11(8):1173–86. https://doi.org/10.1016/j.jcmg.2018.06.003.
Piepoli MF, Abreu A, Albus C, Ambrosetti M, Brotons C, Catapano AL, et al. Update on cardiovascular prevention in clinical practice: a position paper of the European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Prev Cardiol. 2020;27(2):181–205. https://doi.org/10.1177/2047487319893035.
Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89(9):1287–306. https://doi.org/10.1016/j.mayocp.2014.05.013.
Pudil R, Mueller C, Čelutkienė J, Henriksen PA, Lenihan D, Dent S, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1966–83. https://doi.org/10.1002/ejhf.2017.
Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–22. https://doi.org/10.1016/s0735-1097(00)00748-8.
Snowden JA, Hill GR, Hunt P, Carnoutsos S, Spearing RL, Espiner E, et al. Assessment of cardiotoxicity during haemopoietic stem cell transplantation with plasma brain natriuretic peptide. Bone Marrow Transplant. 2000;26:309–13. https://doi.org/10.1038/sj.bmt.1702507.
•• Alvarez-Cardona J, Zhang KW, Mitchell JD, Zaha VG, Fisch MJ, Lenihan DJ. Cardiac biomarkers during cancer therapy practical applications for cardio-oncology. JACC Cardio Oncol. 2020;2(5):791–4. https://doi.org/10.1016/j.jaccao.2020.08.014 Updated review on the topic.
Čelutkienė J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J, et al. The role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22(9):1504–24. https://doi.org/10.1002/ejhf.1957.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15(10):1063–93. https://doi.org/10.1093/ehjci/jeu192.
Lang RM, Badano LP, Mor-AVI V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. https://doi.org/10.1016/j.echo.2014.10.003.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. https://doi.org/10.1016/j.jacc.2012.09.035.
Zhang KW, Finkelman BS, Gulati G, Narayan HK, Upshaw J, Narayan V, et al. Abnormalities in 3-dimensional left ventricular mechanics with anthracycline chemotherapy are associated with systolic and diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11(8):1059–68. https://doi.org/10.1016/j.jcmg.2018.01.015.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8. https://doi.org/10.1161/CIRCULATIONAHA.114.013777.
•• Liu JL, Barac A, Thavendiranathan D, Scherrer-Crosbie M. Strain imaging in cardio-oncology. J Am Coll Cardiol Cardio Oncol. 2020;2:677–89. https://doi.org/10.1016/j.jaccao.2020.10.011 Updated review on the topic.
Brusen RM, Cheng RK, Masri SC, LeedyD SML. Moderate or severe valvular heart disease and outcomes in allogeneic stem cell transplantation. Int J Cardiol. 2019;292:166–70. https://doi.org/10.1016/j.ijcard.2019.05.032.
Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. J Am Coll Cardiol Img. 2016;9:126–38. https://doi.org/10.1016/j.jcmg.2015.05.014.
Pun SC, Landau HJ, Riedel ER, Jordan J, Yu AF, Hassoun H, et al. Prognostic and added value of two-dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J Am Soc Echocardiogr. 2018;31:64–70.
Bravo-Jaimes K, Marcellon R, Varanitskaya L, Kim PY, Iliescu C, Gilchrist SC, et al. Opportunities for improved cardiovascular disease prevention in oncology patients. Curr Opin Cardiol. 2020. https://doi.org/10.1097/HCO.0000000000000767 Online ahead of print.
Mathur P, Paydak H, Thanendrarajan S, van Rhee F. Atrial fibrillation in hematologic malignancies, especially after autologous hematopoietic stem cell transplantation: review of risk factors, current management, and future directions. Clin Lymphoma Myeloma Leuk. 2016;16(2):70–5. https://doi.org/10.1016/j.clml.2015.10.001.
Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Ma-rrow Transplant Survivor Study. Blood. 2005;105(11):4215–22. https://doi.org/10.1182/blood-2005-01-0035.
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92. https://doi.org/10.1182/blood-2007-03-082933.
Baker KS, Armenian S, Bhatia S. Long-term consequences of hematopoietic stem cell transplantation: current state of the science. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S90–6. https://doi.org/10.1016/j.bbmt.2009.09.017.
Scott JM, Armenian S, Giralt S, Moslehi J, Wang T, Jones LW. Cardiovascular disease following hematopoietic stem cell transplantation: pathogenesis, detection, and the cardioprotective role of aerobic training. Crit Rev Oncol Hematol. 2016;98:222–34. https://doi.org/10.1016/j.critrevonc.2015.11.007.
Armenian SH, Sun CL, Vase T, Ness KK, Blum E, Francisco L, et al. WongFL, Forman SJ, Bhatia S. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–12. https://doi.org/10.1182/blood-2012-06-437178.
Chow EJ, Wong K, Lee SJ, Cushing-Haugen KL, Flowers MED, Friedman DL, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(6):794–800. https://doi.org/10.1016/j.bbmt.2014.02.012.
Chow EJ, Mueller BA, Baker KS, Cushing-Haugen KL, Flowers ME, Martin PJ, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. https://doi.org/10.7326/0003-4819-155-1-201107050-00004.
Chow EJ, Baker KS, Lee SJ, Flowers MED, Cushing-Haugen KL, Inamoto Y, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–8. https://doi.org/10.1200/JCO.2013.52.6582.
Tichelli A, Bhatia S, Socié G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142(1):11–26. https://doi.org/10.1111/j.1365-2141.2008.07165.x.
Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation--lessons learned. Haematologica. 2008;93(8):1132–6. https://doi.org/10.3324/haematol.13514.
Bielorai B, Weintraub Y, Hutt D, Hemi R, Kanety H, Modan-Moses D, et al. The metabolic syndrome and its components in pediatric survivors of allogeneic hematopoietic stem cell transplantation. Clin Transpl. 2017;31(3). https://doi.org/10.1111/ctr.12903.
Armenian SH, Chow EJ. Cardiovascular disease in survivors of hematopoietic cell trans-plantation. Cancer. 2014;120(4):469–79. https://doi.org/10.1002/cncr.28444.
D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
Fujimaki K, Maruta A, Yoshida M, Sakai R, Tanabe J, Koharazawa H, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001;27(3):307–10. https://doi.org/10.1038/sj.bmt.1702783.
Armenian SH, Sun CL, Francisco L, Steinberger J, Kurian S, Wong FL, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26(34):5537–43. https://doi.org/10.1200/JCO.2008.17.7428.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–61. https://doi.org/10.1161/CIR.0000000000000509.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8):891–975. https://doi.org/10.1002/ejhf.592.
Rothe D, Cox-Kennett N, Buijs DM, Venner CP, Paterson DI, Gyenes GT, et al. Cardiac rehabilitation in patients with lymphoma undergoing autologous hematopoietic stem cell transplantation: a cardio-oncology pilot project can. J Cardiol. 2018;34(10 Suppl 2):S263–9.
Berleze Penna G, Costa da Silva T, Aparecida Paz A, Ziegler B. Functional capacity, pulmonary function, and quality of life in hematopoietic stem cell transplantation survivors. Support Care Cancer. 2021. https://doi.org/10.1007/s00520-020-05947-3.
Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36:2297–305. https://doi.org/10.1200/JCO.2017.77.5809.
Mohammed J, Smith SR, Burns L, Basak G, Aljurf M, Savani BN, et al. Role of physical therapy before and after hematopoietic stem cell transplantation: white paper report. Biol Blood Marrow Transplant. 2019;25:e191–8. https://doi.org/10.1016/j.bbmt.2019.01.018.
DeFilipp Z, Duarte RF, Snowden JA, Majhail NS, Greenfield DM, Miranda JL, Arat M, Baker KS, Burns LJ, Duncan CN, Gilleece M, Hale GA, Hamadani M, Hamilton BK, Hogan WJ, Hsu JW, Inamoto Y, Kamble RT, Lupo-Stanghellini MT, Malone AK, McCarthy P, Mohty M, Norkin M, Paplham P, Ramanathan M, Richart JM, Salooja N, Schouten HC, Schoemans H, Seber A, Steinberg A, Wirk BM, Wood WA, Battiwalla M, Flowers ME, Savani BN, Shaw BE. Metabolic syndrome and cardiovascular disease following hematopoietic cell transplantation: screening and preventive practice recommendations from CIBMTR and EBMT. Bone Marrow Transplant. 2017;52(2):173-182. doi: https://doi.org/10.1038/bmt.2016.203.
Rotz SJ, Ryan TD, Hayek SS. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J Thromb Thrombolysis. 2020. https://doi.org/10.1007/s11239-020-02344-9.
García AM, Mitroi C, Ramos PM. Stratification and management of cardiovascular risk in cancer patients. A consensus document of the SEC, FEC, SEOM, SEOR, SEHH, SEMG, AEEMT, AEEC, and AECC. Rev Esp Cardiol. 2021. https://doi.org/10.1016/j.recesp.2020.11.014.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455.
Paraskevaidis IA, Makavos G, Tsirigotis P, Psarogiannakopoulos P, Parissis J, Gkirkas K, et al. Deformation analysis of myocardial layers detects early cardiac dysfunction after chemotherapy in bone marrow transplantation patients: a continuous and additive cardiotoxicity process. J Am Soc Echocardiogr. 2017;30:1091–102. https://doi.org/10.1016/j.echo.2017.07.010.
Author information
Authors and Affiliations
Contributions
TLF contributed to conception and design, contributed to analysis and interpretation, and drafted the manuscript, KHB contributed to conception and design and drafted the manuscript, ALG contributed to conception and design and drafted the manuscript, ISB ALG contributed to conception and design and drafted the manuscript. Images and tables are originals
Corresponding author
Ethics declarations
Conflict of interest
Karem Humala Barbier declares that she has no conflict of interest. Ana López de la Guía declares that she has no conflict of interest. Irene Sánchez Vadillo declares that she has no conflict of interest. Teresa López-Fernández reports personal fees from Janssen, Amgen, Pfizer, MSD, Daiichi Sankyo, TEVA, Incyte, Iquone, Philips outside the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
López-Fernández, T., Vadillo, I.S., de la Guía, A.L. et al. Cardiovascular Issues in Hematopoietic Stem Cell Transplantation (HSCT). Curr. Treat. Options in Oncol. 22, 51 (2021). https://doi.org/10.1007/s11864-021-00850-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00850-3