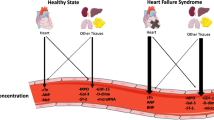
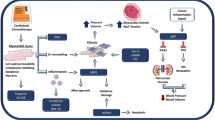
Opinion statement
Improvements in cancer survival have led to the emergence of cardiovascular disease as an important determinant of adverse outcome in survivors. Cancer therapeutics–related cardiac dysfunction is the most well-known form of cardiotoxicity. However, newer cancer therapies bring a broader range of cardiotoxicities. The optimal method to identify patients at risk of these complications is unclear, but circulating biomarkers comprise one possible approach. Troponins and natriuretic peptides have garnered the broadest evidence base for cardiotoxicity risk prediction, but other markers are being investigated. In this review, we explore evidence for circulating biomarkers in cardiotoxicity prediction associated with cancer therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer remains the second most common cause of death in the USA [1]. However, advancements in detection along with a growing arsenal of therapies have contributed to improvements in survivorship [1, 2]. This decline in mortality has led to the emergence of cardiovascular disease as an important cause of adverse outcome in survivors. Rates of cardiovascular mortality are up to tenfold higher in cancer survivors compared with the general US population [3], highlighting the importance of cardiovascular risk evaluation in this population, which necessarily includes the early identification of cardiotoxicities that result from cancer therapies, the most common manifestation being clinical or subclinical cancer therapeutics–related cardiac dysfunction (CTRCD). CTRCD has been variably defined as a decline in left ventricular ejection fraction (LVEF) of ≥ 5–10 to < 50–55% with or without symptoms [4,5,6,7]. Additionally, cancer therapies are associated with higher risks of arrhythmias, hypertension, ischemia, thromboembolism, or myocarditis.
Biomarkers have been proposed as one method of identifying patients at higher risk of cardiovascular consequences and improving the accuracy of risk stratification. A biomarker is “any characteristic that can be objectively measured and evaluated as an indicator of normal biological process, pathogenic process or pharmacological response to intervention” [8]. The definition is therefore broad and not limited to circulating biomarkers, but these have been extensively investigated owing to their potential for rapid and low-cost measurement. In this review, we explore the evidence for circulating biomarkers in cardiotoxicity prediction associated with cancer therapies.
Anthracyclines
Cardiotoxicity
Anthracyclines are widely used in the treatment of solid and hematologic malignancies. First reported in the late 1960s [9], the cardiotoxic effects of anthracyclines are the main contributor to CTRCD. The mechanism of anthracycline cardiotoxicity remains incompletely understood. Cardiomyocyte oxidative stress from excess formation of reactive oxygen species and a decrease in endogenous antioxidants is hypothesized to be an important cause [10]. There is also a significant role for topoisomerase-IIβ which can induce double-stranded breaks in DNA, decrease antioxidant gene transcription, and adversely impact mitochondrial biogenesis and function [11, 12]. Topoisomerase-IIβ deletion in a mouse model was also found to be protective against heart failure [11].
Anthracycline cardiotoxicity is often regarded as irreversible owing to underlying myocyte damage [13]. There is dose-related cardiotoxicity with the cumulative percentage of patients experiencing cardiotoxicity increasing from 7% at a cumulative doxorubicin dose of 150mg/m2 to 9% at 250 mg/m2, 18% at 350 mg/m2, 38% at 450 mg/m2, and 65% at 550 mg/m2 [14]. As a result, reassessment of LVEF after a cumulative dose of doxorubicin or equivalent of 250 mg/m2 is recommended [5]. In addition, age < 18 or > 65 years, cardiovascular comorbidities, prior high-dose chest radiotherapy, renal failure, and female sex are also risk factors for anthracycline cardiotoxicity [12].
Cardiac troponins
Cardiac troponin (cTn) is a component of the sarcomere, the contractile unit of the heart, with isoforms I (cTnI) and T (cTnT) considered cardiac-specific [15]. cTn becomes elevated with cardiomyocyte death. This is most frequently used to diagnose myocardial infarction where diagnosis requires levels exceeding the 99th percentile of a normal population and demonstrating a rising and falling pattern. The latter differentiates acute from chronic myocardial injury which is important when using cTn in cancer therapy, since multiple potential other causes of cTn elevation, such as renal dysfunction or sepsis, may contribute. Troponin assays are not standardized; thus, 99th percentiles often differ.
Early work evaluating cTnI and cardiotoxicity was performed in patients receiving high-dose chemotherapy. Cardinale et al. [16, 17] measured cTnI before and at multiple timepoints within 72 h of chemotherapy and found that, compared with patients who remained cTnI negative, cTnI-positive patients experienced a significant decrease in LVEF from 1 to 3 months which persisted until the end of follow-up at 7 to 12 months [16, 17]. Patients with positive cTnI were more likely to experience a decline in LVEF to < 50% (29% vs 0%, p < 0.001) [16] and heart failure occurred only in cTnI-positive patients [17]. Serum positivity in these early studies was defined by cTnI ≥ 0.5 ng/mL. Subsequent studies from this group using assays with more contemporaneous sensitivity (positivity defined by cTnI ≥ 0.08 ng/mL) [18,19,20] also found cTnI to be prognostic, correlating with the maximal decrease in LVEF (r = 0.78, p < 0.0001) especially if cTnI remained positive 1 month after the end of chemotherapy (r = 0.92, p < 0.0001) [20]. Patients with positive cTnI were also more likely to experience heart failure, asymptomatic decrease in LVEF, arrhythmias, or cardiac mortality than those with persistently normal cTnI. Although these studies form a strong basis for using cTnI to identify cardiotoxicity risk, they require frequent cTn sampling after chemotherapy which is impractical and costly. Additionally, these results have not consistently been replicated in subsequent studies [21, 22].
Relatively fewer studies have used cTnT assays. In one study of hematologic malignancies, cTnT positivity (≥ 0.03 ng/mL) was associated with a greater reduction in LVEF at 6 months (10% vs 2%, p = 0.017) [23]. By contrast, a separate study found no relationship between cTnT positivity (> 0.01 ng/mL) and reduction in LVEF [24]. This latter study was smaller and lower anthracycline doses were used. These factors may account for some of the differences seen but overall data are limited.
There has also been interest in the potential of high-sensitivity cTn assays to predict cardiotoxicity as they are more precise and can detect cTn levels many fold lower than conventional assays. By definition, these assays must provide measurable concentrations above the limit of detection in ≥ 50% of healthy individuals [25]. Thus, many apparently healthy people may have detectable high-sensitivity cTn levels. There is evidence that their use can predict future adverse cardiovascular events in asymptomatic populations [26, 27] driving enthusiasm to investigate their use for cardiovascular risk stratification in cancer patients. In one small study comparing high-sensitivity cTnI in patients receiving anthracycline and non-anthracycline-based therapy, only those receiving anthracyclines had high-sensitivity cTnI elevations [28]. Approximately 94% of detected levels would have been outside the range of detection for standard cTnI assays, indicating previously undetectable levels of cardiomyocyte injury. The added prognostic significance of these lower levels of detection is unclear, owing to small study size and lack of follow-up data. One short-term follow-up study found that high-sensitivity cTnI and cTnT were higher in patients receiving a cumulative epirubicin dose of ≥ 400 compared with those of < 400 mg/m2 but found no association between change in high-sensitivity cTnI and cTnT and change in LVEF [29]. Large-scale prospective studies are needed.
Natriuretic peptides
Amino-terminal proB-type natriuretic peptide (NT-proBNP) and B-type natriuretic peptide (BNP) are members of the natriuretic peptide family [30, 31]. Both are considered of equivalent clinical utility [32]. The main trigger for secretion is wall stretch and wall stress from pressure or volume loading of the cardiac chambers and therefore, both BNP and NT-proBNP have been useful in the diagnosis and prediction of incident heart failure [32,33,34]. Given that CTRCD is the common cardiotoxicity in cancer patients, there has been interest in using natriuretic peptides for cardiotoxicity risk prediction. Upper limits of normal reference values for heart failure are 100 pg/mL for BNP and 300 pg/mL for NT-proBNP in the acute setting and 35 pg/mL for BNP and 125 pg/mL for NT-proBNP in the non-acute setting [32]. Thresholds for prediction and/or detection of CTRCD have not yet been defined.
Natriuretic peptide elevations during chemotherapy have been associated with new left ventricular dysfunction and heart failure. Persistently elevated BNP during the first 72 h of high-dose chemotherapy was associated with a significant decrease in LVEF not seen in patients with no or only transient elevations [35]. These patients experienced a decrease in mean LVEF from 62.8 to 45.6% at 12-month follow-up. Similarly, only persistently elevated NT-proBNP was associated with a significant decrease in LVEF in another group receiving lower dose chemotherapy [21]. In this group, change in NT-proBNP from baseline to peak levels was predictive of decrease in LVEF at 12 months (area under the curve [AUC] 0.77, 95% confidence interval [CI] [0.66–0.86]). The number of cardiac events in this study was small but notably only occurred in patients with persistently elevated NT-proBNP. More recently, BNP above 100 pg/mL measured at the time that half the cumulative anthracycline dose was given was found to be a good predictor of incident heart failure (AUC 0.77, 95% CI [0.65–0.90]) [36]. However, the sensitivity at this cutoff was only 38%. By contrast, other studies have found that increases in BNP and NT-proBNP during chemotherapy are not associated with cardiotoxicity [22, 24, 29]. Direct comparison of studies is often challenging due to differences in definitions of cardiotoxicity, follow-up periods, and, in particular, the variable timing of BNP or NT-proBNP measurement in studies. Head-to-head studies of BNP and NT-proBNP are also lacking.
Novel biomarkers
Data on other circulating biomarkers during anthracycline therapy are limited. Aldosterone, epinephrine, endothelin, galectin-3, and c-reactive protein have been investigated but show no correlation with change in left ventricular function during treatment [22, 29]. Recently, there has been interest in micro-RNA which is involved in myocyte differentiation and development, but appears to be downregulated with anthracycline therapy [37]. Micro-RNA levels during doxorubicin therapy have also shown modest correlation with change in LVEF (r = − 0.531, p < 0.0001) [38]. These early findings require further validation and measurement of micro-RNA is currently a slow and expensive process.
Consensus recommendations
Major consensus recommendations generally support the use of cTn or natriuretic peptides to identify patients at increased risk of cardiotoxicity from anthracyclines (Table 1).
Trastuzumab
Cardiotoxicity
Approximately 20–25% of breast cancers overexpress the human epidermal growth factor receptor 2 (HER2), associated with more aggressive disease and lower disease-free and overall survival [41]. Trastuzumab, a monoclonal antibody targeting HER2, was a significant advancement in therapy and a recent Cochrane meta-analysis found that trastuzumab-containing regimens significantly improved progression-free survival (hazard ratio [HR] 0.61, 95% CI 0.54 to 0.70, p < 0.0001) and overall survival (HR 0.82, 95% CI 0.71 to 0.94) [42]. These improvements in outcomes however need to be balanced against the risk of cardiotoxicity. Rates of asymptomatic decline in LVEF and heart failure have been reported at up to 18.9% [43] and 4% [44], respectively, in clinical trials, while a recent meta-analysis estimated the pooled incidence of decline in LVEF and heart failure to be lower at 8.7% and 1.8%, respectively [45]. The mechanisms of cardiotoxicity remain incompletely understood. HER2 receptors on cardiomyocytes likely have an important role in cardiomyocyte growth and survival signals, and deletion in animal models has been associated with greater risk of developing dilated cardiomyopathy [46]. The risk is higher with prior anthracycline exposure. Other risk factors include older age, lower baseline LVEF, and cardiovascular risk factors. Newer anti-HER2 therapies do not have a high risk of cardiotoxicity [47, 48].
Cardiac troponins
Studies investigating the role of cTn in trastuzumab have largely enrolled patients who have already received anthracyclines. Elevated cTnI both immediately after anthracyclines and after trastuzumab (in patients with normal cTnI following anthracyclines) has been associated with incident cardiotoxicity in the adjuvant setting [19, 49, 50]. However, this may be attributable to the synergistic effects of anthracyclines with trastuzumab. For example, Demissei et al. [51] found that cardiotoxicity occurred in 39% receiving anthracyclines with trastuzumab, 14% receiving anthracyclines alone, and 17% receiving trastuzumab alone, and only patients receiving anthracyclines with or without trastuzumab experienced a significant increase in high-sensitivity cTnT. By contrast, patients not receiving anthracyclines appear to have low rates of cardiotoxicity. Yu et al. [52] followed a cohort receiving paclitaxel, trastuzumab, and pertuzumab for a median of 21 months and found that only 3 of 67 patients had transient increase in cTnI and only 2 of 67 patients experienced cardiotoxicity. In another group receiving neoadjuvant lapatinib and/or trastuzumab prior to surgery, only 5 of 172 patients had elevations in cTnT and none of the 11 patients who experienced a cardiac event had a cTnT elevation [53]. More studies are required to understand the true utility of cTn for trastuzumab therapy without anthracyclines.
Natriuretic peptides
Studies investigating the use of natriuretic peptides to predict cardiotoxicity with trastuzumab therapy have shown mixed results. De Iuliis et al. [54] found that, among patients receiving adjuvant trastuzumab, elevated NT-proBNP levels at 3 months predicted incident left ventricular dysfunction while elevated NT-proBNP at 6 and 12 months predicted mortality at 1 year. Demissei et al. [51] also found that NT-proBNP predicted a decrease in LVEF but this appeared to be driven predominantly by patients receiving serial anthracyclines; in this subgroup, each doubling in NT-proBNP was associated with a ~1.3% decrease in LVEF. By contrast, several studies have found no association between NT-proBNP levels and cardiotoxicity with adjuvant trastuzumab [49, 50, 55].
Novel biomarkers
Myeloperoxidase is being studied to identify cardiotoxicity risk and has a role in the innate immune response generating reactive oxidant species [56]. A doubling in baseline levels of myeloperoxidase was associated with a 30% higher risk of cardiotoxicity [51] and each standard deviation increase at 3 months into therapy was associated with a 34% increased risk of cardiotoxicity [50]. Myeloperoxidase and cTnI together provided additive information: the probability of cardiotoxicity at 15 months with none, one, or both biomarkers elevated was 20.4–23.4%, 31.6–33.9%, and 46.5%, respectively [50]. Importantly, patients enrolled in these studies had received trastuzumab after anthracyclines. Given the role of oxidative stress in anthracycline toxicity, whether these findings reflect the mechanisms of anthracycline or trastuzumab toxicity remains to be elucidated. ST2, growth differentiation factor-15, phosphatidylinositol-glycan biosynthesis class F protein, and galectin-3 have also been investigated but not found to be consistently associated with cardiotoxicity [50, 51].
Consensus recommendations
In comparison with anthracyclines, data are generally less robust for the use of biomarkers in monitoring for trastuzumab-related cardiotoxicity and this is reflected in consensus recommendations (Table 1).
Immune checkpoint inhibitors
Cardiotoxicity
Immune checkpoint inhibitors (ICIs) represent a class of monoclonal antibodies that restore the ability of the immune system to recognize and destroy cancer cells via a T cell–mediated response. Current therapeutic targets of ICIs include CTLA-4, PD-1, and PD-L1 [57]. Though considerable clinical benefit has been seen with use of these agents, there are several toxicities, including rash, pneumonitis, hepatotoxicity, and gastrointestinal side effects. Cardiotoxicity due to ICI therapy is a rare, but serious, complication of these agents, with myocarditis being the defining cardiovascular complication [57, 58]. Clinical manifestations include chest pain, heart failure, arrhythmias, heart block, cardiogenic shock, and even sudden death [58]. The pathophysiology of ICI-myocarditis is felt to be due to “on target” effects of T cells with direct immune infiltration or “off target” cross-reactivity to cardiac proteins [57]. The overall incidence of myocarditis is thought to be between 0.1 and 0.2% [59], and the risk of myocarditis is higher in individuals treated with combination ICI therapy. A recent pharmacovigilance study [60] identified 122 consecutive patients with myocarditis from ICI therapy with an incidence of 0.39%. Importantly, these cases occurred early after exposure to ICI (median 30 days) and were associated with a mortality of 50%.
Cardiac biomarkers
In the same pharmacovigilance study [60], the majority of cases of myocarditis had an elevation in troponins (94%) and natriuretic peptides (66%). Thus, it is recommended that cardiac biomarkers are checked in an individual with clinically suspected ICI-myocarditis [61]. In a multi-center registry of 35 patients with ICI-myocarditis, final or discharge cTnT > 1.5 ng/mL was associated with a fourfold increase risk of major adverse cardiac events [62]. Since concomitant myositis is estimated to be present in 23–25% of patients with myocarditis [57] and cTnT has known cross-reactivity with skeletal muscle [63], cTnI may be more specific for myocardial injury in ICI-myocarditis. However, routine screening with troponins in the early detection of ICI-myocarditis is not currently indicated based on mixed results of troponin surveillance in this setting. One study [64] evaluated screening troponin I and ECG in 76 asymptomatic patients with advanced melanoma on combination ICI therapy. Minimally elevated nondiagnostic troponin levels were seen in 17% of patients and none of the patients in the study developed clinical or subclinical myocarditis. Another study [65] evaluated troponins in 59 patients treated with nivolumab for non-small cell lung cancer. One patient with a sustained troponin elevation consistent with subclinical myocarditis tolerated continued ICI therapy without cardiac events. Ultimately, the diagnosis of ICI-myocarditis is made based on characteristic imaging and pathologic findings in combination with elevated biomarkers and abnormalities seen on electrocardiogram (ECG).
Consensus recommendations
Though no evidence-based algorithm exists, a framework for surveillance of patients treated with ICI using troponin and natriuretic peptides has been proposed (Table 2) [57]. Currently, there is no large-scale data on the use of high-sensitivity troponin to detect myocarditis in this population.
Chimeric antigen receptor T cell therapy
Cardiotoxicity
Chimeric antigen receptor (CAR) T cell therapy uses recombinant fusion proteins that are capable of activating T cells to recognize specific cancer cells in order to more effectively target and destroy them. The use of CAR-T therapy is limited by significant toxicity, with cytokine release syndrome (CRS) and neurotoxicity being the most widely reported [66]. Cardiotoxicity due to CAR-T therapy can manifest with arrhythmias, left ventricular dysfunction, profound hypotension, or shock requiring inotropic support [66]. CAR-T cardiotoxicity is felt to be due to both direct T cell–mediated cytotoxicity and indirect toxicity from CRS and systemic inflammation [67]. A recent retrospective review of 137 adult patients treated with CAR-T therapy [68] found that cardiovascular events occurred in 17 (12%) patients.
Cardiac biomarkers
Of the 137 patients [68], 53 (38%) had either contemporary or high-sensitivity troponin T checked before and after CAR-T cell infusion; 29 (54%) had a troponin elevation after infusion (> 30 ng/L for contemporary and > 14 ng/L for high-sensitivity). Cardiovascular events occurred in 16 of 29 patients (55%) with an elevated troponin compared to 1 of 24 (4.1%) patients with non-elevated troponins post-CAR-T infusion (p < 0.001). A close relationship was noted between the development of CRS, myocardial injury, and cardiovascular events, with all cardiovascular events occurring in patients with grade > 2 CRS and 95% of cardiovascular events occurring in patients with an elevated troponin, suggesting a role for troponin surveillance of cardiovascular events.
Consensus recommendations
No guideline recommendations exist regarding surveillance of cardiotoxicity in patients receiving CAR-T therapy. A cardiac monitoring protocol has recently been proposed by Ghosh et al. [66] consisting of baseline biomarkers with cardiac troponin and natriuretic peptide, ECG, a risk factor questionnaire, and an echocardiogram or cardiac MRI. Seven days post-CAR T cell infusion, patients receive biomarkers, ECG, and echocardiograms. If there is concern for cardiotoxicity, or grade > 2 CRS, an inpatient cardio-oncology evaluation is performed. Patients evaluated for cardiotoxicity, or with abnormalities on the seventh day investigation, have a cardiac MRI performed within 1 month after CAR-T therapy.
Anti-vascular endothelial growth factor therapy
Cardiotoxicity
Angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) signaling pathway (VSP) have emerged as successful therapeutic options in the treatment of several cancers, especially renal cell carcinoma and colorectal cancer. VSP inhibition can be accomplished through two distinct mechanisms. Agents can either [1] interact with the extracellular VSP components by competitively binding to the VEGF receptor (e.g., bevacizumab) or blocking signaling through the VEGF receptor (e.g., ramucirumab) or [2] inhibit tyrosine kinase activity of the intracellular domain (e.g., sorafenib, sunitinib) [69]. Anti-VEGF agents have several known cardiotoxicities associated with their use, the most common being an almost universal increase in blood pressure. A recent meta-analysis of 77 studies noted an overall incidence of severe hypertension in 7.4%, arterial thromboembolism in 1.8%, cardiac ischemia in 1.7%, and cardiac dysfunction in 2.3% of patients treated with anti-VEGF agents [70].
Cardiac biomarkers
There is limited data regarding the use of cardiac biomarkers in the surveillance of cardiotoxicity associated with anti-VEGF use. In a cohort of patients with advanced renal cell carcinoma treated with anti-VEGF therapy, 52 of 159 patients (33%) developed cardiotoxicity when hypertension was excluded as an event [71]. Of the 38 patients who had elevation in NT-proBNP levels during treatment, twelve also developed decreased LVEF. Of these twelve patients, ten developed abnormal natriuretic peptide levels at the same time, while two had a rise in NT-proBNP prior to a drop in LVEF. Troponins were elevated in only 4 of the 159 patients (3%), and only associated with symptoms in one patient. A more recent study prospectively followed patients treated with sunitinib for metastatic renal cell carcinoma [72]. Left ventricular dysfunction occurred in 9.7% of patients and biomarker elevation occurred in a total of 18.9% of patients (6.7% with elevation in high-sensitivity troponin I and 12.2% with an elevation in BNP). However, most of the patients with biomarker elevation did not have a corresponding decline in LVEF. LVEF declines and biomarker elevation occurred early and were not sustained.
Consensus recommendations
In the absence of evidence-based criteria, it is reasonable to obtain natriuretic peptides at baseline and then every 3 months on anti-VEGF therapy. Individuals who are felt to be at higher risk for cardiotoxicity due to the presence of other comorbidities, such as coronary artery disease, diabetes mellitus, hypothyroidism, or hypertension, may benefit from more frequent monitoring [69, 73].
Proteasome inhibitors
Cardiotoxicity
Proteasome inhibitors (PIs) promote the accrual of pro-apoptotic factors, ultimately leading to programmed cell death of cancer cells. Bortezomib forms stable, reversible interactions with the 26S proteasome while carfilzomib forms irreversible interactions with the 20S proteasome, allowing for greater proteasome inhibition [74]. Proteasomes are of particular importance to cardiomyocytes, cells that are under constant physiologic stress with limited regenerative potential [73]. As such, potentially serious cardiovascular complications have been reported with PIs, including heart failure, hypertension, and arrhythmias [74].
Cardiac biomarkers
A recent study found that cardiovascular events occurred in 51% of patients treated with carfilzomib and 17% of those treated with bortezomib. The majority of cardiac events (86%) occurred within the first 3 months after starting therapy [75]. Patients receiving carfilzomib-based therapy with a baseline elevated BNP > 100 pg/mL or NT-proBNP > 125 pg/mL had an increased risk of cardiovascular event (OR 10.8, p <0.001). Elevated natriuretic peptides occurring mid-first cycle of treatment with carfilzomib were also associated with a significantly higher risk of cardiovascular event (OR 36.0, p < 0.001). Another study assessed biomarker fluctuation in 65 consecutive patients treated with bortezomib for AL amyloidosis [76]. NT-proBNP and troponin T were found to significantly increase in patients with stage III/IV AL amyloidosis within the first 3 months of therapy, and these increases eventually stabilized and improved after 4–6 months. Increases in NT-proBNP of > 5000 pg/mL were associated with death after the first year of diagnosis.
Consensus recommendations
There are no formal guidelines with regard to the use of biomarkers such as BNP and NT-proBNP with respect to monitoring for PI-induced cardiotoxicity. Though the above data suggests some predictive utility, natriuretic peptides should be interpreted with caution in patients with multiple myeloma with renal involvement, as they can be elevated in the absence of cardiac dysfunction [77].
Future directions
The heterogeneity of current trial designs, populations, and assays makes synthesizing relevant conclusions challenging. Consideration in this regard for future studies may assist in strengthening the evidence base for circulating biomarkers. Longer follow-up data are also needed. Although high-sensitivity cTn is fast becoming the standard assay globally, most of the studies described are based on traditional cTn assays and more data on high-sensitivity assays are required. Global longitudinal strain has in recent years established a strong evidence base for identifying risk for CTRCD, but a comparison of the utility of strain versus biomarkers and the incremental ability of both together to identify cardiotoxicity risk remains unclear. Finally, an important but unanswered question is whether biomarker-guided management of cardiotoxicity can improve patient outcomes.
Conclusions
Cardiac biomarkers are emerging as important diagnostic tools in the prediction, surveillance, and diagnosis of cardiotoxicity from a wide range of cancer treatments. More evidence is needed to establish standards in care regarding the timing and frequency of testing, thresholds for clinically significant changes, and whether interventions based on these thresholds have meaningful outcomes. Ultimately, the goal of biomarker-based diagnostic algorithms is to identify individuals at high risk for cardiotoxicity to prompt the initiation of treatment strategies that mitigate cardiovascular risk while allowing for effective treatment of the patient’s cancer.
Data availability
Not applicable.
Abbreviations
- BNP:
-
Brain-type natriuretic peptide
- cTn:
-
Cardiac troponins
- cTnI:
-
Cardiac troponin I
- cTnT:
-
Cardiac troponin T
- CTRCD:
-
Cancer therapeutics–related cardiac dysfunction
- HER2:
-
Human epidermal growth factor receptor 2
- LVEF:
-
Left ventricular ejection fraction
- NT-proBNP:
-
Amino-terminal pro-brain-type natriuretic peptide
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85.
Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97.
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–21.
Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–90.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72.
Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95.
Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20(3):333–53.
Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339(13):900–5.
Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42.
Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64(9):938–45.
Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23(13):2900–2.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–79.
Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113(14):1708–18.
Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36(2):517–22.
Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, et al. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13(5):710–5.
Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–81.
Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–6.
Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–54.
Romano S, Fratini S, Ricevuto E, Procaccini V, Stifano G, Mancini M, et al. Serial measurements of NT-proBNP are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer. 2011;105(11):1663–8.
Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G, et al. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148(2):194–8.
Auner HW, Tinchon C, Linkesch W, Tiran A, Quehenberger F, Link H, et al. Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol. 2003;82(4):218–22.
Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008;97(5):318–26.
Sherwood MW, Newby LK. High-sensitivity troponin assays: evidence, indications, and reasonable use. J Am Heart Assoc. 2014;3(1):e000403.
Blankenberg S, Salomaa V, Makarova N, Ojeda F, Wild P, Lackner KJ, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J. 2016;37(30):2428–37.
de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–12.
Jones M, O’Gorman P, Kelly C, Mahon N, Fitzgibbon MC. High-sensitive cardiac troponin-I facilitates timely detection of subclinical anthracycline-mediated cardiac injury. Ann Clin Biochem. 2017;54(1):149–57.
Gulati G, Heck SL, Røsjø H, Ree AH, Hoffmann P, Hagve TA, et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study. J Am Heart Assoc. 2017;6(11).
Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–66.
Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL Jr. Biology of the natriuretic peptides. Am J Cardiol. 2008;101(3A):3–8.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: multi-ethnic study of atherosclerosis. Circ Heart Fail. 2012;5(6):727–34.
Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, et al. N-terminal pro-brain natriuretic peptide and heart failure risk among individuals with and without obesity: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016;133(7):631–8.
Sandri MT, Salvatici M, Cardinale D, Zorzino L, Passerini R, Lentati P, et al. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem. 2005;51(8):1405–10.
Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9(5):e96736.
Oatmen KE, Toro-Salazar OH, Hauser K, Zellars KN, Mason KC, Hor K, et al. Identification of a novel microRNA profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am J Physiol Heart Circ Physiol. 2018;315(5):H1443–h52.
Rigaud VO, Ferreira LR, Ayub-Ferreira SM, Ávila MS, Brandão SM, Cruz FD, et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget. 2017;8(4):6994–7002.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911.
Pudil R, Mueller C, Čelutkienė J, Henriksen PA, Lenihan D, Dent S, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Balduzzi S, Mantarro S, Guarneri V, Tagliabue L, Pistotti V, Moja L, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;2014(6):Cd006242.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31):3792–9.
Eiger D, Franzoi MA, Pondé N, Brandão M, de Angelis C, Schmitt Nogueira M, et al. Cardiotoxicity of trastuzumab given for 12 months compared to shorter treatment periods: a systematic review and meta-analysis of six clinical trials. ESMO Open. 2020;5(1):e000659.
Florido R, Smith KL, Cuomo KK, Russell SD. Cardiotoxicity from human epidermal growth factor receptor-2 (HER2) targeted therapies. J Am Heart Assoc. 2017;6(9).
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43.
Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2011;366(2):109–19.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–80.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imag. 2012;5(5):596–603.
Demissei BG, Hubbard RA, Zhang L, Smith AM, Sheline K, McDonald C, et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc. 2020;9(2):e014708.
Yu AF, Manrique C, Pun S, Liu JE, Mara E, Fleisher M, et al. Cardiac safety of paclitaxel plus trastuzumab and pertuzumab in patients with HER2-positive metastatic breast cancer. Oncologist. 2016;21(4):418–24.
Ponde N, Bradbury I, Lambertini M, Ewer M, Campbell C, Ameels H, et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1-06). Breast Cancer Res Treat. 2018;168(3):631–8.
De Iuliis F, Salerno G, Taglieri L, De Biase L, Lanza R, Cardelli P, et al. Serum biomarkers evaluation to predict chemotherapy-induced cardiotoxicity in breast cancer patients. Tumour Biol. 2016;37(3):3379–87.
Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63(8):809–16.
Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102–11.
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e58.
Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19(3):21.
Zhang L, Jones-O’Connor M, Awadalla M, Zlotoff DA, Thavendiranathan P, Groarke JD, et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Treat Options Cardiovasc Med. 2019;21(7):32.
Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89.
Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64.
Wu AHB, Christenson RH, Greene DN, Jaffe AS, Kavsak PA, Ordonez-Llanos J, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2018;64(4):645–55.
Lee Chuy K, Oikonomou EK, Postow MA, Callahan MK, Chapman PB, Shoushtari AN, et al. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center experience. Oncologist. 2019;24(5):e196–e7.
Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist. 2018;23(8):936–42.
Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM, Roddie C. CAR T cell therapy-related cardiovascular outcomes and management. JACC: Cardio Oncol. 2020;2(1):97–109.
Jamal FA, Khaled SK. The cardiovascular complications of chimeric antigen receptor T cell therapy. Curr Hematol Malig Rep. 2020;15(2):130–2.
Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 2019;74(25):3099–108.
Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13.
Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2017;53:120–7.
Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72–8.
Narayan V, Keefe S, Haas N, Wang L, Puzanov I, Putt M, et al. Prospective evaluation of sunitinib-induced cardiotoxicity in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2017;23(14):3601–9.
Pudil R, Mueller C, Celutkiene J, Henriksen PA, Lenihan D, Dent S, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–83.
Cole DC, Frishman WH. Cardiovascular complications of proteasome inhibitors used in multiple myeloma. Cardiol Rev. 2018;26(3):122–9.
Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37(22):1946–55.
Hussain AS, Hari P, Brazauskas R, Arce-Lara C, Pasquini M, Hamadani M, et al. Changes in cardiac biomarkers with bortezomib treatment in patients with advanced cardiac amyloidosis. Am J Hematol. 2015;90(11):E212–3.
Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Investig. 2014;44(3):303–8.
Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–60.
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Fei Fei Gong declares that she has no conflict of interest. Gregory J. Cascino declares that he has no conflict of interest. Gillian Murtagh is a shareholder and full-time employee of Abbott Laboratories. Nausheen Akhter declares that she has no conflict of interest.
Disclaimer
Abbott had no role in the design of or funding of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Gong, F.F., Cascino, G.J., Murtagh, G. et al. Circulating Biomarkers for Cardiotoxicity Risk Prediction. Curr. Treat. Options in Oncol. 22, 46 (2021). https://doi.org/10.1007/s11864-021-00845-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00845-0