Abstract
Purpose of review
Immunotherapies, particularly immune checkpoint inhibitors (ICI), are revolutionary cancer therapies being increasingly applied to a broader range of cancers. Our understanding of the mechanism, epidemiology, diagnosis, and treatment of cardiotoxicity related to immunotherapies remains limited. We aim to synthesize the limited current literature on cardiotoxicity of ICIs and to share our opinions on the diagnosis and treatment of this condition.
Recent findings
The incidence of ICI-associated myocarditis ranges from 0.1 to 1%. Patients with ICI-associated myocarditis often have a fulminant course with a case fatality rate of 25–50%. The diagnosis of this condition poses many challenges because independently a normal electrocardiogram, biomarkers, or a preserved left ventricular function do not rule out ICI-associated myocarditis. Endomyocardial biopsy should be pursued when clinical suspicion remains despite normal non-invasive tests. Data on optimal screening and surveillance tools are lacking. Cessation of ICIs, combined with high dose corticosteroids and other immunosuppressant approaches are the cornerstones of the treatment of ICI-associated myocarditis. This condition may recur when patients are re-challenged with these agents and the decision to resume ICIs should be made through a multidisciplinary discussion.
Summary
Immunotherapies have changed the landscape of cancer treatment. Recognizing and managing cardiotoxicity related to ICIs is of critical importance. Our understanding of ICI-cardiotoxicity has improved, but large information gaps remain for further research. Due to the high case fatality rate, any type of cardiac symptoms or signs in a patient who has recently started an ICI should prompt consideration of ICI-cardiotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Harnessing the power of the immune system has been recognized as a potential treatment of malignancies for decades. There are multiple approaches available but for this review, we will focus on immune checkpoint inhibitors (ICI). Cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and programmed cell death ligand-1(PD-L1) are the most widely studied and recognized immune checkpoint pathways. Drugs blocking these pathways leverage the immune system to target cancer cells. They are the first in recent decades of immunotherapy research to demonstrate significant clinical benefit with tolerable toxicities and are currently approved for a multitude of solid tumor indications [1,2,3,4]. The first of these agents was ipilimumab [1], a CTLA-4 inhibitor, which was approved in 2011. Trials investigating pembrolizumab (PD-1) [5] and nivolumab (PD-L1) [4, 6] followed shortly after. The use of ICIs is rapidly expanding and moving towards the first-line metastatic and adjuvant settings with multiple ongoing studies testing them and in combination with standard cytotoxic chemotherapies and targeted therapies. For context, as of September 2017, there were 2004 immuno-modulatory agents against 303 targets, from 864 companies in 3042 active clinical trials [7].
Immune-related adverse events (irAEs) are complications that arise presumably from misguided targeting of normal (non-malignant) tissue by the immune system and can involve any organ system. Cardiotoxicity is an uncommon irAE, but it often has a fulminant course. The absence of highly sensitive and specific diagnostic tests and lack of experience and evidence pose some of the many challenges related to this condition.
An ICI-associated myocarditis case
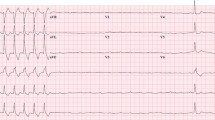
A 69-year-old man with hypertension and diabetes was diagnosed with metastatic renal cell carcinoma. He was treated with nephrectomy. Two years later, the cancer recurred and he was started on bevacizumab and atezolizumab. After receiving two doses of atezolizumab, he presented to the emergency department with fatigue and shortness of breath. His heart rate was 110 and his blood pressure was 100/60. Electrocardiogram (ECG) (Fig. 1a) demonstrated sinus tachycardia and non-specific ST/T wave changes. Conventional troponin T was 1.25 ng/ml and remained stably elevated thereafter. The patient underwent a cardiovascular magnetic resonance (CMR) imaging (Fig. 1b) which showed a markedly decreased left ventricular ejection fraction (LVEF) of 20%. The patient rapidly declined and needed inotropic support due to development of cardiogenic shock. He underwent a coronary angiogram which did not reveal significant obstructive coronary disease and subsequently had an EMB which revealed lymphocytic myocarditis (Fig. 1c). He was treated with antithymocyte globulin and subsequently improved. Several weeks later, he had an echocardiogram which showed recovery of LVEF to 59%.
Electrocardiogram (ECG), cardiac magnetic resonance, and endomyocardial biopsy (EMB) findings in a patient with immune checkpoint inhibitor-associated myocarditis. ECG demonstrated sinus tachycardia and non-specific ST/T wave changes (a). CMR showed a markedly decreased left ventricular ejection fraction of 20% (b). EMB revealed a lymphocytic myocarditis (c).
Immunotherapies and cancer
Since the time of William Coley in the late nineteenth century, harnessing the immune system has been recognized as a potential approach for the treatment of malignancies [8]. Only in the last decade has this strategy become a mainstream clinical reality. Immunotherapies utilize various approaches to activate immune system, generally through one of two mechanisms: (1) stimulating effector mechanisms by vaccination with tumor antigens, augmentation of antigen presentation, adoptive cellular therapy, administration of oncolytic viruses, or the use of antibodies targeting the tumor necrosis factor receptor superfamily; and (2) counteracting immune-inhibitory mechanisms by the use of antibodies to suppress regulatory T cells or antibodies against immune checkpoint molecules. T cells are capable of identifying and destroying malignant cells; however, this mechanism fails in many malignancies. This adaptive immune resistance is a hallmark of malignancies, characterized by downregulation of major histocompatibility complex (MHC) antigen expression, secretion of cytokines that downregulate the immune response, and expression of so-called “checkpoint” molecules [9]. PD-1 and CTLA-4 are proteins on the surface of regulatory T cells with a role in regulating the immune system by downregulating the immune response. In some malignant cells, programmed death-ligand 1 (PD-L1) proteins are overexpressed to inhibit the function of anti-tumor T cells by combining with PD-1. Checkpoint inhibition refers to the mechanism by which drugs block the function of these checkpoint molecules, thereby leading to the activation of cytotoxic T cells and their targeting of malignant cells. The most clinically notable checkpoint inhibitors block PD-1, the receptor for PD-L1, and CTLA-4.
Immune checkpoint inhibitors and heart—basic science
PD-1 signaling axis has a clear role in the protection of the heart from autoimmunity. PD-L1 is expressed on both murine and human heart. Certain mouse strains lacking the PD-1 gene develop a severe dilated cardiomyopathy [10] secondary to the development of autoantibodies to cardiac troponin I [11, 12]. As a result, it was hypothesized that PD-1 plays an important role in autoimmune cardiac pathologies. Indeed, a fatal and diffuse lymphocytic myocarditis developed in the PD-L1 null (−/−) MRL mice [13]. Notably, the loss of the PD-1 gene led to the development of antibodies specific for cardiac myosin [14]. In a mouse model of T cell-mediated myocarditis, PD-1 protected against T cell-mediated myocarditis [15] through the upregulation of cardiac endothelial PD-L1 [16]. More recently, studies have suggested that PD-1/PD-L1 interactions are important in modulating immune response and subsequent immune-mediated cardiac damage following myocardial infarction and ischemia-reperfusion injury [17].
CTLA-4 is known to play a distinct, though less specific, role in cardiovascular biology. CTLA-4 acts as a key negative regulator of T cell activation, with knockout models demonstrating diffuse, lethal T cell-mediated toxicity across organ systems. These models have also been demonstrated to spontaneously produce lymphoproliferation and lymphocytic infiltrates in multiple organs, including the heart where they cause severe myocarditis [18]. Further demonstrating the cardioprotective role of CTLA-4, inoculation of T cells deficient in CTLA-4 and augmented with IL-12 led to significant intracardiac infiltration of lymphocytes, prominent lymphoproliferation within intracardiac nodes, and development of a near-fatal myocarditis [19].
Immunotherapies and immune-related adverse events
Toxicities associated with immunotherapies are novel, in comparison to toxicities associated with traditional therapies [20]. As regulatory elements of immune response is inhibited, the potential for immune related adverse events (irAEs) occurs. It has been estimated that irAEs (of any severity) occur in up to 70% of ICI recipients, with grade 3–4 events reported in 10–15% [21, 22]. When anti-CTLA4 and anti-PD-1/PD-L1 agents are used in combination, irAEs appear to be more frequent and more severe [3]. Presentation of these toxicities is variable. Though irAEs mostly occur during the first 3–6 months of treatment [23, 24], delayed immune effects have been reported, in some cases up to 2 years after treatment [25]. The most commonly affected tissues have been the gastrointestinal tract, skin, endocrine organs, liver, and lung. Such a pattern may fit with the immunological characteristics of these tissues, as they each have high exposure to foreign antigens [26].
Incidence of cardiotoxicity
While there has been increased reporting of ICI-associated myocarditis [27], the true incidence is likely underestimated due to challenges in diagnosis, lack of awareness, and the limitations of current systems of reporting adverse events in clinical trials. Myocarditis from ICIs was first noted in case reports and phase II and III clinical trials of ICIs, and later in case series [3, 28,29,30,31,32,33,34]. Johnson and colleagues analyzed the Bristol-Myers Squibb corporate safety database to report the incidence of myocarditis in patients being treated with nivolumab, ipilimumab, or both. Among 20,594 patients, myocarditis was reported in 0.06% of patients who received nivolumab alone, and 0.27% of patients who received combination therapy [31]. We previously reported that among 964 patients who received ICIs at the Massachusetts General Hospital, either as monotherapy or combination therapy, ~ 1% developed myocarditis [33]. This range may be explained, in part, by the wide potential presentations as it is important to note that there are other potential ICI-cardiotoxicity beyond myocarditis, including cardiomyopathy, conduction defects (heart block), atrial and ventricular tachyarrythmias, and acute coronary syndromes [35,36,37]. For example, pericarditis is also underrecognized, underreported, and undertreated, and is associated with a relatively high case fatality rate (around 13%) [38]. A recent meta-analyses of 22 clinical trials involving single-agent PD-1 or PD-L1 inhibitors in non-small cell lung cancer suggested incidence rates of 2.0% for heart failure, 1.0% for myocardial infarction, 1.0% for cardiac arrest, 0.7% for cardiac tamponade, and 0.5% for myocarditis. This report suggested a 5.2% overall rate of significant cardiac events during a brief follow-up period [37].
Several other challenges may also lead to underestimation of incidence of cardiotoxicity. First, the current non-invasive tests are lacking in sensitivity. In our experience, a relatively normal ECG, lack of major symptoms, or a normal LVEF are not sufficient to rule out ICI-associated myocarditis [33]. Second, subclinical myocarditis can be challenging as demonstrated by a recent case of a metastatic melanoma patient treated with ipilimumab and nivolumab; cardiac involvement was clinically unapparent, but patchy fibrosis and diffuse mononuclear infiltrates of myocardium was found in postmortem autopsy [39]. Lastly, an EMB should be strongly considered when clinical suspicion remains despite normal results from non-invasive testing. However, EMB is often underutilized due to its invasive nature.
Presentation
Consistent data suggest that most cases of ICI-associated myocarditis occur early after starting treatment [27, 32, 33]. For example, Escudier and colleagues reported that the median time to presentation of cardiotoxic effects was 65 days from the start of treatment (equivalent to three ICI cycles), but with a wide variation of 2 to 454 days [32]. Moslehi and colleagues reviewed 101 cases of ICI-associated myocarditis from the World Health Organization’s Vigibase and found that 64% of myocarditis occurred after the first or second ICI dose, and 76% occurred within the first 6 weeks of treatment [40]. Mahmood et al. reported a median time from the first ICI dose to the onset of myocarditis of 34 days and 81% of cases presented within 3 months of the first dose [33]. However, it is important to recognize that late-onset ICI-associated myocarditis also occurs in a small percentage of patients, in some cases up to several months after starting ICI therapies [32, 41]. It is unclear whether these late-onset cases represent the late diagnosis of myocarditis that had begun much earlier or a truly delayed development of myocarditis after ICI administration.
The risk factors for ICI-associated myocarditis are generally poorly understood. Patients with underlying autoimmune disease, pre-existing cardiovascular disease, and diabetes mellitus may be at increased risk [20, 33, 42, 43]. Consistent data showed that patients receiving combination ICI therapies are at more than the double risk [31, 33]. Screening with cardiovascular testing is often considered in the presence of a cardiotoxicity; however, limited early data suggests that there is no difference in ECG or echocardiogram findings between cases and controls prior to starting an ICI [33]. Patients with ICI-associated myocarditis can have pre-existing or concomitant non-cardiac irAEs [27, 32, 33]. Concomitant myositis may be found in 25% of patients [27, 32]. Moslehi and colleagues reported that myasthenia gravis occurred in 11 of 101 patients with myocarditis [27]. In another report describing ten patients with immune-related myositis, four patients also had evidence of myocarditis [34]. These data suggest that patients with ICI-related myositis should undergo evaluation to exclude cardiac involvement.
Presentation of ICI-associated myocarditis may range from nonspecific to fulminant. Patients can present with fatigue, dyspnea, orthopnea, myalgia, palpitation, chest pain, lower extremity edema, lightheadedness, syncope, or change in mental status [33, 44]. Severe cases can present with cardiogenic shock or cardiac arrest. Contributing to the diagnostic challenges is that symptomatic patients may present to oncology, cardiology, or general medicine providers in an outpatient setting, or to acute or emergency care in a hospital setting. Any relevant alerting symptoms should trigger immediate testing and referral to cardiology/cardio-oncology specialists if necessary [33].
Diagnosis—ECG, echocardiogram, biomarkers, CMR, EMB
Due to its potentially fulminant course, a prompt and accurate diagnosis is critical so that ICIs are immediately discontinued and effective treatment can be initiated [33]. Commonly used diagnostic tests of ICI-associated myocarditis are summarized in the Table 1.
An ECG is an inexpensive and widely available test and should be performed immediately upon presentation. On ECG, findings range from a normal ECG to a range of conduction abnormalities and atrial or ventricular arrhythmias [32, 45]. In our cohort of 35 ICI-associated myocarditis patients, the ECG was abnormal in 89% of patients [33]. Although ECG abnormalities, such as tachycardia, conduction abnormalities, ST segment/T-wave abnormalities, QT prolongation, presence of Q wave, or atrial or ventricular arrhythmias are nonspecific, their identification can be instrumental, especially if new, towards triggering further investigation. Importantly, a normal ECG does not rule out myocarditis. As patients with ICI-associated myocarditis frequently develop tachy- and brady-arrhythmias, clinically suspected patients should be closely monitored with a cardiac telemetry or an ambulatory ECG monitor.
Serum biomarkers are commonly used in the assessment of myocarditis. Troponin levels, indicating the degree of myocardial damage, and B-type natriuretic peptide (BNP), a marker suggesting wall stress, are instrumental in the diagnosis and prognosis of myocarditis. Our data revealed that 94% of patients with ICI-associated myocarditis had an elevated troponin, and 66% had an elevated N-terminal (NT)-pro BNP [33]. However, Escudier and colleagues reported that 46% of patients with various types of ICI-cardiotoxicity had elevated troponin and 100% had an abnormal BNP [32]. Variation in these results could be due to differences in patient cohorts and timing of the tests/presentation with the time to presentation being significantly longer in the Escudier’s study. Similar to ECG, a normal biomarker is not sufficient to rule out myocarditis. It is also prudent to rule out coronary ischemia with either coronary angiography, coronary computed tomography angiography, or stress testing if clinically indicated; however, among patients presenting early after starting ICI, myocarditis should be considered the principle diagnosis and excluded.
Echocardiogram is the first-line non-invasive imaging test in the assessment of ICI-associated myocarditis. Unlike myocarditis due to other etiologies, more than half of ICI-associated myocarditis patients (51%) have a preserved LVEF [33], and severe LV dysfunction (LVEF ≤ 35%) was not commonly seen [32]. Wall motion abnormalities, indices of diastolic function, and valvular function in ICI-associated myocarditis have not been investigated and represent important topics of future research. Pericardial effusions are found in 7–17% of patients with ICI-associated myocarditis [32, 33], in some representing an occurrence of perimyocarditis, typically with the presentation of pericardial pain. Myocardial strain assessment using speckle tracking echocardiography has been applied in the monitoring of traditional chemotherapy-associated cardiotoxicity [46]. Early unpublished experience from our group suggests that global longitudinal strain may have utility in the diagnosis and prognosis of patients with ICI-associated myocarditis.
CMR is the gold-standard noninvasive test for the diagnosis and risk prediction of myocarditis [47, 48]. The use of advanced tissue characterization in CMR such as late gadolinium enhancement (LGE) and the presence of myocardial edema provides a unique opportunity for differentiating myocarditis from other myocardial diseases [47]. Beyond research letters and case reports, there are no data on the use of CMR in the diagnosis of ICI-associated myocarditis [32]. Escudier and colleagues reported that LGE was presented in 23% of 13 patients in their cohort [32]. In comparison, LGE is present in about 80% of cases of non-ICI myocarditis [49, 50]. The limited availability, high cost, and difficulty of obtaining in critically ill patients may limit the use of CMR in some clinical scenarios.
EMB remains the gold-standard test for the diagnosis of myocarditis. There are established criteria using a right ventricular biopsy to diagnose myocarditis [51], and when five or more samples are available, the accuracy is increased but cases may still be missed [52, 53]. EMB should be performed by experienced interventionists in specialist centers under echocardiography/fluoroscopic guidance after weighing the benefit of diagnostic clarification against the inherent risks of EMB. However, because of its invasive nature and potential complications (overall rate 6%), EMB is rarely pursued as the first-line test [54]. There are limited data on EMB in ICI-associated myocarditis. Escudier and colleagues reported that 89% (8/9) of patients had lymphocytic infiltration [32]. Based on unpublished experience from our group, all suspected ICI-associated myocarditis patients who underwent EMB had histopathology, demonstrating a lymphocytic infiltration, and 56% had myocardial fibrosis. Immunohistochemistry for cell-specific markers such as T lymphocytes (CD3), macrophages (CD68), or human leukocyte antigens may be additive and improve the sensitivity of the test and the understanding of the disease [55].
Treatments
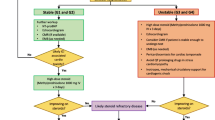
There are no prospective studies or randomized trials evaluating treatment options for ICI-associated myocarditis. Based on early experience-based studies, prompt discontinuation of ICI therapies, and initiation of corticosteroids, and other immunosuppression are the cornerstones of the treatment (Fig. 2). With suspected myocarditis, patients should be admitted to an oncology unit/medicine unit with telemetry or cardiac care unit depending on the severity of their presentation. Cardiology or cardio-oncology consultation should be obtained, and in relevant cases, an advanced heart failure consultation [56]. Guideline-directed medical therapy for patients with heart failure should be initiated [57]. ICIs should be discontinued, and high-dose corticosteroids, as the first-line therapy, should be initiated promptly in unstable patients without any delay for confirmatory tests (1000 mg methylprednisolone/day). In unstable patients who do not respond to high-dose steroids, antithymocyte globulin or intravenous immunoglobulin should be considered together with inotropic agents and/or temporary mechanical circulatory support in patients with cardiogenic shock. The latter should be done in consultation with an advanced heart failure specialist who are experienced in the use of immunosuppression [58,59,60]. In stable patients, it may be reasonable to wait for confirmatory testing by CMR or EMB. Stable patients should also be treated with 1000 mg of methylprednisolone once the diagnosis is confirmed. A taper of steroids should be performed when the patient is stable or biomarkers (especially troponin) have started to decline. When stable patients do not respond to corticosteroids, second-line interventions including infliximab, mycophenolate mofetil, and plasma exchange could be considered [61, 62]. There are several reports on the use of infliximab as a second-line therapy [31, 33]; however, it has to be used with caution in patients with heart failure, and in our experience, the success rate is modest [63]. Intravenous steroids are followed by oral prednisone (1–2 mg/kg) and a careful tapering plan (depending on clinical response) [43, 44, 56]. The optimal duration of these therapies is unknown, but it is reasonable to continue the treatment until resolution of symptoms and normalization of troponin, LVEF, and conduction abnormalities, which is usually over 4–6 weeks. Prophylactic strategies, such as pneumocystis pneumonia prophylaxis, proton-pump inhibitor, and nystatin for oral candidiasis are recommended while patients are on high-dose corticosteroids therapy. In our registry, steroids were administered in 89% of 35 patients. The mean time from admission to steroid administration in patients without a major adverse cardiac event (MACE) was 18.3 days, shorter than in patients who developed a MACE (27.2 days). A higher initial dose of steroids was associated with lower rate of MACEs and lower discharge serum troponin levels [33]. The data on additional strategies are more limited. In a systemic review including 73 studies and 88 cases with cardiotoxicity related to ICIs, infliximab, mycophenolate, intravenous immunoglobulin, antithymocyte globulin, and/or plasmapheresis were used in 12 patients and 9 of them survived (75%) [38].
For patients with cardiovascular risk factors and cardiotoxicity of high grade, a longer follow-up time and routine surveillance of cardiac function is reasonable. In our registry, there have been no recurrence after the initial diagnosis of ICI-associated myocarditis over a median follow-up time of 209 days [33]. Although it is recommended to definitively discontinue ICIs in cases of severe (grade 3) or life-threatening (grade 4) toxicities [56], re-challenging patients with a single agent can be carefully considered when no effective alternative oncologic therapy exists provided the patient fully comprehends the risk of recurrent and potentially fulminant cardiotoxicity. The risk of recurrence of cardiotoxicity when patients are re-challenged with a ICI is unknown, but previous research on other irAEs revealed that in 38 patients retreated with anti-PD-1/L1 agents, 18 (48%) patients had no subsequent irAEs, 10 (26%) had recurrence of the initial irAE, 10 (26%) had a new irAE, and most of the irAEs were mild and manageable [64]. If re-initiation of immunotherapies is considered necessary, monotherapy with a different specific agent and close cardiac monitoring may be a reasonable approach. Our experience suggested that monotherapy with an anti-PD-1 agent was related to a lower risk of cardiotoxicity [33]. Escudier reported that immune therapy was administered again after the first episode of cardiotoxicity in the 4 out of 30 patients in their cohort without any recurrences [32]. More broadly, retrospective studies have indicated that after severe irAEs related to anti-CTLA-4 monotherapy or combination therapy with CTLA-4 and PD-1 blockade, the majority of patients tolerated re-challenge with an anti-PD-1 agent well [65, 66].
Outcomes
ICI-associated myocarditis can have a fulminant course and is potentially fatal. In our registry, 46% of the 35 myocarditis cases experienced a MACE: cardiovascular death (n = 6), cardiogenic shock (n = 3), cardiac arrest (n = 4), or CHB (n = 3) [33]. Eight out of the 35 patients (23%) died of cardiovascular causes during the median follow-up time of 102 days. Escudier and colleagues reported a case fatality rate of 27% [32]. According to the World Health Organization pharmacovigilance database, comprising more than 16,000,000 adverse drug reactions, myocarditis had the highest fatality rate of 40% (52 out of 131 reported cases), whereas 2–17% of toxic effects of other organ-system had fatal outcomes [67]. In contrast, among 670 patients admitted with non-ICI myocarditis, MACEs occurred in 15% of patients, and death occurred in 4% of patients during a median follow-up time of 4.7 years [48]. Table 2 summarizes the difference between ICI-associated myocarditis and non-ICI myocarditis.
Predictors of adverse outcomes in ICI-associated myocarditis are not well characterized. Escudier and colleagues reported that cardiovascular mortality was significantly associated with conduction abnormalities (80% versus 16%; p = 0.003) and ipilimumab-nivolumab combination therapy (57% versus 17%; p = 0.04) [32]. We found that a cardiac troponin T of more than 1.5 ng/mL was associated with a fourfold increased risk of MACE, and early administration of corticosteroids could potentially lead to less MACE [33]. We also found that the mean LVEF during the index admission in patients who did not develop a MACE was 49%, higher than in patients who had a MACE in the follow-up period (41%); however, the difference was not statistically significant [33].
Unresolved issues
Our understanding of cardiotoxicity of immunotherapies and ICI-associated myocarditis is rapidly improving, but remains very limited. Screening and surveillance are important to risk stratify and to monitor patients before the occurrence of these conditions. However, our data suggest that neither a screening ECG nor LVEF assessment by echocardiogram prior to ICIs would be sensitive enough to identify patients at risk of ICI-associated myocarditis [33]. Whether surveillance should be performed in all patients on ICIs, and if so, the optimal approach, are unknown. Based on our experience, most patients have an abnormal ECG or elevated troponin levels on presentation [33], so, a surveillance approach with serial ECGs and troponins should be strongly considered, especially in those at higher risk (for example, combination therapy). As most ICI-associated myocarditis patients present within the first 3 months of starting ICIs, obtaining a baseline ECG and troponin before initiation of ICI and again before each cycle can be considered for at least the first 8–12 weeks [43, 44]. As clinical trials are testing the adjuvant or neoadjuvant use of ICIs, as well as their roles alongside traditional cytotoxic or targeted therapies with cardiotoxicity, surveillance may be especially useful as a part of the clinical trial design and clinical care.
The pathophysiological mechanisms of cardiotoxicity of immunotherapies remain unclear. This information is key towards developing a tailored, effective, and less toxic therapeutic approach, rather than broad immunosuppression that is currently employed. Preventive measures targeting specific pathways should also be identified to help decrease the occurrence of ICI-cardiotoxicity, especially for those at heightened risk. In addition, there is uncertainty around the use of wearable defibrillator or implantable cardioverter defibrillator in these patients who frequently manifest ventricular arrhythmias during the active phase because the long-term risk of sudden cardiac death is unclear. Lastly, whether the use of high-doses immunosuppression for treating cardiotoxicity will affect cancer outcomes is unknown.
Future directions
Immunotherapies, specifically ICIs, have revolutionized cancer treatment, and their clinical use is rapidly expanding, leading to a new and exciting era of oncology care. Managing and reducing cardiotoxicity is critical towards allowing the safe delivery of these effective cancer therapies. Our understanding of the cardiotoxicity of immunotherapies and, in particular, ICI-associated myocarditis, remains limited. The key knowledge gaps that should drive further research include (1) understanding mechanistic pathways of pathogenesis of cardiotoxicity related to immunotherapies; (2) describing genetic, clinical, and immunological risk factors for immunotherapy-related cardiotoxicity; (3) establishing a sensitive and cost-effective approach to monitor cardiotoxicity; (4) identifying diagnostic strategies with superior sensitivity and specificity; and (5) discovering effective and targeted cardiac interventions. Lastly, multidisciplinary efforts between primary care physicians, oncologists, cardiologists, cardio-oncologists, and immunologists will be a key component to advance our understanding in this area [71]. We are hopeful that the ongoing intense efforts of the oncology and cardiology research communities will help to resolve these issues, and thereby allow immunotherapies to achieve their maximum potential impact.
References and Recommended Reading
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/NEJMoa1003466.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. https://doi.org/10.1056/NEJMoa1104621.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. https://doi.org/10.1056/NEJMoa1504030.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. https://doi.org/10.1056/NEJMoa1305133.
Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomized, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–84. https://doi.org/10.1016/s1470-2045(15)70076-8.
Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29(1):84–91. https://doi.org/10.1093/annonc/mdx755.
Coley WBII. Contribution to the Knowledge of Sarcoma. Ann Surg. 1891;14(3):199–220.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science (New York, NY). 2001;291(5502):319–22. https://doi.org/10.1126/science.291.5502.319.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–83. https://doi.org/10.1038/nm955.
Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol (Baltimore, Md: 1950). 2008;181(4):2513–21.
Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–52. https://doi.org/10.1093/intimm/dxq026.
Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol (Baltimore, Md: 1950). 2012;188(10):4876–84. https://doi.org/10.4049/jimmunol.1200389.
Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T cell mediated injury in the heart. Circulation. 2007;116(18):2062–71. https://doi.org/10.1161/circulationaha.107.709360.
Baban B, Liu JY, Qin X, Weintraub NL, Mozaffari MS. Upregulation of programmed death-1 and its ligand in cardiac injury models: interaction with GADD153. PLoS One. 2015;10(4):e0124059. https://doi.org/10.1371/journal.pone.0124059.
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7.
Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res. 2007;101(3):248–57. https://doi.org/10.1161/circresaha.106.147124.
Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–68. https://doi.org/10.1056/NEJMra1703481.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. https://doi.org/10.1056/NEJMoa1200690.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. https://doi.org/10.1056/NEJMoa1200694.
Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–82. https://doi.org/10.1002/cncr.27969.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(10):1020–30. https://doi.org/10.1200/jco.2013.53.0105.
Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–90. https://doi.org/10.1056/NEJMc1505197.
Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer (Oxford, England: 1990). 2016;54:139–48. https://doi.org/10.1016/j.ejca.2015.11.016.
Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet (London, England). 2018;391(10124):933. https://doi.org/10.1016/s0140-6736(18)30533-6.
Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. https://doi.org/10.1371/journal.pone.0053745.
Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. https://doi.org/10.1186/s40425-015-0057-1.
Geisler BP, Raad RA, Esaian D, Sharon E, Schwartz DR. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer. 2015;3:4. https://doi.org/10.1186/s40425-015-0048-2.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55. https://doi.org/10.1056/NEJMoa1609214.
Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–7. https://doi.org/10.1161/circulationaha.117.030571.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64. https://doi.org/10.1016/j.jacc.2018.02.037.
Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):e985–e94. https://doi.org/10.1212/wnl.0000000000006124.
Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer (Oxford, England: 1990). 2016;60:210–25. https://doi.org/10.1016/j.ejca.2016.02.024.
Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. https://doi.org/10.1186/s40425-016-0152-y.
Hu YB, Zhang Q, Li HJ, Michot JM, Liu HB, Zhan P, et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6(Suppl 1):S8–s20. https://doi.org/10.21037/tlcr.2017.12.10.
Mir H, Alhussein M, Alrashidi S, Alzayer H, Alshatti A, Valettas N, et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol. 2018;34(8):1059–68. https://doi.org/10.1016/j.cjca.2018.03.012.
Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13. https://doi.org/10.1186/s40425-016-0117-1.
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. 2008.
Yamaguchi S, Morimoto R, Okumura T, Yamashita Y, Haga T, Kuwayama T, et al. Late-onset fulminant myocarditis with immune checkpoint inhibitor nivolumab. Can J Cardiol. 2018;34(6):812.e1–3. https://doi.org/10.1016/j.cjca.2018.03.007.
Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234–40. https://doi.org/10.1001/jamaoncol.2015.4368.
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e58. https://doi.org/10.1016/s1470-2045(18)30457-1.
Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23(8):879–86. https://doi.org/10.1634/theoncologist.2018-0130.
Reddy N, Moudgil R, Lopez-Mattei JC, Karimzad K, Mouhayar EN, Somaiah N, et al. Progressive and reversible conduction disease with checkpoint inhibitors. Can J Cardiol. 2017;33(10):1335.e13–5. https://doi.org/10.1016/j.cjca.2017.05.026.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. https://doi.org/10.1016/j.echo.2014.07.012.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–76. https://doi.org/10.1016/j.jacc.2018.09.072.
Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–76. https://doi.org/10.1016/j.jacc.2017.08.050.
Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(15):1581–90. https://doi.org/10.1161/circulationaha.105.606509.
Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study. J Am Coll Cardiol. 2017;70(16):1977–87. https://doi.org/10.1016/j.jacc.2017.08.044.
Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21(4):245–74. https://doi.org/10.1016/j.carpath.2011.10.001.
Cunningham KS, Veinot JP, Butany J. An approach to endomyocardial biopsy interpretation. J Clin Pathol. 2006;59(2):121–9. https://doi.org/10.1136/jcp.2005.026443.
Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235–45.
Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol. 1992;19(1):43–7.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48, 48a-48d. https://doi.org/10.1093/eurheartj/eht210.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(17):1714–68. https://doi.org/10.1200/jco.2017.77.6385.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. https://doi.org/10.1016/j.jacc.2017.04.025.
Jain V, Mohebtash M, Rodrigo ME, Ruiz G, Atkins MB, Barac A. Autoimmune myocarditis caused by immune checkpoint inhibitors treated with antithymocyte globulin. J Immunother (Hagerstown, Md: 1997). 2018;41(7):332–5. https://doi.org/10.1097/cji.0000000000000239.
Kobashigawa J, Crespo-Leiro MG, Ensminger SM, Reichenspurner H, Angelini A, Berry G, et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2011;30(3):252–69. https://doi.org/10.1016/j.healun.2010.11.003.
Rodriguez ER, Skojec DV, Tan CD, Zachary AA, Kasper EK, Conte JV, et al. Antibody-mediated rejection in human cardiac allografts: evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant. 2005;5(11):2778–85. https://doi.org/10.1111/j.1600-6143.2005.01074.x.
Renlund DG, Gopinathan SK, Kfoury AG, Taylor DO. Mycophenolate mofetil (MMF) in heart transplantation: rejection prevention and treatment. Clin Transpl. 1996;10(1 Pt 2):136–9.
Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56. https://doi.org/10.1016/j.healun.2010.05.034.
Kwon HJ, Cote TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138(10):807–11.
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–9. https://doi.org/10.1158/2326-6066.Cir-17-0755.
Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. https://doi.org/10.1093/annonc/mdw443.
Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–5. https://doi.org/10.1093/annonc/mdx642.
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.3923.
Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2015;386(9995):743–800. https://doi.org/10.1016/s0140-6736(15)60692-4.
Pinamonti B, Alberti E, Cigalotto A, Dreas L, Salvi A, Silvestri F, et al. Echocardiographic findings in myocarditis. Am J Cardiol. 1988;62(4):285–91.
Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, et al. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation. 2017;136(6):529–45. https://doi.org/10.1161/circulationaha.117.026386.
Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, et al. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist. 2018;23(8):874–8. https://doi.org/10.1634/theoncologist.2018-0157.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lili Zhang, Maeve Jones-O’Connor, Magid Awadalla, and Daniel A. Zlotoff each declare no potential conflicts of interest.
Paaladinesh Thavendiranathan reports consulting fees from Takeda, BI, Janssen, and Amgen unrelated to the contents of this manuscript.
John D. Groarke has received research support from Amgen.
Alexandra-Chloe Villani reports an Innovation Award from Damon Runyon-Rachleff.
Alexander R. Lyon has received speaker, advisory board or consultancy fees and/or research grants from Pfizer, Novartis, Servier, Amgen, Clinigen Group, Takeda, Roche, Eli Lily, Eisai, Bristol Myers Squibb, Ferring Pharmaceuticals and Boehringer Ingelheim, and Stealth Peptides.
Tomas G. Neilan reports consulting fees from Parexel, Intrinsic Imaging, and Takeda, unrelated to the contents of this manuscript. Dr. Neilan reports being a member of a scientific advisor board to Bristol-Myers Squibb related to ICI myocarditis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Zhang, L., Jones-O’Connor, M., Awadalla, M. et al. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr Treat Options Cardio Med 21, 32 (2019). https://doi.org/10.1007/s11936-019-0731-6
Published:
DOI: https://doi.org/10.1007/s11936-019-0731-6