Opinion statement
Despite its history as one of the most impactful toxicities associated with cytotoxic cancer therapy, oral mucositis (OM) remains an unmet clinical need which affects hundreds of thousands of patients. Descriptions of its complex pathogenesis have provided mechanistic targets which are being exploited to develop an effective therapeutic intervention. Favorable results of recently completed clinical trials in which agents focused on interrupting the early stages of the mucositis biological cascade were assessed provide reason for optimism, not only for oral mucositis but also for halo indications which share its pathobiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) remains a significant side effect of cytotoxic anti-cancer chemotherapy and radiotherapy. Of the 1.8 million patients who will be diagnosed with malignancies this year in the USA, almost half will suffer some degree of mucositis. For a lucky minority, OM manifestations will be limited and easily controlled transient mouth pain. But for many, mucositis will be of such severity as to cause major diet modifications and weight loss, necessitate opioid analgesics, require supplemental nutrition, and disrupt optimal cancer therapy [1]. For patients whose chemotherapy regimens (CT) are myelosuppressive, mucositis poses the additional threat of bacteremia and sepsis as it creates systemic portal of entry for microorganisms [2]. Patients with OM are more likely to have negative treatment outcomes, poorer quality of lives, and incur more costs than patients who do not develop the condition [3].
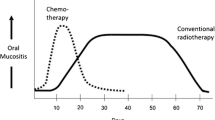
OM has a predictable clinical trajectory that is determined by cancer regimen [4]. CT-associated OM becomes clinically apparent about 4 days after infusion when manifestations of mucosal atrophy, primarily sensitivity and erythema are noted. The tissue continues to deteriorate, and ulceration occurs a few days later, peaking at 2 weeks and persisting for 1–2 weeks after which it typically resolves spontaneously. It is the ulcerative phase that is most painful and associated with poor health outcomes. In contrast, OM associated with radiation regimens (RT) used to treat head and neck cancer (HNC) has a slower onset in response to the cumulative effects of daily fractions of 2 Gy (ref). While patients complain of burning mouth after a week of treatment, ulceration and more severe, opioid-requiring pain usually develops around week 4 and may extend over subsequent weeks, ultimately healing 4–6 weeks after the completion of RT (usually patients undergo 7 weeks of RT).
Even with aggressive cancer regimens, patients are at equal risk of developing OM. Germ line genomics are thought to be especially important in predisposing patients to OM, although epigenetics, the microbiome and metabolomics may also contribute [5•].
Current management practices for oral mucositis
To date, OM management has focused on symptom control using topical or systemic analgesics and the application of barrier agents to cover injured mucosa as a salve or ointment might cover irritated skin [6]. Such devices have been available for years and are most effective during early phases of OM when symptoms are most mild. Examples of these agents are GelClair, MuGard, and sucralfate suspension [6]. Magic mouthwash, a generic term to describe a class of institutionally developed rinses which include a coating agent such as kaopectate or milk of magnesia as a base. A variety of additives (usually based on institutional folklore) complete the suspension and include options like lidocaine, anti-fungals, topical steroids or antibiotics the utility of these formulations is marginal [7, 8].
Tooth remineralizing solutions such as Caphosol have been aggressively marketed as mucositis interventions. Results of clinical trials in different patient populations (stem cell transplant recipients and patients being treated with chemoradiation [CRT] for HNC) failed to confirm their value [9, 10].
Cryotherapy has been advocated as an OM intervention for certain CT regimens, including conditioning regimens prior to stem cell transplant [11]. Typically delivered as ice chips held in patients’ mouths during infusion, it is believed that cold-induced vasoconstriction limits tissue levels of stomatotoxic agents and thereby reduces mucosal damage (ref). An ice chip-alterative cold delivery device has been developed, is commercially available and being studied in an ongoing clinical trial (A Trial Testing Chemo Mouthpiece Device and Best Supportive Care Against Best Supportive Care Only for Symptoms of Oral Mucositis in Patients Receiving Chemotherapy; NCT04595838).
Studies assessing low level laser therapy (photobiomodulation) to control OM have produced a substantial body of literature comprised mostly of investigator-initiated, single institution trials. Variations in technique and energy parameters have been problematic and results are not uniform [12, 13]. Inconsistent reports relative to PBM’s effect on tumor response raise unanswered questions regarding its impact on long term effects, not dissimilar to concerns associated with palifermin [14]. A multicenter trial of 69 patients is currently beginning (NCT 03972527; Prospective, multicenter, randomized, double-blind, placebo-controlled, adaptive sample size, two-treatment parallel, pivotal clinical study).
Past strategies and interventions—lessons learned
A role for the oral microbiome’s role in mucositis pathogenesis has been speculated following the observation that cancer therapy results in an alteration in the oral flora’s composition [15]. The finding that the oral bacterial load increased subsequent to the development of OM-associated ulceration lead to speculation that secondary colonization might be important in extending the duration or increasing the intensity of existing mucositis [4].
In response to a hypothesis reduction in oral bacteria load would favorably impact OM, a number of clinical trials were conducted in the early 2000s. In a double-blind, randomized, placebo-controlled international study, iseganan was evaluated in patients receiving aggressive myeloablative, stomatotoxic chemotherapy. Iseganan is a structured analog of porcine-derived protegrin and broad-spectrum antibiotic having extended salivary antimicrobial activity and good mucosal adherence. The trial randomized 323 patients [16]. A 5 times daily swish and swallow dosing regimen beginning on the day of infusion and continuing for 21–28 days resulted in a trend (p < 0.067) toward a reduced incidence of ulcerative mucositis (using CTCv2 criteria). While iseganan did not modify OM development, its effect was observed during the later stages of mucositis when secondary colonization would be expected to be most impactful.
Randomized studies of antimicrobial strategies have produced less favorable results in patients being treated with RT for HNC. In one of the largest mucositis clinical trials ever completed (n = 545 patients), iseganan was assessed in HNC patients being treated with RT. Iseganan, administered in the same swish and swallow formulation noted above, failed to impact the severity or incidence of mucositis [17].
Other anti-microbials have also been ineffective in the same patient group. A 1994 randomized trial (n = 52 patients) found that topical application of chlorhexidine worsened the course of OM [18]. Two other unsuccessful studies evaluated 4 antimicrobial troches. A lozenge comprised of bacitracin, clotrimazole, and gentamicin failed to impact time to onset or extent of mucositis in a two-arm, randomized study of 137 patients receiving radiotherapy for a range of HNCs [19]. A differently formulated lozenge (polymyxin, tobramycin, amphotericin) failed to prevent the development of severe mucositis in a similar group of patients [20].
The disparity in outcomes of microbial manipulation observed between patients receiving CT those receiving RT for HNC points to OM’s multifactorial nature and the fact that OM’s pathoetiology is impacted by the patient’s systemic state. Before the biological complexity of mucositis was realized, it was believed to be simply the unavoidable consequence of non-specific epithelial basal stem cell destruction caused by the cytotoxic effects of CT or RT [4]. Early mechanistic approaches to OM intervention were based on countering direct non-specific basal cell destruction, for which growth factors such as keratinocyte growth factor-1 (KGF1; palifermin, Kepivance) and fibroblast growth factor-20 (Velafermin) were representative examples.
Palifermin is the only drug or biological approved as a mucositis intervention in the USA. While its use is restricted to OM prevention in patients with hematologic malignancies receiving conditioning regimens in preparation for hematopoietic stem cell transplant, it has also shown to be efficacious in patients being treated with CRT for HNC [21,22,23]. It was hypothesized that palifermin’s ability to stimulate keratinocyte proliferation would result in mucosal hyperplasia, thereby increasing tissue tolerance to subsequent challenge, minimize atrophy and thus reduce the likelihood of ulcer development at best, or at least reduce its duration [24]. The major challenge with the approach was the concern that the proliferation noted in normal tissue would be replicated in KGF1-receptor-bearing tumor cells and negatively impact their behavior. Critically, any mucositis intervention cannot risk impugning anti-cancer therapies or negatively impact tumor behavior.
Since palifermin’s early development in the 1990s, much has been learned about mucositis pathogenesis. We now understand that direct non-specific toxicity of epithelial basal cells is not the major biological driver of OM, rather, injury precipitated by a CT- or RT- induced biological cascade which terminates with the release of a range of damaging mediators [4, 25]. In fact, palifermin’s stimulation of epithelial stem cell proliferation is but a small component of its diverse biological potential as it initiates a range of actions which are consistent with tissue protection based on known pathways associated with mucositis [26]. Especially relevant to its observed OM efficacy are its effects on the oxidative stress response, the innate immune response, and pro-inflammatory cytokines. KGF1 increases Nrf2 activity, effects TLR4 and impacts pro-inflammatory cytokine levels. Each of these actions, as discussed below, has been used as the basis for drugs currently under development for OM.
Current mechanistic targets for mucositis being investigated for oral mucositis (Table 1)
Biological targets most likely to impact the OM’s course and severity of are those which occur early in its pathogenesis. Thus, one must consider pathways during the initiation and/or amplification phase as being particularly vulnerable [4, 27•, 28]. Indeed, agents which focus on downstream OM mediators are often too little and too late to block or reverse the snowballing tissue destroying cascade that characterizes OM development. This is especially true in the case of radiation-associated mucositis where the biological challenge is ongoing with each radiation fraction.
The significant unmet clinical need for a mucositis intervention in a growing patient pool has stimulated the quest for a successful treatment. Further catalyzing the commercial enthusiasm for OM are its estimated current and growing market size (over one billion USD; [29]), and the recognition by regulatory agencies of its as impact on patients’ quality of life and ability to tolerate optimum cancer treatment. Consequently, many agents in development have obtained fast-track and/or breakthrough status. Additionally, OM’s shared pathobiology with a myriad of other regimen-related toxicities (i.e., radiation-induced dermatitis and proctitis, fibrosis, and pneumonitis) suggests that a drug that is effective in preventing or attenuating mucositis will likely be efficacious for other indications.
Common features of drugs under development and considerations for assessment
While several exploratory, investigator-initiated, one-center studies are ongoing, the following discussion focuses on small molecules in clinical development by the pharmaceutical industry.
By far, the most common indication being evaluated in clinical trials is OM associated with standard concomitant chemoradiation (CRT) regimens used to treat mouth, oropharyngeal and nasopharyngeal cancers. The uniformity in OM incidence and trajectory associated with CRT provides investigators assurance in power calculations and a threshold for results with clinical meaningfulness. Severe forms of OM occur in 60%–70% of CRT-treated patients. Since treatment requires daily weekday hospital visits clinical trial compliance is enhanced. Additionally, the severity of symptoms, OM’s impact of quality of life and nutrition, and health resource use are measurable endpoints. One of the challenges in this patient population, especially for studies in which incidence is the primary efficacy endpoint, is the extended cancer treatment period (typically 7 weeks). For study drug formulations which are administered topically or by mouth, patient oral or swallowing discomfort (especially in placebo-recipients) and chemotherapy-induced nausea risk contributing to study discontinuation. Conversely, the necessity of IV infusions among patients who receive parental formulations presents a different set of challenges. In the current clinical trial environment, formulations delivered by all routes are being successfully evaluated.
Key factors in assessing and comparing trial outcomes
Historically, one of the challenges in assessing and comparing clinical trial outcomes was the lack of a standardized measurement of mucositis severity. Many grading scales are available [30]. The most common (WHO, CTCAE, and RTOG) were originally developed as measures of adverse events. While WHO OM grading criteria have remained consistent since its inception, CTCAE and RTOG scales have undergone periodic changes thereby hindering comparisons of study outcomes. Currently, the WHO scale is the gold standard and is most common in drug development trials. Clinician assessment is a critical component of the WHO scale. Hence, assuring that individuals charged with this responsibility receive uniform and effective training with competency measurements is required to optimize inter-observer and inter-site outcome consistency [31, 32].
Inclusion criteria for trials typically include a requirement for pathologically confirmed diagnoses of squamous cell carcinoma of the oral cavity (OC) and oropharynx (OPC). Depending on the trial, tumors of the nasopharynx and hypopharynx may be allowed. Most important is the need to assure that studied patients receive equivalent radiation doses to anatomic sites at risk for OM. This objective is typically met by mandating that a minimum number of at-risk sites are in the radiation field which is planned to receive a cumulative dose of at least 50 Gy to 55 Gy, and further assured by independent review of the radiation plan.
In agreement with treatment guidelines, the overwhelming number of patients who receive RT for HNC receive concomitant chemotherapy as a radiosensitizer in which cisplatin is the agent of choice, administered as weekly infusions of 40 mg/m2 or tri-weekly high-dose infusions of 80–100 mg/m2 [33]. The low-dose regimen is the newer of the two, and its use was motivated but its lower rate of toxic events, particularly cisplatin-associated renal toxicity. The impact of treatment regimen on tumor response is mixed and may be dependent on whether the primary tumor site is in the OC or OPC.
Whereas the prevalence of OC in the USA is declining, HPV-related OPC is on the rise. The OPC cohort, especially non-smokers, has a high response rate to CRT [34]. OC and OPC smokers do less well. Data suggest that for OC, the tri-weekly high-dose regimen may be superior, whereas for OPC, tumor outcomes between the two regimens are equivalent [35, 36]. There may be some variability in mucositis risk between the two regimens, although that is unclear. Typically, OM clinical trials allow both treatment regimens; typically, these are stratified in the randomization and analyses. As reported in a large phase 2 trial, OPC was markedly more common than OC and the low-dose regimen was used in almost two-thirds of cases [37•].
Small molecules targeting oxidative stress
Oxidative stress induced by radiation and/or chemotherapy is a primary initiating event in both direct DNA damage and the more critical indirect pathways in the pathobiological cascade leading to mucosal injury. Two pharmacological strategies to attenuate ROS impact are being evaluated, supplementation of physiological antioxidant defense mechanisms and stimulation of transcription factors which induce naturally occurring ROS control.
Superoxide dismutase mimetic: avasopasem manganese
Oxidative stress is a constant threat to cell survival and health. Among the intrinsic mechanisms effectively mitigating this challenge, the superoxide dismutases (SODs) play a compelling role in maintaining oxidative homeostasis [38]. However, in the context of regimen-related toxicities, particularly those associated with fractionated radiation schemes, the accumulating excessive and repeated generation of superoxides overwhelms the ability of naturally occurring anti-oxidative SOD enzymes to sufficiently control the challenge. Consequently, superoxides are is a critical driver of cancer regimen-related tissue damage.
One clinical strategy to alleviate this problem is the provision of SOD supplementation, an approach that was attempted using naturally occurring SODs over two decades ago [39]. However, characteristics of naturally occurring dismutases limited their utility and efficacy. In response, SOD mimetics were developed which, in addition, to having superior qualities are at least as active than as naturally occurring enzymes.
An early pre-clinical study [40] suggested the efficacy of one such mimetic. An analog, avasopasem manganese, has since been developed for OM mitigation by Galera Therapeutics.
In a 223-patient, double-blind, randomized, placebo-controlled phase 2b clinical trial in patients with (locally advanced) nonmetastatic squamous cell carcinoma of the head and neck receiving concurrent radiotherapy (NCT02508389 available at clinicaltrials.gov), avasopasem (GC4419), 90 mg/day, significantly reduced the duration of severe oral mucositis (SOM) by 92%, compared to placebo, including a reduction in median SOM duration from 19 to 1.5 days. The incidence of SOM and the incidence of Grade 4 OM were also significantly reduced (by 34% and 47%, respectively) in patients treated with avasopasem. No significant safety signals were observed, demonstrating avasopasem is well-tolerated when added to a standard radiotherapy regimen [37•]. The drug is now in a phase 3 trial (NCT03689712) in the United States North America and a phase 2 open-label study in Europe (NCT04529850), both for reduction of SOM, and a phase 2, US open-label study for reduction of chemoradiotherapy esophagitis (NCT04225026).
Nrf-2 modulators: RRx-001 and ST-617
In contrast to Galera’s approach, two companies have recently completed early clinical trials of drugs which target the transcription factor Nrf2. Nrf2 is a robust controller of an array of genes, including those involved in the physiologic response to oxidative stress [41]. Specifically, Nrf2 stimulation activates genes associated with the production of anti-oxidative enzymes.
In a small phase 2a trial of Prothex’s RRx-001, a dinitroazetide (NCT03515538) was evaluated in patients receiving concomitant chemoradiation for cancers of the mouth or oropharynx, at 12 US sites [42]. The trial was open label and compared three different dosing schedules of study drug (n = 11–13/group) against a standard-of-care control arm (n = 10). In all arms, the first dose was administered 2 weeks prior to the first radiation dose. Rx-001 was delivered by IV infusion after mixing with patients’ blood. Overwhelming study patients were being treated for OPCs (71%). Although the cohort sizes were small, efficacy trends favored RRx-001-treated patients vs. placebo controls. Median duration of SOM from the start to the last day of radiation (through 70 Gy) was reduced in the test arms (5 days, 13 days, 9 days) compared to the placebo cohort (duration 23 days). Rx001 also increased time to onset of SOM from a median of 26 days in the placebo arm to 33 to 38 days in the active arm (pooled 36 days). Of the 3 treatment schedules tested, the one in which RRx-001 was delivered only prior to CRT favorably affected incidence of most severe forms (grade 4) mucositis. Whereas 30% of patients in the placebo cohort developed grade 4 mucositis (unable to eat or drink anything), none of the patients in the pre-treatment only arm was so impacted. Consequently, gastrostomy use in the pre-treatment only population was reduced compared to placebo patients (60% vs. 33%). Further clinical trials are planned.
Using a rinse/swallow formulation, Supportive Therapeutics evaluated the safety, pharmacokinetics, pharmacodynamics and efficacy of their small molecule Nrf2 activator, ST-617, in an ongoing small, multi-national phase 1b dose-finding study (phase 1B, international, open-label trial to evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of ST-617 for the attenuation of Oral Mucositis in patients receiving Chemoradiation for Head and Neck Cancer) in which patients were treated with three doses of ST-617 [43]. As of October 2020, a total of 16 patients receiving concomitant chemoradiation for OC and OPC were enrolled in 3 dosing arms, 50 mg (n = 7), 100 mg (n = 6), and 150 mg (n = 3; ongoing) at study sites (n = 7) in South Africa and Australia. Based on recently reported results (ESMO; ASTRO), it appeared that ST-617 was safe and well-tolerated. Analyses of tissue (oral mucosa) and blood demonstrated that ST-617 successfully reduced ROS and RNS in a dose-dependent manner. Promisingly, compared to historical controls, patients receiving the 50 mg and 100 mg doses of ST-617 demonstrated attenuation of SOM. Results of the 150 mg cohort are pending. A phase 2 trial is planned.
Small molecules targeting the innate immune response
The innate immune response is a key element in the initiation phase of oral mucositis. Two small molecules are in development for which the innate immune response is specifically targeted.
Dusquetide
Dusquetide, a small molecule under development by Soligenix, Inc., modulates the innate immune response by binding to p62 (SQSTM1) thereby impacting innate immune activation by DAMPS, PAMPS, and CRAMPS [44•] and is in late clinical development. It is administered as a 4-min IV infusion, twice weekly, beginning 3 days after the first radiation dose and continuing for 2 weeks following the last dose of radiation therapy. Results of a multicenter phase 2a study [44•] in which 108 patients were randomized to 3 efficacy cohorts (PL, 1.5 mg/kg, 6.0 mg/kg) demonstrated a 50% reduction in SOM duration in patients receiving the lower dose (1.5 mg) (9 days; placebo 18 days; n = 38). In contrast to data reported in Galera’s phase 2 study in which SOM was unaffected by the choice of cisplatin regimen, the phase 2 dusquetide results found that both the duration and incidence of SOM was greater in patients for whom their chemoradiation regimen included high-dose cisplatin (80 mg/kg-100 mg/kg) compared to those who received weekly low-dose cisplatin.
Enrollment was recently completed for a phase 3 trial of dusquetide (A Pivotal, Double-Blind, Randomized, Placebo-Controlled, Multinational Study of SGX942 (Dusquetide) for the Treatment of Oral Mucositis in Patients Being Treated With Concomitant Chemoradiation for the Treatment of Squamous Cell Carcinoma of the Head and Neck) (NCT03237325) which enrolled 268 patients at 53 study sites in the USA and Europe. Patients in the active arm received the best informed by the phase 2. Unique to the phase 3 study design was the limitation of enrollment to patients whose concomitant chemotherapy was restricted to tri-weekly, high-dose cisplatin (80–100 mg/kg), which contrasts with other trials which also include low-dose weekly cisplatin regimens. Duration of severe oral mucositis, rather than incidence, is the primary efficacy endpoint. Topline results are expected in late 2020.
EC-18
EC-18 is a synthesized monoacetyldiglyceride based on a naturally occurring molecule that is common in seed oils, milk fat and an extract isolated from the antlers of silk deer. The naturally occurring compound has a long history as a component of oriental medicines. EC-18 has been reported to have a range of biological activities [45], many of which are consistent with potential targets for radiation- and chemotherapy-induced tissue injury, particularly its mitigation of activation of the innate immune response. EC-18 is being developed by Enzychem for multiple indications, including OM.
EC-18 is amid a 2-stage, phase 2 multi-institutional, randomized, placebo-controlled study at 20 sites in the USA (NCT03200340). One hundred four patients being treated with concomitant chemoradiation (cisplatin) for cancers of the OC, OPC nasopharynx and hypopharynx are being enrolled. The first stage of the study (completed) consisted of a dose-ranging comparison for safety and toleration in which three doses of EC-18 (n = 6 per arm) were evaluated against an equally sized placebo cohort. The drug was administered in a capsule formulation in which total daily doses of 500 mg, 1000 mg, and 2000 mg were administered twice daily. In the absence of safety concerns, the 2000 mg dose was determined to be optimal for the efficacy component of the trial (n = 86) and is currently the dose being assessed in the ongoing trial. Enrollment is expected to be completed in Q1 of 2021.
Small molecules targeting NF-κB and pro-inflammatory cytokine production
Brilacidin
Brilacidin is a fully synthetic defensin mimetic which regulates immune responsiveness and inflammation through its modulation of the cAMP pathway and subsequent inhibition of PDE4 and PDE3 to mitigate pro-inflammatory responses and activate anti-inflammatory activity [46, 47]. A topical rinse formulation is under development by Innovation Pharmaceuticals.
A small, US-centric, multi-institutional phase 2 trial was completed in patients receiving CRT for cancers of the mouth and oropharynx (Phase 2 Study to Evaluate the Efficacy & Safety of Brilacidin Oral Rinse Administered Daily for 7 Weeks in Attenuating Oral Mucositis in Patients With Head & Neck Cancer Receiving Chemoradiation; NCT02324335) in which patients rinsed with test solution three times per day for the duration of their cancer therapy. Results were reported in ClinicalTrials.gov.
Sixty-one patients were randomized; 46 in the modified intent-to-treat population were evaluated for efficacy (at least one dose of study drug and having been treated with a cumulative radiation dose of 55 Gy). The primary study efficacy endpoint was the incidence of SOM (WHO grades 3 or 4). Of patients in the placebo arm (n = 25), 60% (n = 15) developed SOM vs. 42.9% (n = 9) of patients who received brilacidin. Interestingly, the drug was more active in patients who received high-dose cisplatin every 3 weeks, than in patients being treated with weekly low-dose infusions. A subset analysis in this small group of patients suggested more activity than was observed overall: incidence in the placebo cohort (n = 14) was higher 71.4% than was noted in the 8 patients who received active drug (25%; n = 2).
A phase 3 study is planned.
Validive
In addition to its action as an anti-hypertensive, clonidine’s activity as an α-2 adrenergic receptor agonist modulates NF-κB function to attenuate pro-inflammatory cytokine production. Using clonidine as an active agent, BioAlliance/Onxeo developed a mucobuccal tablet (Validive) which had a longer dissolution time than had been previously described for their Lauriad technology. The tablet, when placed in a patient’s mucobuccal fold enables high local and sustained levels of the drug.
A phase 2 (A Phase II, Multi-center, Randomised, Double-blind, Placebo-controlled Study Comparing the Efficacy and Safety of Clonidine Lauriad® 50 μg and 100 μg Mucoadhesive Buccal Tablet (MBT) Applied Once Daily to Those of Placebo in the Prevention and Treatment of Chemoradiation Therapy Induced Oral Mucositis in Patients With Head and Neck Cancer; NCT01385748) completed enrollment in late 2014 [48•]. One hundred eighty-three patients (n = 183) were randomized to one of three cohorts (placebo n = 62), low-dose clonidine (n = 56) or high-dose clonidine (n = 65). The study’s primary efficacy endpoint was the incidence of SOM.
SOM incidence in the aggregate clonidine-treated cohorts was less (45%) than was reported in placebo-treated patients (60%; p = 0.06). Likewise, the threshold of cumulative radiation associated with SOM onset was higher in patients in the consolidated active arms (60 Gy) than in PL patients (48 Gy), and this was reflected in SOM time-to-onset (45 days vs. 36 days). Reversible hypotension was noted in 6.7% of patients in the active arms.
Validive was licensed to Monopar in 2019. Additional clinical trials are planned for late 2020.
IZN6N4
IZN6N4 is a polymolecular biologically active blend derived from Sambucus nigra, Centella asiatica, and Echinacea purpurea being developed for the mitigation of SOM by Izun Pharmaceuticals [49]. The blend has immunomodulatory, antioxidant, anti-inflammatory and wound-healing activities, and has been successfully applied as an intervention of periodontal disease and diabetic foot ulcers.
A multi-national (USA, Israel) trial (Safety and Efficacy of IZN-6N4 Oral Rinse for the Prevention of Oral Mucositis in Patients With Head and Neck Cancer; NCT1400620) conducted at 12 centers enrolled 110 patients with HNC scheduled to receive standard CRT regimens. IZN6N4 or placebo was used as an expectorated rinse 3 times daily throughout the course of radiation. Results reported in late 2017 demonstrated that, compared to placebo controls, patients treated with IZN-6N4 had less mouth and throat pain and soreness and were more able to maintain their weights throughout the course of radiotherapy. Although not statistically significant, treatment with IZN-6N4 also reduced the incidence of severe SOM compared to placebo. Additional clinical studies are planned.
Summary and conclusions
OM remains a clinically significant toxicity of the most common forms of cancer therapy. It is particularly devastating in patients who are treated with chemoradiation regimens for HNC and with myeloablative forms of chemotherapy. Mucositis is among the most studied and biologically understood of regimen-related toxicities. Since the elements which drive its pathogenesis are shared with other forms of radiation and chemotherapy side effects, a successful intervention for OM will likely to pave the way for halo indications such as radiation-associated dermatitis, proctitis and pneumonitis, fibrosis and even chemotherapy-induced cognitive dysfunction and chemotherapy- and radiation-induced fatigue.
The recognition of the biological complexities which underlie mucositis and the understanding that many of the events perpetuated by radiation or chemotherapy are not the consequence of direct epithelial cell damage, but the result of secondary signaling events has provided a series of potential therapeutic targets. Of these, oxidative stress, the innate immune response, and pro-inflammatory cytokines have been the targets of choice. While the results of phase 2 trials have been a cause for optimism, it will probably be at least another year before definitive data are available.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Lalla RV, et al. Oral mucositis due to high-dose chemotherapy and/or head and neck radiation therapy. J Natl Cancer Inst Monogr. 2019;2019(53):lgz011.
Panghal M, Kaushal V, Kadayan S, Yadav JP. Incidence and risk factors for infection in oral cancer patients undergoing different treatments protocols. BMC Oral Health. 2012. Jul 20;12:22. https://doi.org/10.1186/1472-6831-12-22.
Berger K, Schopohl D, Bollig A, Strobach D, Rieger C, Rublee D, et al. Burden of oral mucositis: a systematic review and implications for future research. Oncol Res Treat. 2018;41(6):399–405.
Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009;45:1015–20.
• Bachour P, Sonis ST. Predicting the risk of mucositis associated with cytotoxic cancer treatment regimens: rationale, complexity and challenges. Curr Opinion Support Palliat Care. 2018;12:1998–210 Mucositis risk is not uniform. This paper discusses risk prediction, especially in the context of genomics.
Saunders DP, et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer. 2020. May;28(5):2473–84.
Chan A, Ignoffo RJ. Survey of topical oral solutions for the treatment of chemo-induced oral mucositis. J Oncol Pharm Pract. 2005;11:139–43.
Dodd MJ, Dibble SL, Miaskowski C, MacPhail L, Greenspan D, Paul SM, et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:39–47.
Treister N, Nieder M, Baggott C, Olson E, Chen L, Dang H, et al. Caphosol for prevention of oral mucositis in pediatric myeloablative haematopoietic cell transplantation. Br J Cancer. 2017. Jan 3;116(1):21–7.
Rao NG, Trotti A, Kim J, Schell MJ, Zhao X, Amdur RJ, et al. Phase II multicenter trial of Caphosol for the reduction of mucositis in patients receiving radiation therapy for head and neck cancer. Oral Oncol. 2014;50:765–9.
Park SH, Lee HS. Meta-analysis of oral cryotherapy in preventing oral mucositis associated with cancer therapy. Int J Nurs Pract. 2019. Oct;25(5):e12759.
Zecha JA, et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24:2781–92.
Pearlman R, Zaki M, Laszewski P, et al. Does low level laser therapy improve clinical outcomes related to oral mucositis in patients treated for head and neck cancer. Int J Radiat Oncol Biol Phys 2020; 108, S3: E812.
Sonis ST, Hashemi S, Epstein JB, Nair RG, Raber-Durlacher JE. Could the biological robustness of low-level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients? Oral Oncol. 2016;54:7–14.
Vasconcelos RM, Sanfilippo N, Paster BJ, Kerr AR, Li Y, Ramalho L, et al. Host-microbiome crosstalk in oral mucositis. J Dent Res. 2016;95:725–33.
Giles FJ, Miller CB, Hurd DD, Wingard JR, Fleming TR, Sonis ST, et al. A phase III, randomized, double-blind, placebo-controlled, multinational trial of Iseganan for the prevention of oral mucositis in patients receiving stomatotoxic chemotherapy (PROMPT-CT trial). Leuk Lymphoma. 2003;44:1165–72.
Trotti A, et al. A multinational, randomized phase III trial of Iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head and neck malignancy. Int J Radiat Oncol Phys. 2004;3:674–81.
Foote RL, Loprinzi CL, Frank AR, O’Fallon JR, Gulavita S, Tewfik HH, et al. Randomized trial of a chlorhexidine mouthwash for alleviation of radiation-induced mucositis. JCO. 1994;12:2630–3.
El-Sayed S, et al. Prophylaxis of radiation-associated mucositis in conventionally treated patients with head and neck cancer: a double-blind, phase III, randomized, controlled trial evaluating the clinical efficacy of an antimicrobial lozenge using a validated mucositis scoring system. J Clin Oncol. 2002;20:3956–63.
Stokman MA, Spijkervet FKL, Burlage FR, Dijkstra PU, Manson WL, de Vries EGE, et al. Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: a double-blind randomized trial. Brit J Cancer. 2003;88:1012–6.
Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–8.
Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–20.
Le QT, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–14.
Borges L, Rex KL, Chen JN, Wei P, Kaufman S, Scully S, et al. A protective role for keratinocyte growth factor in a murine model of chemotherapy and radiotherapy-induced mucositis. Int J Radiat Oncol Biol Phys. 2006;66:254–62.
Russi EG, Raber-Durlacher JE, Sonis ST. Local and systemic pathogenesis and consequences of regimen-induced inflammatory responses in patients with head and neck cancer receiving chemoradiation. Mediators Inflamm 2014; 518261. https://doi.org/10.1155/2014/518261.
Blijlevens N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol. 2007;18:817–26.
• Bowen J, et al. The pathogenesis of mucositis: updated perspectives and emerging targets. Support Care Cancer. 2019;27:4023–33 This recent review provides an update in the pathogenesis of mucositis and provides a perspective for the mechanistic basis for a number of agents under development.
Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84.
Oral mucositis market size, growth and trends by cause (oral mucositis caused by chemotherapy, oral mucositis caused by radiotherapy, oral mucositis caused by hematopoietic stem cell transplantation, and others), end-user (hospitals, dental clinics, oncology hospitals, and research institutes), and region (Americas, Europe, Asia-Pacific, and Middle East & Africa)—global forecast till 2025; ID: MRFR/Pharma/1002-HCR | September 2020.
Sonis ST. A comparison and assessment of scoring scales for mucositis. In Sonis, ST, Oral Mucositis. Springer Handbook Series 2012; pps 39–46.
Stokman MA, Sonis ST, Dijkstra PU, Burgerhof JGM, Spijkervet FKL. Assessment of oral mucositis in clinical trials: impact of training on evaluators in a multi-centre trial. Eur J Cancer. 2005;41:1735–8.
Quinn B, Stone R, Uhlenhopp M, et al. Ensuring accurate oral mucositis assessment in the European Group for Blood and Marrow Transplantation Prospective Oral Mucositis Audit. Eur J Oncol Nurs. 2007;11 Suppl 1:S10–8.
Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, et al. NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Cancer Netw. 2018;16:479–90.
Vawda N, Banerjee RN, Debenham BJ. Impact of smoking on outcomes of HPV-related oropharyngeal cancer treated with primary radiation or surgery. Int J Radiat Oncol Biol Phys. 2019;103:1125–31.
Noronha V, Joshi A, Patil VM, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. 2018;36:1064–72.
Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. 2017;22:1056–66.
• Anderson CM, et al. Phase 2b, randomized, double-blind trial of GC4419 vs placebo to reduce severe oral mucositis in head and neck cancer patients receiving concurrent radiotherapy and cisplatin. J Clin Oncol. 2019;37:3256–65 Results of a phase 2 trial testing the efficacy of a superoxide dismutase mimetic. The clinical trial design is representative of most of studies being done for an OM indication. The efficacy data support the importance of superoxide formation as a driver of OM and validate superoxide dismutases’ potential in mitigating its pathogenesis.
Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217:1915–28.
Escribano A, García-Grande A, Montañés P, Miralles L, García A. Aerosol orgotein (Ontosein) for the prevention of radiotherapy-induced adverse effects in head and neck cancer patients: a feasibility study. Neoplasma. 2002;49:201–8.
Murphy CK, Fey EG, Watkins BA, Wong V, Rothstein D, Sonis ST. Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin Cancer Res. 2008;14:4292–7.
Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. JBC. 2009;284:13291–5.
Sher DJ, Bonomi M, Blakaj DM, et al. Results of a randomized, open label, multicenter trial assessing the safety, dose and schedule of RRx-001 (R001) in reducing severe oral mucositis (SOM) in patients receiving chemoradiation (CRT) for oral cavity/oropharynx squamous cell carcinoma (OSCC). Int J Radiat Oncol Biol Phys 2020; 108, S3:S107–108.
Osei-Fofie D, et al. Phase1b, international, dose-escalation study to evaluate the safety, pharmacokinetics (PK) and efficacy of ST-617, a dithiolethione, to attenuate oral mucositis (OM) in patients receiving chemoradiation for head and neck cancers. ESMO 2020; Meeting Abstract 3692.
• Kudrimoti M, et al. Dusquetide: reduction in oral mucositis associated with enduring ancillary benefits in tumor resolution and decreased mortality in head and neck cancer patients. Biotechnol Rep (Amst). 2017;15:24–6 The pre-clinical and clinical results described support the importance of the innate immune response in the initiation of mucositis. The difference in response based on the concomitant chemoradiation regimen is noteworthy.
Choi S, Shin SH, Lee HR, Sohn KY, Yoon SY, Kim JW. 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol ameliorates chemoradiation-induced oral mucositis. Oral Dis. 2020;26:111–21.
Innovation Pharmaceuticals Press Release. Innovation Pharmaceuticals Phase 2 Oral Mucositis Trial Additional Data Show Brilacidin-OM Demonstrated A Significant Reduction in the Incidence of Severe Oral Mucositis in Patients with Head and Neck Cancer (HNC) Receiving Aggressive Chemotherapy Regimen. April 9, 2018.
Scott, R.W., Sonis, S., Korczak, B., Brilacidin, Host Defense Peptide Mimetic, One of a New Class of Immunomodulatory Agents That Can Target Multiple Disease Indications et al. 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2015).
• Giralt J, et al. Randomized phase 2 trial of a novel clonidine mucoadhesive buccal tablet for the amelioration of oral mucositis in patients treated with concomitant chemoradiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2020;106:320–6 This paper describes the results of a clinical trial in which a topically delivered formulation impacted the course and severity of mucositis development.
Grbic J, Wexler I, Celenti R, Altman J, Saffer A. A phase II trial of transmucosal herbal patch for the treatment of gingivitis. J Am Dent Assoc. 2011;142:1168–75.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Stephen T. Sonis is an employee of Biomodels LLC and Primary Endpoint Solutions, LLC; both companies assist industry (including companies described in this paper), government, and academics to study and enable drugs, biologicals, and devices to treat patients for a wide range of indications including oral mucositis. Dr. Sonis does not have equity in any of the companies with which he works, nor does he receive direct compensation from them. Dr. Sonis is also a founder of Inform Genomics, Inc.; and is listed as an inventor on the following issued patents: 6458777, 6663850, 6713463, 6841578B2, 7297123, and 10,475539.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Palliative and Supportive Care
Rights and permissions
About this article
Cite this article
Sonis, S.T. Treatment for Oral Mucositis—Current Options and an Update of Small Molecules Under Development. Curr. Treat. Options in Oncol. 22, 25 (2021). https://doi.org/10.1007/s11864-021-00823-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00823-6