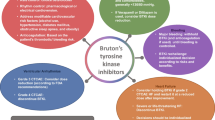
Opinion statement
There has been a significant shift in the management of B cell malignancies over the past decade. Initial strategies involving the use of systemic chemotherapies have been gradually replaced by more targeted therapies to improve survival and overall tolerability. Bruton’s tyrosine kinase inhibitors are breakthrough drugs that have been approved to treat many B cell malignancies. Despite their demonstrated benefits, unintended events still occur including various cardiotoxicities. In this review, we discuss the rationale behind developing these agents, their common cardiovascular toxicities, and associated management challenges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional approaches to treating B cell malignancies include chemotherapy, immunotherapy, and a combination of both. Initial approaches consisted of monotherapy with alkylating agents such as chlorambucil, which was the gold standard for decades, purine analogs such as fludarabine, and anti-CD20 antibodies such as rituximab [1, 2]. Combinations of chemotherapy and immunotherapy have been shown to have synergistic effects, such as rituximab with fludarabine or fludarabine-cyclophosphamide [3]. The combination of fludarabine, cyclophosphamide, and rituximab (FCR) was the first-line therapy for young patients with symptomatic chronic lymphocytic leukemia (CLL) [4], with an overall response rate of 95% in previously untreated patients and a complete remission rate of 44%, double than those with fludarabine and cyclophosphamide alone (44% vs 22%) [5]. However, most of these therapies require intravenous infusion and safety monitoring due to their cytotoxic side effects. Patients with CLL who are older than 65 years of age are less likely to tolerate fludarabine-based therapy, with increased toxicity often leaving them unable to complete the necessary cycles [5]. In addition, patients with certain mutations, such as the deletion of the short arm of chromosome 17 (del 17p13.1), have a decreased overall response rate and decreased progression-free survival [6]. This led to the paradigm shift in cancer moving towards targeted treatments, thus leading to the development of Bruton’s tyrosine kinase (BTK) inhibitors [6].
BTK, a member of the Tec kinase family, is an essential enzyme in the activation of downstream signaling pathways required for survival and proliferation of malignant B cells [7, 8]. The pathogenesis of CLL and various other B cell malignancies consists of the upregulation of this B cell receptor pathway [9, 10]. Inhibition of BTK in malignant B cells leads to diminished proliferation, decreased tumor survival, and impaired adhesion and migration of the malignant B cells to their growth-promoting microenvironment [11, 12]. As such, two BTK inhibitors, ibrutinib and acalabrutinib, have gained Food and Drug Administration (FDA) approval for the treatment of various B cell malignancies, with several others currently under active clinical investigation and/or development.
Ibrutinib
Ibrutinib is an oral, once-a-day, BTK inhibitor that works by forming an irreversible covalent bond with a cysteine residue, demonstrating an improvement in overall and progression-free survival in various B cell malignancies [13, 14]. Unlike other regimens used for B cell malignancies, this BTK inhibitor does not have toxic effects on normal T cells [15]. The randomized phase 3 RESONATE trial in patients with previously treated CLL or small lymphocytic lymphoma (SLL) demonstrated a statistically significant 57% reduction in the rate of death vs. ofatumumab, with an overall survival rate of 90% at 12 months, and increased overall response rate (42.6% vs 4.1%) [16]. Similarly, in a phase 2, open-label, multicenter study that assessed the activity of ibrutinib in 145 patients with relapsed or refractory CLL with del17p or SLL who had experienced poor response rates after chemoimmunotherapy, 120 patients (83%) had an overall response at a median follow-up of 27.6 months. The 24-month progression-free survival was 63%, while the 24-month overall survival was 75% [17]. In a phase 2 study that included 111 patients with relapsed or refractory mantle cell lymphoma, patients treated with ibrutinib had a complete response rate of 21% and a partial response rate of 47% [18]. In another phase 3 trial, 150 patients with Waldenström’s macroglobulinemia (WM) were assigned to receive ibrutinib plus rituximab or placebo plus rituximab; it was found that at 30 months, the progression-free survival rate was 82% with ibrutinib-rituximab versus 28% with placebo-rituximab [19]. Similarly, a multicenter, open-label study evaluating the efficacy of ibrutinib in patients with chronic graft versus host disease (cGVHD) demonstrated an overall response rate of 67% at a median follow-up of 13.9 months [20].
As a result of these various studies, ibrutinib is currently approved for the treatment of CLL, SLL, mantle cell lymphoma, WM, 17p deletion CLL, and cGVHD [21]. Despite its many clinical benefits, ibrutinib has several cardiotoxic effects including atrial and ventricular arrhythmias and hypertension, which have been associated with increased morbidity and mortality in this population [22••]. Moreover, ibrutinib is also associated with increased bleeding which further complicates the management of these patients [22••]. As such, it is imperative that both cardiologists and hematologists/oncologists recognize and appropriately manage these issues to allow for the continued optimal cancer treatment while mitigating long-term cardiovascular risk.
Atrial fibrillation and other atrial arrhythmias
Atrial fibrillation is one of the most common cardiac side effects related to ibrutinib therapy (Table 1) [29]. Incidence of atrial fibrillation has been reported in up to 16% of patients treated with ibrutinib [30]. Although patients with a prior history of atrial fibrillation are more prone to recurrence with this medication, patients with no known history of atrial fibrillation can also develop this arrhythmia. In the RESONATE trial, 3% of patients developed grade 3 or higher atrial fibrillation, while another 2% of patients developed grade 1 or 2 atrial fibrillation [16]. After a 19-month median follow-up, a total of 7% of patients developed atrial fibrillation with a 5.1-month median time to onset [23]. In another randomized phase 3 trial (RESONATE-2), 269 patients age 65 years or older with previously untreated CLL or SLL were randomized to receive either ibrutinib or chlorambucil, with 6% of ibrutinib-treated patients developing atrial fibrillation after a median exposure of 17.4 months in contrast to only 1 patient in the chlorambucil group [13]. The 5-year follow-up of the RESONATE-2 trial showed a total of 16% had developed atrial fibrillation at any time, with 5% having developed a grade 3 event. Interestingly, only 4% had continued episodes of AF at 5 years [25]. In a phase 2, open-label, multicenter study of 144 patients with relapsed or refractory CLL or SLL with the 17p deletion, 6% developed atrial fibrillation after a median of 27 months of follow-up, similar to the reported rate in RESONATE [17].
The excess risk of developing atrial fibrillation with ibrutinib has been shown in multiple studies. In a retrospective study, 137 patients diagnosed with B cell malignancies treated with ibrutinib were compared with 106 patients treated with chemotherapy for the same cancers. The incidence of atrial arrhythmias was 14% in the ibrutinib group, compared with 3% in patients treated with chemotherapy (p = 0.009). In multivariable analysis, patients treated with ibrutinib had a fivefold increased risk of developing atrial arrhythmias (odds ratio = 5.18, 95% confidence interval 1.42 to 18.89) and ibrutinib was an independent risk factor for developing atrial arrhythmias [26]. Additionally, in a randomized trial of 150 patients, comparing placebo-rituximab and ibrutinib-rituximab, atrial fibrillation occurred in 15% of patients treated with ibrutinib vs 3% in the placebo group. Notably, 27% of patients in the ibrutinib group had a prior history of atrial fibrillation [19]. In a retrospective case-control study of 183 patients with CLL treated with ibrutinib, 11.3% developed atrial fibrillation at a median time of 7 months from ibrutinib initiation [28]. In another study where 43 CLL patients were assessed before starting ibrutinib therapy, atrial fibrillation occurred in 16.3% of patients after a median follow-up of 8 months. Of those, 57.1% were started in anticoagulation, and 71.4% were started on beta blockers or amiodarone [27]. Lastly, a recent observational retrospective analysis, which was the largest and most extensive clinical characterization of cardiovascular adverse drug reactions, identified 303 cardiovascular deaths related to ibrutinib out of 13,572 individual case safety reports. In addition, the median time from initiation of treatment with ibrutinib to onset of supraventricular arrhythmia (SVA) was approximately 2–3 months with a total of 959 (7.07%) cases, 93% of which were atrial fibrillation. The reported odds ratio was 23.1 with associated rates of heart failure and death in 11.9 and 10% respectively [22••].
Mechanism of atrial fibrillation
Although the actual mechanism of ibrutinib-related atrial fibrillation is still unknown, it is thought to be related to the inhibition of cardiac PI3K-Akt signaling [31]. However, the effects on PI3K-AKT signaling alone may not be sufficient to explain the mechanism of ibrutinib-induced atrial fibrillation as only a few cases of atrial fibrillation were documented with acalabrutinib, a second-generation, selective, irreversible inhibitor of Burton’s tyrosine kinase inhibitor [32]. In a mouse model, potential mechanisms for ibrutinib-associated atrial fibrillation included atrial structural remodeling and fibrosis, dysregulated calcium handling in atrial myocytes, enhanced delayed afterdepolarization, and increased activity of CaMKII, an enzyme kinase implicated in many cardiac pathologies [33].
Risk factors
Multiple risk factors for developing atrial fibrillation with ibrutinib therapy have been identified.
Brown and colleagues evaluated over 1500 ibrutinib-treated patients with CLL and mantle cell leukemia from four randomized trials and found that a history of atrial fibrillation, ibrutinib therapy, and older age (> 65 years) were independent risk factors in multivariate analyses for developing atrial fibrillation, while hypertension and hyperlipidemia were additional risk factors in a univariate model [30].
In a “real-world” retrospective study where patients with prior history of atrial fibrillation were excluded, ibrutinib use, age, hypertension, and previous use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and aspirin were independently associated with incident arrhythmias in univariate analyses [26]. A retrospective case-control study of 183 consecutive patients with chronic lymphocytic leukemia treated with ibrutinib identified left atrial enlargement on pre-ibrutinib EKG as a significant predictor of atrial fibrillation development. Interestingly, diabetes mellitus and coronary artery disease were not shown to be strong predictors for atrial fibrillation in this study [28]. In another study, left atrial volume index of ≥ 40 mL/m2 on transthoracic echocardiogram at ibrutinib initiation identified patients at risk for developing atrial fibrillation [27].
Management of ibrutinib-associated atrial fibrillation
Management of ibrutinib-associated atrial fibrillation can be challenging; however, discontinuation of ibrutinib solely due to atrial arrhythmias is generally not recommended [34]. When considering a rate control strategy (primarily for those patients with minimal symptoms), beta blockers are preferred due to fewer drug-drug interactions. Due to the inhibition of cytochrome P450 3A4, non-dihydropyridine calcium-channel blockers such as diltiazem and verapamil are generally avoided as they can increase concentrations of ibrutinib [35, 36]. Conversely, ibrutinib can affect digoxin metabolism via p-glycoprotein leading to potential digoxin toxicity due to its narrow therapeutic window [32]. From a rhythm control perspective, amiodarone should be used with caution due to its effects on CYP 3A4 metabolism. Little data exists regarding other antiarrhythmic medications. Moreover, the safety and efficacy of ablation have not been specifically evaluated in this population [37].
Stroke risk prediction
The most commonly used stroke risk prediction tool is the CHADS VASc score, which has been extensively validated in the general cardiology patient population [24]. However, its accuracy in predicting stroke risk in the cancer population is unclear, as it does not incorporate certain characteristics such as the presence of malignancy and life expectancy into its calculations [32]. In a recent study from Denmark, patients in a nationwide registry with cancer and AF had higher rates of thromboembolism at low CHADS VASc scores (0–1) but lower rates with scores of 2+ when compared with non-cancer patients [38]. Similarly, in a study from Taiwan, higher CHADS VASc scores had less impact on predicting the risk of ischemic stroke in the setting of AF and cancer [39].
Stroke risk reduction
The antiplatelet effects of ibrutinib make it challenging to choose the appropriate stroke risk reduction strategy in the setting of atrial fibrillation. Ibrutinib is known to increase the risk of bleeding by inhibiting glycoprotein VI collagen-activation pathway and glycoprotein 1b–mediated platelet function, thus decreasing platelet aggregation and adhesion to von Willebrand factor [40,41,42]. In a phase II study with mantle cell lymphoma, almost 4% of patients developed subdural hematoma following episodes of trauma, while on aspirin or warfarin, leading to the discouragement of warfarin use [43]. The use of direct oral anticoagulants appears to be generally safe; however, careful monitoring is necessary given the potential for drug-drug interactions [44]. For example, ibrutinib can increase concentrations of direct oral anticoagulants metabolized by CYP 3A4, including apixaban and rivaroxaban, which can lead to an increased risk of bleeding. In addition, ibrutinib can increase the levels of dabigatran, a direct thrombin inhibitor, via its effects on the P-glycoprotein system [37]. Although bleeding risk scores such as HAS-BLED are used to assess bleeding risk in patients receiving anticoagulation, its applicability in cancer patients lacks validation [45].
Ventricular arrhythmia
Ibrutinib use has been associated with ventricular arrhythmias but with less frequency than atrial fibrillation. Ibrutinib-associated ventricular arrhythmias are not driven by QT prolongation and early afterdepolarizations as is the case with many other cancer therapeutics [46,47,48]. The incidence of ibrutinib-associated ventricular arrhythmias was recently reported in a study of 582 patients. After a median follow-up of 32 months, 1% developed symptomatic ventricular arrhythmias related to ibrutinib exposure. In a subset of those without prior history of coronary artery disease or heart failure, the incidence of ventricular arrhythmia events was estimated to be 596 per 100,000 person-years [49••]. In the HELIOS study, a randomized, double-blind, phase 3 study that assessed the safety of adding ibrutinib to bendamustine plus rituximab for previously treated CLL or SLL, 2% of patients developed grade 3 or more ventricular arrhythmias, cardiac arrests, and sudden deaths in the ibrutinib-containing arm, consistent with 1991 events per 100,000 person-years [46, 50, 51].
Hypertension
Ibrutinib exposure has also been linked to the development of hypertension. In the HELIOS trial, the incidence of hypertension was 3.5% in patients treated with ibrutinib after a median follow-up of 17 months [51]. After a three-year follow-up study of 101 refractory/relapsed (R/R) CLL/SLL patients and 31 treatment-naïve (TN) CLL/SLL patients, 20% of RR patients and 23% of TN patients developed grade 3 or grade 4 hypertension [21]. In the RESONATE trial, with up to 71 months of follow-up after the initiation of ibrutinib therapy, 21% of patients developed hypertension of grade 3 or higher [52]. In a safety analysis of four randomized controlled trials that included 756 patients on ibrutinib and 749 patients on the comparator drug, grade 3 or higher hypertension was reported in 4% versus 1%, respectively. The median time to onset of hypertension was 4.6 months. While no patient required dose reduction due to hypertension, 78% of patients required initiation or escalation of antihypertensive medications [53]. In a study of 562 patients over an average period of 30 months, 78.3% of patients developed new or worsening hypertension, with a mean increase in systolic pressure of 5.2 mmHg. New hypertension developed in 71.6% of patients on ibrutinib. Of those who did not have a previous history of hypertension, 17.7% developed high-grade hypertension with BPs of 160/100 mmHg or greater. New or worsening hypertension was associated with a twofold increase in major adverse cardiac events. Although initiation of antihypertensive medications was associated with a lower risk of major adverse cardiac events, no specific class was identified as superior in preventing or controlling ibrutinib-associated blood pressure elevations [54••]. While the pathophysiology of ibrutinib-associated hypertension is not yet established, it does not appear to be VEGF (vascular endothelial growth factor) mediated.
Acalabrutinib
Acalabrutinib is a novel BTK inhibitor that was designed to improve on the safety and efficacy of first-generation BTK inhibitors such as ibrutinib [55]. In preclinical studies, acalabrutinib did not inhibit epidermal growth factor receptor (EGFR), interleukin-2-inducible T cell kinase (ITK), T cell X chromosome kinase, or tyrosine kinase expressed in hepatocellular carcinoma (TEC) family proteins. This translates into less off-target activity and likely explains the overall improved side effects profile of acalabrutinib [56].
Acalabrutinib was initially granted accelerated approval for the treatment of relapsed or refractory mantle cell lymphoma, based on the efficacy data from an open-label, phase 2 study of 124 patients with mantle cell lymphoma who had received at least one prior therapy [57]. There were no reported cases of atrial fibrillation in this study.
Recently, the FDA granted acalabrutinib Breakthrough Therapy designation for the treatment of adults with CLL or SLL. The approval was based on two randomized clinical trials. The phase 3 ELEVATE TN trial recruited 535 patients with previously untreated CLL, in which patients were randomized in a 1:1:1 fashion to acalabrutinib alone, acalabrutinib combined with intravenous obinutuzumab, or obinutuzumab plus oral chlorambucil. Patients who received acalabrutinib had a longer progression-free survival compared with patients receiving other standard treatments, with atrial fibrillation incidence of less than 4% (60). The second clinical trial (ASCEND) included 310 patients with previously treated CLL and showed that patients receiving acalabrutinib also had longer progression-free survival than patients receiving rituximab in combination with idelalisib or rituximab in combination with bendamustine. The incidence of atrial fibrillation in the acalabrutinib group in this study was 5% (61). Interestingly, bleeding of any grade with acalabrutinib occurred in 40% in ELEVATE TN and 26% in ASCEND.
Conclusion
The introduction of targeted BTK inhibitors, such as ibrutinib, has revolutionized the management of B cell malignancies. Nevertheless, unique cardiovascular toxicities have been identified including atrial and ventricular arrhythmias and hypertension. These toxicities are primarily associated with ibrutinib and do not appear to be class-related adverse events as low rates are observed with other BTK inhibitors. Atrial fibrillation is now a well-recognized adverse effect of ibrutinib, and while management can be challenging, especially regarding anticoagulation strategies given the enhanced bleeding with ibrutinib, most patients can continue receiving the drug with careful monitoring. Ventricular arrhythmias are quite rare but can be life threatening often requiring treatment discontinuation. Finally, hypertension is commonly identified and can be managed with typical antihypertensive medications. The exact mechanism of these toxicities remains unclear; basic and translational research to understand the pathophysiology is an area of significant future need in order to develop better clinical risk prediction and mitigation strategies so that patients can continue receiving these effective therapies with minimal cardiovascular morbidity.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst. 1999;91:861–8.
Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92:946–65.
Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–9.
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74.
Badar T, Burger JA, Wierda WG, O’Brien S. Ibrutinib: a paradigm shift in management of CLL. Expert Rev Hematol. 2014;7:705–17.
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94.
Anderson JS, Teutsch M, Dong Z, Wortis HH. An essential role for Bruton’s [corrected] tyrosine kinase in the regulation of B-cell apoptosis. Proc Natl Acad Sci U S A. 1996;93:10966–71.
Davids MS, Brown JR. Ibrutinib: a first in class covalent inhibitor of Bruton’s tyrosine kinase. Future Oncol. 2014;10:957–67.
de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102:1629–39.
Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9.
Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28:649–57.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42.
Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96.
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23.
O’Brien S, Jones JA, Coutre SE, Mato AR, Hillmen P, Tam C, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17:1409–18.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16.
Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenstrom’s macroglobulinemia. N Engl J Med. 2018;378:2399–410.
Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–50.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506.
•• Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with Ibrutinib. J Am Coll Cardiol. 2019;74:1667–78 Large study evaluating adverse cardiovascular events with ibrutinib and demonstating increaed mortality in the setting of atrial arrhythmais.
Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32:83–91.
Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GYH, Dorian P, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34:170–6.
Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2019.
Fradley MG, Gliksman M, Emole J, Viganego F, Rhea I, Welter-Frost A, et al. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol. 2019;124:539–44.
Reda G, Fattizzo B, Cassin R, Mattiello V, Tonella T, Giannarelli D, et al. Predictors of atrial fibrillation in ibrutinib-treated CLL patients: a prospective study. J Hematol Oncol. 2018;11:79.
Mato AR, Clasen S, Pickens P, Gashonia L, Rhodes J, Svoboda J, et al. Left atrial abnormality (LAA) as a predictor of ibrutinib-associated atrial fibrillation in patients with chronic lymphocytic leukemia. Cancer Biol Ther. 2018;19:1–2.
Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6:e001049.
Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–805.
McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–30.
Alomar M, Fradley MG. Electrophysiology translational considerations in cardio-oncology: QT and beyond. J Cardiovasc Transl Res. 2019.
Jiang L, Li L, Ruan Y, Zuo S, Wu X, Zhao Q, et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374–82.
Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol. 2019;136:56–63.
Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4:1491–500.
Thompson PA, Levy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175:462–6.
Rhea IB, Lyon AR, Fradley MG. Anticoagulation of cardiovascular conditions in the cancer patient: review of old and new therapies. Curr Oncol Rep. 2019;21:45.
D’Souza M, Carlson N, Fosbol E, et al. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–8.
Hu WS, Lin CL. Impact of atrial fibrillation on the development of ischemic stroke among cancer patients classified by CHA2DS2-VASc score-a nationwide cohort study. Oncotarget. 2018;9:7623–30.
Vrontikis A, Carey J, Gilreath JA, Halwani A, Stephens DM, Sweetenham JW. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology (Williston Park). 2016;30:970–4 980–1, C3.
Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783–7.
Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124:3991–5.
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–45.
Rhea I, Burgos PH, Fradley MG. Arrhythmogenic anticancer drugs in cardio-oncology. Cardiol Clin. 2019;37:459–68.
Sanz AP, Gomez JLZ. AF in cancer patients: a different need for anticoagulation? Eur Cardiol. 2019;14:65–7.
Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, Banerji V, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–4.
Tomcsanyi J, Nenyei Z, Matrai Z, Bozsik B. Ibrutinib, an approved tyrosine kinase inhibitor as a potential cause of recurrent polymorphic ventricular tachycardia. JACC Clin Electrophysiol. 2016;2:847–9.
Beyer A, Ganti B, Majkrzak A, Theyyunni N. A perfect storm: tyrosine kinase inhibitor-associated polymorphic ventricular tachycardia. J Emerg Med. 2017;52:e123–7.
•• Guha A, Derbala MH, Zhao Q, et al. Ventricular arrhythmias following Ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72:697–8 Important study quatifying the burden of ventricular arrhythmias associated with ibrutinib exposure.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11.
Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–63.
O’Brien S, Hillmen P, Coutre S, Barr PM, Fraser G, Tedeschi A, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:648–57 e15.
•• Dickerson T, Wiczer T, Waller A et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019. Large study reporting the incidence of hypertension with ibrutinib and its association with adverse cardiovascular outcomes.
Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26:e233–40.
Vreman RA, Geenen JW, Hovels AM, Goettsch WG, Leufkens HGM, Al MJ. Phase I/II clinical trial-based early economic evaluation of acalabrutinib for relapsed chronic lymphocytic leukaemia. Appl Health Econ Health Policy. 2019;17:883–93.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ricardo Pineda-Gayoso declares that he has no conflict of interest.
Mohammed Alomar declares that he has no conflict of interest.
Dae Hyun Lee declares that he has no conflict of interest.
Michael Fradley has received research funding from Medtronic and has received compensation from Novartis for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Pineda-Gayoso, R., Alomar, M., Lee, D.H. et al. Cardiovascular Toxicities of Bruton’s Tyrosine Kinase Inhibitors. Curr. Treat. Options in Oncol. 21, 67 (2020). https://doi.org/10.1007/s11864-020-00764-6
Published:
DOI: https://doi.org/10.1007/s11864-020-00764-6