Opinion statement
Neuroendocrine tumors (NETs) are relatively rare, with 12,000–15,000 new cases diagnosed annually in the USA. Although NETs are a diverse group of neoplasms, they share common molecular targets that can be exploited using nuclear medicine techniques for both imaging and therapy. NETs have traditionally been imaged with SPECT imaging using 111In-labeled octreotide analogs to detect neoplasms with somatostatin receptors. In addition, certain NETs (pheochromocytomas, paragangliomas, and neuroblastomas) are also effectively imaged using 123I- or 131I-labeled metaiodobenzylguanidine (MIBG), an analog of guanethidine. More recently, PET imaging with 68Ga-labeled somatostatin receptor (SSR) analogs allows neuroendocrine tumors to be imaged with much higher sensitivity. 68Ga-DOTATATE was approved as a PET tracer by the FDA in June 2016. In addition to imaging, both MIBG and DOTATATE can be labeled with therapeutic radionuclides to deliver targeted radiation selectively to macroscopic and microscopic tumor sites. The incorporation of the same molecular probe for imaging and therapy provides a radio-theranostic approach to identifying, targeting, and treating tumors. Over the years, several centers have experience treating NETs with high-dose 131I-MIBG. 177Lu-DOTATATE was approved by the FDA in 2018 for treatment of gastroenteropancreatic NETs and constitutes a major advancement in the treatment of these diseases. In this paper, we provide an overview of imaging and treating neuroendocrine tumors using MIBG and SSR probes. Although uncommon, neuroendocrine tumors have provided the largest experience for targeted radionuclide imaging and therapy (with the exception of radioiodine treatment for thyroid disease). In addition to benefitting patients with these rare tumors, the knowledge gained provides a blueprint for the development of future paired diagnostic/therapeutic probes for treating other diseases, such as prostate cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumors are a relatively uncommon but diverse family of neoplasms, which can originate in the lung, thymus, GI tract, or pancreas. Other tumors with neuroendocrine features include pheochromocytoma, paraganglioma, and neuroblastoma. The incidence of neuroendocrine tumors has increased from 1.09 per 100,000 persons in 1973 to 6.98 per 100,000 in 2012 [1]. This increase may be related in part to earlier detection and diagnosis. Patients often present with non-specific symptoms and treated for more common etiologies before the diagnosis of NET is made. Sites of NETs can be small and can be difficult to characterize with conventional anatomic imaging (CT and MRI). Targeted imaging techniques greatly improve the specificity for diagnosing, and the newest PET imaging is also very sensitive for detecting small neuroendocrine tumors. Well-differentiated NETs typically do not exhibit high glucose metabolism and are therefore not well visualized on FDG-PET scans. High-grade or poorly differentiated neuroendocrine tumors, on the other hand, can exhibit high activity on FDG-PET and demonstrate low activity on targeted imaging.
Molecular imaging and therapy approaches have been utilized in the management of neuroendocrine tumors for many years, with promising results. Until recently, clinical studies have been limited because neuroendocrine tumors are uncommon and reflect a heterogeneous set of diseases. In addition, treatment regimens have varied between institutions. Nonetheless, the radio-theranostic concept of using a molecular marker for both imaging and treatment has shown great promise in the treatment of neuroendocrine tumors and serves as a model for developing targeted probes for other tumor sites.
Imaging neuroendocrine tumors
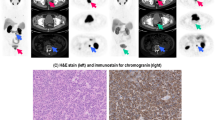
Examples of molecular techniques for imaging neuroendocrine tumors are shown in Fig. 1. Overall, somatostatin receptor imaging is the modality of choice and offers the highest accuracy across the different neuroendocrine tumor subtypes. PET/CT imaging with 68Ga-DOTATATE and similar somatostatin receptor analogs has been shown to outperform SPECT/CT imaging with 111In-octreotide and is being widely adapted into clinical practice. MIBG imaging with SPECT/CT provides high sensitivity for imaging pheochromocytoma, paraganglioma, and neuroblastoma.
Neuroendocrine tumor imaging. a 123I-MIBG images of a patient with MEN2 syndrome, demonstrating high MIBG uptake in a medullary thyroid lesion as well as a right adrenal pheochromocytoma. b 111In-Octreotide images (24 h after injection) demonstrating liver metastases from NET. c MIP image from a 68Ga-DOTATATE PET scan in a patient with negative CT scan, demonstrating multiple small somatostatin receptor avid metastatic sites.
123I-MIBG and 131I-MIBG
MIBG (metaiodobenzylguanidine) is a guanethidine analog of norepinephrine which accumulates in neuroendocrine tumors through the norepinephrine transporter mechanism, where it accumulates in neurosecretory granules [2]. MIBG is avidly accumulated in NETs rich in sympathetic adrenergic tissues, particularly pheochromocytoma, paraganglioma, and neuroblastoma. Other NETs such as carcinoids can also take up MIBG, but with more variability [2].
Prior to MIBG imaging, the patient should discontinue medications (e.g., tricyclic antidepressants, sympathomimetics, neuroleptics) that could potentially interfere with MIBG uptake [3]. Potassium iodide or other iodide preparation is administered orally prior to MIBG injection to block physiologic thyroid uptake from dissociated iodine. MIBG imaging can be performed using 123I-MIBG or 131I-MIBG. 123I-MIBG has a half-life of 13 h and photon emission energy of 159 keV, which is favorable for gamma camera imaging, and is the preferred radiotracer for imaging. 131I-MIBG is more readily available than 123I-MIBG, and the longer half-life of 131I (8 days) allows longer delayed imaging (48 h or more) to enhance sensitivity. However, the photon energy of 364 keV is not optimal for imaging with gamma cameras, and the beta emission (which is utilized for therapy) and long half-life limits the amount of activity that can be administered for diagnostic purposes. Imaging is typically performed 24 h following injection of 123I-MIBG or 48 h following 131I-MIBG administration. Optimally, both planar and SPECT/CT images are acquired. The combination of SPECT and CT allows areas of MIBG uptake to be localized and correlated with anatomic imaging and enables attenuation-correction of the MIBG SPECT images. An example of planar 123I-MIBG images on a patient with multiple endocrine neoplasia (MEN 2A) is illustrated in Fig. 1, showing MIBG-avid pheochromocytoma and medullary thyroid cancer. 123I-MIBG imaging is useful for initial staging and follow-up of pediatric patients with neuroblastoma. Because MIBG is not normally taken up in bone or marrow, it is particularly suited for discerning osseous involvement in this pediatric population. Diagnostic 123I-MIBG and 131I-MIBG scans are useful to determine the disease burden of MIBG-avid disease and to evaluate the potential benefit of treating with 131I-MIBG. Following injection of a small dose of 131I-MIBG (~ 74 MBq), imaging can also be obtained over several days for dosimetry calculations prior to administering the therapeutic dose.
Somatostatin receptor imaging: 111In-pentetreotide and 68Ga-DOTATATE
Until recently, the mainstay of somatostatin receptor evaluation has been imaging with 111In-pentetreotide. Patients are injected and planar images obtained 4 h and 24 h following injection, with further delayed imaging as needed. Additional SPECT/CT imaging is very helpful for localizing disease, but also adds substantial time to the test and only covers a pre-determined portion of the body.
Over the past decade, several 68Ga-labeled somatostatin receptor analogs have been investigated for PET imaging, mainly 68Ga-DOTANOC, 68Ga-DOTATOC, and 68Ga-DOTATATE. All of these PET tracers provide significantly higher sensitivity and specificity for identifying receptor-positive NETs compared to imaging with pentetreotide [4]. Five somatostatin receptor subtypes have been identified (sst1–sst5), which are expressed by neuroendocrine tumors. 68Ga-DOTATOC and 68Ga-DOTATATE have high affinity for sst2. 68Ga-DOTANOC has lower affinity for sst2, but has additional binding to sst3 and sst5 receptors, which may improve sensitivity [5]. 68Ga-DOTATATE has been approved by the FDA for use in the USA and is therefore the most commonly employed. 68Ga has a half-life of 68 min, making distribution of 68Ga PET tracers more challenging than 18F-labeled PET tracers, which have a 2-h half-life. However, 68Ga-DOTATATE is available commercially in some areas and can also be synthesized locally by purchasing a 68Ge/68Ga generator and using a synthesis kit. The expanding availability of 68Ga-DOTATATE combined with the superior sensitivity of 68Ga-DOTATATE PET/CT over 111In-pentetreotide SPECT/CT imaging has compelled the NCCN to now recommend 68Ga-DOTATATE PET/CT as the preferred imaging modality to determine somatostatin receptor status of NETs [6]. 68Ga-DOTATATE can identify lesions that are small or not well visualized on CT. Several studies have examined the impact of 68Ga-DOTATATE PET/CT imaging on clinical decision making. Hofman et al. studied 76 consecutive studies in 59 patients and found that 68Ga-DOTATATE PET/CT had a high impact in 48% and moderate impact of 10% of the patients [7].
In our practice, 68Ga-DOTATATE PET/CT has completely supplanted 111In-pentetreotide SPECT/CT for imaging patients with neuroendocrine tumors. In addition to superior imaging and high clinical impact, the study is completed within 2 h (rather than 24 h or more), and with less radiation dose to the patient. The uptake time of 1 h is similar to FDG-PET, enabling 68Ga-DOTATATE PET/CT studies to be readily integrated into our busy oncology PET/CT schedule. We have found that combining the DOTATATE PET with diagnostic contrast-enhanced CT to be an extremely valuable combination. Contrast-enhanced CT enables smaller lesions to be identified and localized. Perhaps more importantly, it enables identification of disease sites that are not DOTATATE-avid (e.g., negative for somatostatin receptors). This information can be critically important when considering somatostatin receptor-targeted radionuclide therapy. 68Ga-DOTATATE PET/MRI is a new modality that could potentially improve the ability to delineate neuroendocrine tumors that are somatostatin receptor-positive and those that are de-differentiated. Further research is required to determine if advanced MRI combined with 68Ga-DOTATATE PET can contribute to response assessment.
Radionuclide therapy for neuroendocrine tumors
Primary radionuclide treatments for neuroendocrine tumors include 131I-MIBG and peptide receptor radionuclide therapy (PRRT). Although several different PRRT agents have been used, the current discussion is limited to 177Lu-DOTATATE, as this is currently most widely used clinically. General features and differences between these two therapeutic radiopharmaceuticals are summarized in Table 1.
131I-MIBG therapy
Pheochromocytomas and paragangliomas are tumors that originate from chromaffin cells, either in the adrenal glands or the extra-adrenal sympathetic nervous system, respectively. Given the structural similarities between norepinephrine and MIBG, 131I-MIBG is transported into pheochromocytoma and paraganglioma tumor cells via a combination of receptor-mediated and non-receptor-mediated mechanisms [8, 9]. Pheochromocytomas and paragangliomas can sometimes occur as part of familial syndromes, such as MEN 2A and 2B, where they present with other neuroendocrine tumors, such as carcinoid and islet cell tumors. Unlike pheochromocytomas and paragangliomas, neuroendocrine tumors usually express high levels of somatostatin receptors and demonstrate high uptake of somatostatin analogs and variable uptake of MIBG [10].
Prior to treatment with 131I-MIBG, patients are given potassium iodide or other medication to protect the thyroid gland by blocking uptake of any free 131I. 131I-MIBG is typically administered intravenously over a period of 15 to 60 min (either by shielded infusion pump or gravity drip infusion), with frequent blood pressure monitoring during the infusion to monitor for acute changes in blood pressure. Prophylactic anti-emetic medication may be administered prior to 131I-MIBG injection. Additionally, intravenous fluids can be administered to promote renal excretion of 131I-MIBG and reduce radiation exposure to patients [11]. Later side effects of 131I-MIBG administration include myelosuppression, hypothyroidism, xerostomia, and nephrotoxicity [11]. Currently, the role of 131I-MIBG in the treatment of neuroendocrine tumors is as a salvage therapy that is used for inoperable tumors, palliative pain control from bone metastases, or for patients who are unable to receive peptide receptor therapy (PRRT) [9]. Contraindications for 131I-MIBG therapy include pregnancy, breastfeeding status, bone marrow failure, and renal failure. For 131I-MIBG treatments, patients are generally admitted to the hospital under radiation safety precautions, until they meet release criteria under U.S. Nuclear Regulatory Commission guidelines 10 CFR 35.75 (usually 1–2 days).
Overall, the response rates of 131I-MIBG for pheochromocytoma and paragangliomas by RECIST criteria are reported to range from 0 to 50%. (Due to the lack of interpretative value of stable disease [7], for the purpose of this review, “response rate” represents the sum of complete and partial response rates from the referenced studies.) The largest study was by Pryma et al., which evaluated the safety and efficacy of high specific activity 131I-MIBG in patients with advanced pheochromocytoma and paragangliomas. In this study, the response rate for 14 patients receiving one therapy of 18.5 GBq was 0%, but increased to 30% in 50 patients receiving two therapies of 18.5 GBq 131I-MIBG (with individual treatment doses of ~ 18.5 GBq) [12•]. Noto et al. showed a similar relationship between increasing cumulative dose of I-131 MIBG and response rates for pheochromocytoma/paraganglioma, by reporting a 0% response rate in patients receiving < 18.5 GBq 131I-MIBG and 29% in patients receiving > 18.5 GBq 131I-MIBG [13]. In other smaller studies (n < 50), the response rates ranged from 17 to 43%. In these studies, the reported cumulative dose for treatment of pheochromocytoma and paragangliomas with 131I-MIBG ranged from 18.5 to 33.1 GBq [12•, 13,14,15,16]. Progression-free survival was infrequently reported for patients receiving 131I-MIBG for pheochromocytoma, but has been reported to be as high as 85 months [14].
As described above, the neuroendocrine tumors demonstrate variable uptake of 131I-MIBG, compared to pheochromocytomas and paragangliomas. Overall, the response rates of 131I-MIBG for neuroendocrine tumors ranged from < 1 to 48% [17,18,19]. The largest study was by Kane et al., which reported a response rate of 20% in 125 patients with stage IV pulmonary and gastroenteropancreatic MIBG-positive neuroendocrine tumors, receiving 1–4 therapies of 131I-MIBG (first treatment dose of 18.5 GBq; a subset of patients received multiple treatments (1–4) with a median cumulative dose of 33.7 GBq) [10]. In this study, the radiographic progression-free survival was reported to be 20.4 months, with a median duration of symptomatic response of 12 months. Additional studies evaluating the use of 131I-MIBG for neuroendocrine tumors reported progression-free survival times ranging from 13 to 34 months [18, 19]. Two studies evaluated the use of 131I-MIBG for carcinoid tumors, which reported response rates of 0 and 11% at mean cumulative doses of 22.2 GBq and 22.7 GBq, respectively (mean individual treatment dose of 7.3 GBq and 11.1 GBq, with patients receiving a range of 1–8 treatments and 1–4 treatments, respectively) [16, 20].
131I-MIBG leads to both radiographic and symptomatic response in patients with limited treatment option for paragangliomas, pheochromocytomas, and other neuroendocrine tumors. Currently, published reports suggest multiple rounds of treatment, rather than a single treatment session, leads to improved response to pheochromocytomas and paragangliomas [12•, 13]. Given the rare nature of these tumors, large-scale clinical trials and comparative studies (surgery, chemotherapy) to determine appropriate dosing regimens, appropriate timing for initiating treatment, and treatment response are difficult to perform. In regard to treatment response, a major confounder is the stable disease category of RECIST. It is unclear if stability is a result of therapy or a consequence of natural tumor behavior [14, 21]. Additionally, there are likely multiple genetic factors, including succinate dehydrogenase complex subunit B (SDHB), which impact treatment response and are largely unaccounted for due to the rarity of the disease [12•].
Of note, 131I-MIBG has also been utilized for the treatment of progressive or chemoresistant neuroblastomas in the pediatric population. Neuroblastoma is the most common extracranial malignancy of childhood. Prognostic markers for neuroblastomas include DNA ploidy, MYCN gene amplifications, and chromosomal aberrations (specifically at 11q, 17q, and 1p) [22]. Similar to other neuroendocrine tumors, treatment options for neuroblastomas include surgical resection, chemotherapy (most commonly carboplatin, cyclophosphamide, doxorubicin, and etoposide), external beam radiation, immunotherapy (dinutuximab), and radiotherapies (131I-MIBG).
Ninety percent of neuroblastoma tumor cells show expression of norepinephrine receptors [23]. As mentioned above, MIBG is a ligand for the norepinephrine receptor, making 131I-MIBG a potential therapeutic option for neuroblastomas. Currently, 131I-MIBG is mostly used in addition to chemotherapies in patients with neuroblastomas. The response rates of 131I-MIBG (with or without additional chemotherapy) for the treatment neuroblastomas range from 29 to 69%. In general, administered doses per treatment ranged from 0.1 to 0.7 GBq/kg, with patients receiving between 1 and 4 treatments. A study by Johnson et al. explored the utility of multiple 131I-MIBG treatments of 0.7 GBq/kg and showed utility in additional treatment with 131I-MIBG (first infusion response rate 30%; 29% of patient’s receiving a second infusion showed an additional 29% response rate) [24]. A phase II trial by Matthay et al. evaluated the response of refractory neuroblastomas to 0.4 and 0.7 GBq/kg of 131I-MIBG (groups were separated based on the availability of hematopoietic stem cells). This study showed a response rate of 25% in patients receiving 0.4 GBq/kg and a response rate of 37% in patients receiving 0.7 GBq [25].
Given that 131I-MIBG is generally utilized in conjunction with other therapies, it is difficult to fully isolate the effects of MIBG therapy on long-term survival of patients with neuroblastomas. Additionally, the degree of myelosuppression experienced by the patient population significantly restricts the ability to fully characterize its full protention. Given these limitations, there is high variability in 131I-MIBG dosing regimens (multiple versus single dose, body weight dose versus whole-body dose), overall therapeutic plan (131I-MIBG therapy versus chemotherapy +131I-MIBG therapy), and outcome reporting (objective response versus symptomatic response, lack of long-term survival data). Within these limitations, 131I-MIBG offers a treatment modality for chemoresistant neuroblastomas and/or for palliative care [2]. A large phase 3 clinical trial (COG ANBL1531) is currently underway to study the effects of adding 131I-MIBG therapy to intensive therapy in children with newly diagnosed high-risk neuroblastoma. This trial will also evaluate the effect of adding crizotinib as a targeted agent in patients with ALK-aberrant tumors.
Peptide receptor radionuclide therapy with 177Lu-DOTATATE
Well-differentiated gastroenteropancreatic neuroendocrine tumors typically express high levels of somatostatin receptors and consequently demonstrate an avidity for somatostatin analogs [10]. Targeted therapies utilized to treat these tumors are divided into non-radiolabeled somatostatin analogs (SSAs) and radiolabeled SSA, or peptide receptor radionuclide therapy (PRRT). Similar to 131I-MIBG, PRRT involves the attachment of a therapeutic radionuclide (such as 177Lu or 90Y, which are β− emitters), combined with a somatostatin analog (DOTATATE, DOTATOC, DOTANOC) to bind the receptors [26]. Given the comparable efficacy, better hematologic toxicity profile, and commercial availability of 177Lu-DOTATATE, this review will focus on the use of 177Lu-DOTATATE for the treatment of neuroendocrine tumors.
Similar to 131I-MIBG, PRRT currently serves as a salvage therapy for neuroendocrine tumors, with the primary treatment being surgical resection. Indications for PRRT include inoperable or metastatic neuroendocrine tumor and positive expression of somatostatin receptor (sst2) by 111In-pentetreotide or 68Ga-labeled somatostatin receptor PET imaging. Contraindications for the use of PRRT include pregnancy, severe acute concomitant illness, GFR < 60% of mean adjusted normal value [27]. Side effects of PRRT administration include myelosuppression, nephrotoxicity, and gastrointestinal symptoms (nausea, diarrhea). The most serious late complication of 177Lu-DOTATATE therapy is secondary hematopoietic malignancy. In a study of 274 patients treated with 177Lu-DOTATATE, 8 patients (3%) developed hematopoietic malignancy (myelodysplastic syndrome, acute myeloid leukemia, or myeloproliferative neoplasm), and 3 (1%) developed bone marrow failure as a delayed complication [28]. To prevent nephrotoxicity associated with 177Lu-DOTATATE, an IV infusion of positively charged amino acids (lysine and arginine) is administered concomitantly for 4 h, starting 30–60 min before the start of 177Lu-DOTATATE infusion. 177Lu-DOTATATE is administered by IV drip or pump over a period of 30–60 min, with the amino acid infusion continuing for a total of 4 h. The 177Lu-DOTATATE and amino acids are administered concurrently using two separate IV sites. At our facility, radiation safety personnel are present during the administration. They interview and provide patients with written post-treatment instructions. Following therapy, the treatment room and bathroom are surveyed for any radioactive contamination.
Few published studies have evaluated the utility of 177Lu-DOTATATE for pheochromocytomas and paragangliomas. A study published by Nastos et al. compared the efficacy of 131I-MIBG (mean cumulative dose 19 GBq) and PRRT (individual and cumulative treatment doses of 3.2 GBq and 10.1 GBq for 90Y-DOTATATE, and 7.4 GBq and approximately 21 GBq for 177Lu-DOTATATE, respectively) for the treatment of metastatic or inoperable pheochromocytomas and paragangliomas (n = 22). This study showed improved response rates and PFS in patients treated with PRRT (90Y-DOTATATE, 177Lu-DOTATATE, or a combination of PRRT and 131I-MIBG) versus 131I-MIBG (MIBG 62.5%, 20.6 months; PRRT 100%, 38.5 months). Interestingly, there was also a difference in overall survival when only the treated paragangliomas were compared separately (131I-MIBG 22.8 months versus PRRT 60.8 months) [29•]. Given that patients with advanced pheochromocytomas and paragangliomas respond better to multiple 131I-MIBG treatments, with cumulative doses > 18.5 GBq, this comparison is somewhat limited (as described in the study by Pryma et al.).
Apart from a few small-scale studies, 177Lu-DOTATATE is primarily utilized for the treatment of gastroenteric neuroendocrine tumors. 177Lu-DOTATATE on average has a higher incidence of response compared to 131I-MIBG for treatment of neuroendocrine tumors, with response rates ranging from 0 to 70%. The largest of these studies is reported by Brabander et al., which evaluated the use of 177Lu-DOTATATE in patients with gastroenteropancreatic and bronchial neuroendocrine tumors (midgut, hindgut, pancreatic, bronchial) with overall response rates ranging from 30 to 55% in patients treated with 27.8–29.6 GBq of 177Lu-DOTATATE. Between the different types of neuroendocrine tumors, bronchial neuroendocrine tumors demonstrated the lowest progression-free survival and response rates (midgut 30 months, 31%; hindgut 29 months, 33%; pancreatic 30 months, 55%; bronchial 20 months, 30%; foregut 25 months, 41%) [30]. Additional study published by Strosberg et al., specifically evaluated the response of well-differentiated, metastatic midgut neuroendocrine tumors to 177Lu-DOTATATE (individual dose of 7.4 GBq, distributed over 4 treatments with a cumulative dose of 29.6 GBq) compared to octreotide (60 mg every 4 weeks), and reported a response rate of 18% with a PFS at month 20 of 65.2% for the 177Lu-DOTATATE group, and a response rate of 3% with a PFS at month 20 of 10.8% (NETTER-1 trial) [31••]. Interestingly, a study by Zhang J. explored the impact of Ki-67 on response rates of grade 3 NET to 177Lu-DOTATATE and reported an average rate of 49.1% with a PFS of 28 months. Patients with a Ki-67 index < 55% demonstrated a response rate of 52% with a PFS period of 11 months, while patients with a Ki-67 index > 55% demonstrated a response rate of 20% with a PFS period of 4 months [32].
Like pheochromocytomas and paragangliomas, other neuroendocrine tumors are also relatively rare, which limits the ability to perform large-scale clinical trials and comparative studies. Overall, PRRT appears to demonstrate both radiographic and symptomatic response in patients with other neuroendocrine tumors, especially in patients with a Ki-67 index < 55%. Additionally, the NETTER-1 trial demonstrated that PRRT was superior to high-dose octreotide in the treatment of other neuroendocrine tumors. In regard to the treatment of paragangliomas and pheochromocytomas, PRRT appears to be an effective treatment for paragangliomas and demonstrates a better efficacy than 131I-MIBG [29•, 33]. Moving forward, more prospective trials are warranted to further assess the role of PRRT in the treatment of paragangliomas.
While clinical results using 177Lu-DOTATATE are extremely promising, more research is needed in terms of tailoring the treatment to meet the needs of each patient. For example, rather than a fixed dose of 7.4 MBq, it might be better that the administered activity be adjusted based on the patient’s tumor burden as defined by the 68Ga-DOTATATE PET/CT scan. Currently, the standard treatment regimen is four 7.4 MBq doses of 177Lu-DOTATATE. Post-treatment and early response evaluation following 177Lu-DOTATATE therapy have not yet been clearly defined. In the future, imaging and serum biomarkers may help guide the number of therapies that a patient needs on an individualized basis. Identifying cases where the administered dose and/or number of 177Lu-DOTATATE treatments can be reduced without affecting efficacy could also reduce the potential for late hematological toxicities.
While the current standard practice is four 177Lu-DOTATATE treatments, strategies for interim imaging during therapy and optimum time points for follow-up evaluation with 68Ga-DOTATATE PET have yet to be determined. The photon emissions (113 and 208 keV) from 177Lu enable planar and SPECT/CT imaging to be performed following the treatment dose administration, and the potential value of obtaining these images is being investigated. More work is needed to determine if interim imaging can be useful for tailoring the administered dose and/or number of treatments for each patient.
Conclusion
Paragangliomas, pheochromocytomas, and other neuroendocrine tumors represent a group of rare tumors that have the potential to cause significant morbidity and mortality secondary to uncontrollable mass effect and/or excreted catecholamines. In addition to the rare nature of these tumors, they demonstrate significant phenotypic and genetic heterogeneity (receptor status, radionuclide uptake, individual mutations), especially at more advanced stages. Combining contrast-enhanced CT with the somatostatin/MIBG receptor PET aids substantially in the identification and characterization of lesions. The identification of receptor status is crucial for patients being considered for receptor-based therapy.
Radio-theranostic approaches combining imaging and therapy by utilizing a common molecular probe (e.g., MIBG, DOTATATE) labeled with a diagnostic radionuclide for imaging and a therapeutic radionuclide for therapy offer a novel method for treating these tumors. For neuroendocrine tumors, radio-theranostic probes include MIBG (123I for imaging and 131I for therapy) and DOTATATE (68Ga for imaging and 177Lu for therapy). MIBG has been shown to be effective for progressive/inoperable pheochromocytomas, paragangliomas, and neuroblastomas, while PRRT has been shown to be effective for inoperable/progressive GI neuroendocrine tumors.
Although small-scale studies evaluating inoperable/progressive neuroendocrine tumors generally show treatment response to radionuclide therapy, the inherent genetic heterogeneity within an already rare set of tumors has precluded large-scale clinical trials to accurately characterize treatment response. Evaluation of treatment response is further limited by poor understanding of natural tumor behavior. Specifically, there is significant uncertainty regarding the “stable disease” criteria in RECIST, and whether this is attributable to natural tumor behavior versus treatment response. In addition to tumor heterogeneity, radionuclide therapies are generally combined with other treatment modalities (surgery versus chemotherapy), which limits the ability to accurately characterize dosing regimens.
Overall, available evidence shows that 131I-MIBG can be an effective therapy for non-operable/palliative treatment of pheochromocytomas and paragangliomas, and PRRT can be effective for non-operable/palliative treatment of GI neuroendocrine tumors (including paragangliomas), as demonstrated by rates of symptomatic and radiographic response. Further studies are needed to characterize the relationship between genetic makeup, radiotracer uptake, and treatment response to allow for optimal patient distribution among the different treatment modalities with optimized dosing regimens.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncology. 2017;3:1335–42. https://doi.org/10.1001/jamaoncol.2017.0589.
Pandit-Taskar N, Modak S. Norepinephrine transporter as a target for imaging and therapy. J Nucl Med. 2017;58:39S–53S. https://doi.org/10.2967/jnumed.116.186833.
Taïeb D, Timmers HJ, Hindié E, Guillet BA, Neumann HP, Walz MK, et al. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1977–95. https://doi.org/10.1007/s00259-012-2215-8.
Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016;57:708–14. https://doi.org/10.2967/jnumed.115.163865.
Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54:364–72. https://doi.org/10.2967/jnumed.112.111724.
Waldmann CM, Stuparu AD, Dam RMV, Slavik R. The search for an alternative to [68Ga]Ga-DOTA-TATE in neuroendocrine tumor theranostics: current state of 18F-labeled somatostatin analog development. Theranostics. 2019;9:1336–47. https://doi.org/10.7150/thno.31806.
Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–7. https://doi.org/10.1111/j.1754-9485.2011.02327.x.
Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001;134:315–29. https://doi.org/10.7326/0003-4819-134-4-200102200-00016.
Vöö S, Bucerius J, Mottaghy FM. I-131-MIBG therapies. Methods. 2011;55:238–45. https://doi.org/10.1016/j.ymeth.2011.10.006.
Kwekkeboom DJ, Herder WWD, Kam BL, Eijck CHV, Essen MV, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. https://doi.org/10.1200/jco.2007.15.2553.
Grünwald F, Ezziddin S. 131I-Metaiodobenzylguanidine therapy of neuroblastoma and other neuroendocrine tumors. Semin Nucl Med. 2010;40:153–63. https://doi.org/10.1053/j.semnuclmed.2009.11.004.
• Pryma DA, Chin BB, Noto RB, et al. Efficacy and safety of high-specific-activity I-131 MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2018. https://doi.org/10.2967/jnumed.118.217463 Study outlining the improved response rates of pheochromocytoma and paragangliomas to higher doses of 131I-MIBG.
Noto RB, Pryma DA, Jensen J, Lin T, Stambler N, Strack T, et al. Phase 1 study of high-specific-activity I-131 MIBG for metastatic and/or recurrent pheochromocytoma or paraganglioma. J Clin Endocrinol Metab. 2017;103:213–20. https://doi.org/10.1210/jc.2017-02030.
Kotecka-Blicharz A, Hasse-Lazar K, Handkiewicz-Junak D, Gawlik T. 131-I MIBG therapy of malignant pheochromocytoma and paraganglioma tumours - a single-centre study. Endokrynologia Pol. 2018;69:246–51. https://doi.org/10.5603/EP.a2018.0024.
Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27:4162–8. https://doi.org/10.1200/JCO.2008.21.3496.
Mukherjee JJ, Kaltsas GA, Islam N, Plowman PN, Foley R, Hikmat J, et al. Treatment of metastatic carcinoid tumours, phaeochromocytoma, paraganglioma and medullary carcinoma of the thyroid with 131I-meta-iodobenzylguanidine (131I-mIBG). Clin Endocrinol. 2001;55:47–60.
Kane A, Thorpe MP, Morse MA, Howard BA, Oldan JD, Zhu J, et al. Predictors of survival in 211 patients with stage IV pulmonary and Gastroenteropancreatic MIBG-positive neuroendocrine tumors treated with 131I-MIBG. J Nucl Med. 2018;59:1708–13. https://doi.org/10.2967/jnumed.117.202150.
Navalkissoor S, Alhashimi DM, Quigley A-M, Caplin ME, Buscombe JR. Efficacy of using a standard activity of 131I-MIBG therapy in patients with disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2010;37:904–91. https://doi.org/10.1007/s00259-009-1326-3.
Bomanji J, Wong W, Gaze MN, Cassoni A, Waddington W, Solano J, et al. Treatment of neuroendocrine tumours in adults with 131I-MIBG therapy. Clin Oncol. 2003;15:193–8.
Ezziddin S, Sabet A, Logvinski T, Alkawaldeh K, Yong-Hing CJ, Ahmadzadehfar H, et al. Long-term outcome and toxicity after dose-intensified treatment with 131I-MIBG for advanced metastatic carcinoid tumors. J Nucl Med. 2013;54:2032–8. https://doi.org/10.2967/jnumed.112.119313.
Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C, et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98:4006–12. https://doi.org/10.1210/jc.2013-1907.
Pastor ER, Mousa SA. Current management of neuroblastoma and future direction. Crit Rev Oncol Hematol. 2019;138:38–43. https://doi.org/10.1016/j.critrevonc.2019.03.013.
Matthay KK, George RE, Yu A. Promising therapeutic targets in neuroblastoma. Clin Cancer Res. 2012;18:2740–53. https://doi.org/10.1158/1078-0432.CCR-11-1939.
Johnson K, McGlynn B, Saggio J, Baniewicz D, Zhuang H, Maris JM, et al. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. 2011;57:1124–9. https://doi.org/10.1002/pbc.23062.
Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to Iodine-131-Metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–60. https://doi.org/10.1200/JCO.2006.09.3484.
Maqsood MH, Din ATU, Khan AH. Neuroendocrine tumor therapy with lutetium-177: a literature review. Cureus. 2019. https://doi.org/10.7759/cureus.3986.
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, et al. Erratum to: the joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;41:584. https://doi.org/10.1007/s00259-013-2454-3.
Bergsma H, Lom KV, Raaijmakers MH, Konijnenberg M, Kam BBL, Teunissen JJ, et al. Persistent hematologic dysfunction after peptide receptor radionuclide therapy with177Lu-DOTATATE: incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2017;59:452–8. https://doi.org/10.2967/jnumed.117.189712.
• Nastos K, Cheung VT, Toumpanakis C, Navalkissoor S, Quigley A-M, Caplin M, et al. Peptide receptor radionuclide treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol. 2017;115:425–34. https://doi.org/10.1002/jso.24553 Study comparing the effectiveness of 131I-MIBG and PRRT for pheochromocytomas and paragangliomas.
Brabander T, Zwan WAVD, Teunissen JJ, Kam BL, Feelders RA, Herder WWD, et al. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23:4617–24. https://doi.org/10.1158/1078-0432.CCR-16-2743.
•• Strosberg J, El-Haddad G, Wolin E, Hendifar A, Et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. The New England Journal of Medicine 2017:376:125–135. DOI: https://doi.org/10.1056/NEJMoa1607427. Large clinical trial demonstrating the effectiveness of 177Lu-DOTATATE for midgut neuroendocrine tumors.
Zhang J, Kulkarni HR, Singh A, Niepsch K, Müller D, Baum RP. Peptide receptor radionuclide therapy in grade 3 neuroendocrine neoplasms: safety and survival analysis in 69 patients. J Nucl Med. 2018;60:377–85. https://doi.org/10.2967/jnumed.118.215848.
Pinato DJ, Black JRM, Ramaswami R, Tan TM, Adjogatse D, Sharma R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol. 2016;33:47. https://doi.org/10.1007/s12032-016-0737-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lower Gastrointestinal Cancers
Rights and permissions
About this article
Cite this article
Desai, H., Borges-Neto, S. & Wong, T.Z. Molecular Imaging and Therapy for Neuroendocrine Tumors. Curr. Treat. Options in Oncol. 20, 78 (2019). https://doi.org/10.1007/s11864-019-0678-6
Published:
DOI: https://doi.org/10.1007/s11864-019-0678-6