Opinion statement
Dermatofibrosarcoma protuberans (DFSP) is a slow growing tumor with a very low metastatic potential but with significant subclinical extension and great capacity for local destruction. Thus, the first surgeon approached with such challenging tumor must attempt to cure the patient with a method that spares healthy tissue and ensures an optimal oncological, functional, and esthetic result. The treatment of DFSP often requires a multidisciplinary approach. Depending on location, dermatologic surgeons, surgical oncologists, head and neck surgeons, neurosurgeons, plastic surgeons, and occasionally medical oncologists may be involved with the management. Mohs micrographic surgery (MMS) is the preferred method when available. In our institution, most of the DFSP cases are often advanced cases; thus, dermatologic surgeons obtain clear margins peripherally and other surgical specialties assist with resection of the fascia and any critical deeper structures. When MMS is not available, wide local excision (at least 2- to 3-cm margins of resection) with exhaustive pathologic assessment of margin status is recommended, and it is best to confirm tumor extirpation prior to any reconstruction. Subclinical extension of the tumor could be related to the size; how long it has been growing or histological markers that are unknown right now. No clinical trials comparing MMS vs WLE are available, and further research should be focused on these subjects as well as the use of imatinib and other targeted therapies for recurrent and metastatic tumors and for neoadjuvant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare tumor with a high potential for local invasion and recurrence. It is almost invariably associated with a chromosomal translocation that ultimately drives oncogenesis by mitogen activation. Molecular methods for a precise diagnosis in equivocal cases are discussed as well as ongoing research on physiopathology. Recent articles describe the use of dermoscopy and confocal microscopy. Once patients have a confirmed histological diagnosis, contrast MRI is the imaging modality of choice to better characterize its extension; imaging should be considered in recurrent and large tumors or cases with a fibrosarcomatous change. There is no standard staging system for DFSP but multiple ones being used will be reviewed. Surgery is the treatment of choice. Due to its potential to recur, its eccentric tentacle-like projections, meticulous histopathological assessment of the margins is critical. MMS is the preferred option but conventional surgery with en face pathological margin assessment, when MMS is not available, may be an alternative. No randomized clinical trials have been performed to compare surgical treatment options; there are only few comparative nonrandomized case series and selected reviews in the literature. Radiation is used mainly in cases of unresectable DFSP or cases where negative margins cannot be obtained. Imatinib has also been used in unresectable cases.
History, epidemiology, genetics, and physiopathology
DFSP is a rare neoplasm of intermediate malignancy. Taylor first described it in 1890 but Darier was credited with establishing DFSP as a distinct clinicopathological entity in 1924, and finally, Hoffman established the term in 1925 [1,2,3,4].
Overall annual incidence has been estimated to be 4.2 per million, and the tumor accounts for approximately 0.1% of all malignancies. The incidence is almost double among blacks compared to whites and women have a higher incidence rate than men [5••]; the highest age-specific annual incidence rates are observed between the ages of 30 and 50 years. Most occur on the trunk (42%), followed by the upper extremities (23%), lower extremities (18%), and head and neck (16%) [6•]. Infrequently, it may affect the genitalia [7, 8]. DFSP in children has been reported to represent around 8% of cases with a male to female ratio of 0.86, 15% of them being congenital. The distribution is similar to that in adults [9].
Approximately 10% of DFSP report prior trauma, surgical or burn scars, and even immunizations at the site of disease but a causative relationship is unclear [4].
DFSP is characterized by translocation of t(17;22)(q22;q13) either in the form of supernumerary ring chromosomes or unbalanced linear translocation der [10]. Both ring chromosomes or linear der [10] contain a fusion of collagen type I alpha 1 (COL1A1) and platelet-derived growth factor subunit B (PDGFB) [11]. It is believed that the translocation is an early event and transfection with the chimeric sequence can transform normal cells into neoplastic ones [12]. The gene fusion places PDGFB under the control of the COL1A1 promoter [11]. PDGFB is a potent mitogen for mesenchymal cells; when constantly expressed, it activates PDGFB receptor leading to its autocrine activation and tumor development [13•].
Other oncogenic mechanisms have been described, but little is currently understood about COL1A1-PDGFB negative DFSP which represents around 8% of cases [13•]. A novel COL1A2-PDGFB fusion gene has also been described [14]. Recently, hormone receptor expression in DFSP was determined, looking for a potential role for antihormone therapies in the treatment of patients with DFSP. Loss of receptor expression was observed in all recurrent tumors warranting further study [15]. Cyclin-dependent kinase inhibitor 2A (CDKN2A)/p16 loss has been implicated in imatinib-resistant DFSP and inhibition of cyclin dependent kinase 4 (CDK4) could be a potential therapeutic target for this type of tumor [16].
Understanding the molecular events of DFSP tumorigenesis led PDGFB receptor to become the therapeutic target. [17] Imatinib mesylate is a tyrosine kinase inhibitor which inhibits PDGFRA/B and is currently used for unresectable, recurrent, or metastatic DFSP [13•, 18].
Clinical assessment and histology
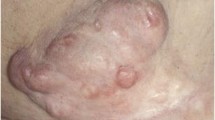
DFSP presents as an asymptomatic slowly growing violaceous nodule or plaque. On dermoscopy, multiple structures have been described including delicate pigmented network (87%), vessels (80%), structureless light brown areas (73%), white streaks (67%), pink background (67%), and structureless hypo- or depigmented areas (60%) [19, 20].
On confocal microscopy, loss of normal “edge papillae” with elongated bright cells corresponding to tumor cells has been described [21]. However, the role of dermoscopy and confocal microscopy for diagnosis of DFSP has not been established.
Clinical differential diagnosis includes neurofibroma, leiomyoma, malignant melanoma, morpheaform basal cell carcinoma, keloid, desmoid tumors, Kaposi sarcoma, fibrosarcoma, dermatofibroma, nodular fasciitis, and sarcoidosis [22•].
Incisional or excisional biopsy should be performed upon a suspicious lesion [4]. DFSP is a cellular neoplasm composed of storiform spindle cells with elongated nuclei, minimal cytological atypia, and a low mitotic count within a collagenous stroma. Tumor cells often spread along the septae of the subcutaneous fatty tissue (known as fat-trapping) [10]. Histologically, the differential diagnosis are other fibrous tumors like dermatofibroma, fibrosarcoma, pleomorphic sarcoma of the skin, leiomyosarcoma, malignant peripheral nerve sheath tumors, rare variants of spindle cell malignant melanoma, atypical fibroxanthoma, and nodular fasciitis [22•, 23••]. On immunohistochemistry, DFSP shows strong and diffuse expression of CD34; it is also positive for vimentin, nestin, and apolipoprotein D and is negative for cytokeratins, smooth muscle actin (SMA), S100, CD56 Factor XIIIa, Stromelysin 3, and Cathepsin K [4]. Histological subtypes include myxoid, pigmented or Bednar tumor, atrophic, sclerosing, granular cell, giant cell fibroblastoma, and tumors that have undergone fibrosarcomatous transformation (DFSP-FS) [4, 10].
Histologic characteristics associated with fascia invasion are the presence of a sheet-like pattern, a high degree of cellular pleomorphism, and more than one mitotic figure [24]. DFSP-FS is the most aggressive and is associated with the highest risk of local recurrence, distant metastasis, and even death [25••].
In difficult cases, to confirm DFSP fluorescence, in situ hybridization or multiplex reverse transcriptase polymerase chain reaction to detect translocation and gene fusion may be useful [23••, 26, 27, 28•].
Work up, imaging, staging, and prognosis
Although small lesions may be treated without obtaining imaging studies, imaging may provide a better understanding of the extension of disease and more precise surgical planning in larger lesions [4]. An extensive workup is not routinely recommended since metastases are extremely rare [29]. It is recommended for patients with a clinical examination that is suspicious for metastases, recurrent disease, or DFSP-FS. Chest Xray and abdominal and lymph node ultrasound are recommended [23••]. Since DFSP-FS has a 14.5% risk of metastasis, usually to the lungs, routine CT or MRI may be warranted [25••, 30•].
On CT/MRI scans, DFSP presents as a noncalcified, superficial, nodular mass arising from the skin, which is isodense to muscles on CT and on MRI shows a T2 high and T1 low signal [31, 32]. It shows intermediate to marked enhancement on contrast CT/MRI [32]. A “claw” sign has been described at the lesion/skin interface that is evidenced in various imaging modalities [33]. MRI is the study of choice for the preoperative setting as well as postoperative surveillance [4]. 18F-FDG PET/CT has been used to predict aggressive behavior and response to imatinib in metastatic DFSP [34].
Although there is no standard staging system for DFSP, the European consensus interdisciplinary guidelines, a unique collaboration of multidisciplinary experts from the European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization of Research and Treatment of Cancer (EORTC) consider the primary tumor stage I, lymph node metastasis stage II, and distant metastasis stage III [23••]. Some authors recommend staging according to the American Joint Commission on Cancer for sarcomas which classifies the tumor as T1 if the largest dimension is of 5 cm or less and T2 if the largest dimension is more than 5 cm; N0 if there is no nodal metastases and N1 with nodal metastases; histologic grade as G1 if it is low grade or well differentiated, G2 intermediate grade (moderately well differentiated), and G3 high grade (undifferentiated); and M0 if there is no distant metastasis, M1 if there is presence of distant metastasis. Most DFSP are stage I (Any T, N0M0G1). Stage II is defined as T1N0M0, G2 or G3, or T2N0M0G2; stage III T2N0M0G3 or any T, N1M0; and stage IV any T, any N, M1 [35, 36]. Other reports limit DFSP staging data to LRD staging system: localized, regional, or distant [5••].
The relative 5, 10, and 15-year survivals have been reported to be 99.2, 99.1, and 97.2% [5••, 6•]. Higher all-cause mortality was associated with increased age, male sex, tumor size, black race, and anatomic location of the limbs and head compared to the trunk [5••, 37••].
Fibrosarcomatous (FS) change has been reported in 3–20% of cases. When analyzing outcome by the presence or absence of FS change, the risk of local recurrence, metastasis, and death is higher in this group. The risk of local recurrence was 29.8% for the DFSP-FS group vs. 13.7% for DFSP; the risk for metastasis was 14.4 vs. 1.1% and death from disease was 14.7 vs. 0.8% [25••]. The outcomes were not statistically different depending on the proportion of FS change within the tumors [25••]. One year and 5-year recurrence-free survival for DFSP was 94 and 86% while for DFSP-FS it was 86 and 42%, respectively [38••].
Risk factors for recurrence include FS change, less than 1 mm to positive margins, increased cellularity, increased mitotic rate, and age older than 50 years [39]. Another risk factor for distant metastasis is tumor size; metastatic cases are significantly larger (most metastatic cases being larger than 10 cm) compared to nonmetastatic cases; this could be related to FS changes within the tumor. Local recurrence was not found to be related to metastases [40]..
Metastasis is detected on a mean time of 14.8 months and within a range from the initial visit to less than 5 years after the treatment [40]. Brain, pleura [41], pancreas [42], cervical lymph nodes [43], and orbit metastasis have been reported [44]..
Treatment
Complete surgical resection with microscopically negative margins is the cornerstone of treatment for localized DFSP [10, 45•]. Adjuvant radiotherapy has a role for unresectable DFSP and in those cases with positive margins when re-excision is not feasible. Radiation can also be considered as adjuvant treatment in recurrent cases. Imatinib is the first effective systemic therapy for advanced DFSP and could potentially be used for reducing tumor size in those considered initially unresectable so surgery may be feasible [45•].
Surgery
Conventional surgery
Initially, recurrence with conventional surgery was reported to be up to 50–60% due to conservative margins [46, 47]. In the earlier studies, there were no unified standards for margin of resection and patients rarely underwent adequate margin-controlled tumor extirpation [4]. When undefined or conservative margins were used, local recurrence rates ranged from 26 to 60% with a total recurrence rate of 39.7% [30•]. However, recurrence rates dropped with wider margins of 2–5 cm, to around 6% [48, 49].
The reason for recurrence is that microscopic projections are not removed adequately or assessed satisfactorily; the wider the margin, the higher the probability the tumor will be removed completely [10] and margin status has been shown to be an important predictor of recurrence [50]. Using 1-cm margins around the primary tumor leaves residual microscopic tumor in more than 70% of patients, 2-cm margins in 20–40% of patients, 3-cm in 9–15.5% of patients, and 5-cm in 5% of patients [51,52,53]. Peripheral margins of 5 cm have a close to 0% recurrence rate [54]. However, tumors are not circumferential and are asymmetric; DFSP mapping with Mohs micrographic surgery (MMS) has shown long tentacle-like projections that extend beyond 3 cm. Wider margins unnecessarily remove healthy tissue, increase the risk for complications, and may lead to suboptimal functional and cosmetic results [10, 55]. Wide resections may also not be practical in patients with tumors located in critical areas like the head and neck [30•], and intraoperative frozen section assessments have not been reliable for determining margin status [55]. When smaller peripheral margins are used, meticulous margin assessment is critical to avoid recurrences. Deep margins should always include excision of the deep fascia [23••]. In our personal experience when using 2-cm lateral margins, the deep margin is most commonly involved by tumor, and not infrequently, the complete resection of the tumor requires excision of the external outer table in the cranium; muscle in trunk and extremities; peritoneum in thin patients with DFSP located on the abdomen; sternum, clavicles; and vertebral apophysis when located on the thorax.
Due to subclinical extension and its tentacle-like projections, routine step-section pathological assessment of surgical margins is a potential pitfall [30•, 56•]. Bread loafing allows the assessment of 10% of the margin which may lead to false-negative results [4]; en face evaluation of surgical specimens allows for a more thorough assessment of surgical margins [57•]. Compared to the 6% recurrence rates described with wide local excision, en face pathologic evaluation with the use of 2-cm margins reported a local recurrence less than 1% [57•]. This approach cannot be cataloged as wide local excision (WLE) since it allows for the analysis of all the margins just as MMS. MMS assesses 100% of the margins, resulting in less healthy tissue removal and adequate margin assessment making it a better choice [52••] (see Fig. 1).
Mohs micrographic surgery
MMS has become the alternative to wide local excision. It analyzes 100% of the margins. Consequently, it detects the microscopic extensions and enables the surgeon to remove them more accurately. It uses, instead of representative vertical sectioning (used in conventional surgery), sequential horizontal sectioning with immediate microscopic examination of resected frozen tissue [51, 58]. Modified Mohs uses paraffin embedded sections [59] and has been used for DFSP with good outcomes and few recurrences [60•].
Table 1 lists selected review papers evaluating recurrence rates for WLE and MMS. The relative risk of recurrence for WLE vs. MMS patients was 15.9 (95% CI 7.2–35.5) [62•].
The disadvantages of MMS are that tumor cells can be confused with normal spindle cells of the dermis in frozen sections and CD34 staining of frozen sections, which can help, has high variability, and thus, some authors do not consider the technique entirely reliable [67, 68]. To circumvent this issue, some authors recommend excising an additional layer and sending it for permanent paraffin-embedded evaluation after achieving negative frozen margins [52••]. MMS is also time consuming, complex and a highly specialized method that requires considerable training and can be costly. Most reviews, however, suggest a lower recurrence with MMS than with WLE [30•, 51, 52••, 62•, 63•, 66•, 69••, 70, 71]. Randomized controlled trials are lacking; nonetheless and as previously stated, WLE is an acceptable treatment [10] if MMS is unavailable. Combined recurrence rate for WLE during the past 20 years is 7.3% compared with 1.1% for MMS [52••] (see Table 1).
MMS should be the preferred approach in anatomically challenging areas [51, 66•, 72, 73] and in the treatment of children since children have a smaller body surface area [9].
When using techniques that allow pathological tridimensional control of all margins starting with a lateral safety margin of 1–1.3 cm may be sufficient [23••, 74]. NCCN guidelines recommend 2-cm margins [75••]. If standard histopathological procedures are available, a lateral safety margin of 3 cm is advisable [23••].
Some authors recommend determining the peripheral margin based on the tumor’s size and recurrence status (1 extra cm for tumors larger than 5 cm in diameter or recurrent tumors) [76]; others demonstrate tumors <2 cm will be cleared with 1.5-cm margins while >2-cm tumors will require 2.5-cm margins [53].
Referral to a multidisciplinary team that has broad knowledge and experience with DFSP, including the biology of the tumor and especially technical expertise on management, is mandatory [4]. The choice of surgical approach should be individualized, and the goals are to completely excise the tumor with negative margins, preserve function, optimize cosmesis, and minimize morbidity as well as minimize costs for the healthcare systems [65]. If MMS is unavailable, WLE with surgical margins of 3 cm is usually sufficient [23••]. Pathological tridimensional study of all margins is preferred and reconstruction should be delayed until margins are confirmed clear [23••].
Surgery is the first option for recurrent tumors [39, 75••].
Reconstruction
In cases of complex closures, a reconstructive surgeon must be consulted in order to preserve pedicles that may be required for flaps [49]. Undermining should be avoided since it can result in seeding of the tumor [49], or in cases of modified MMS when a second layer is necessary, scar tissue can make the interpretation difficult. Negative pressure dressings can be used before reconstruction while waiting for margin status [77].
MMS allows greater preservation of tissue [69••]. The final defect size has been reported to be smaller when using MMS vs. WLE [49] reflected in that primary closure is reported to be used more frequently when MMS is performed whereas with WLE flaps, grafts, or other closures are more commonly used [64••]. A demonstrable reconstructive benefit can be seen in 80% of patients treated with MMS when compared to WLE [78]. WLE compared to MMS had incomplete margins in 24% of cases, less optimal reconstruction (more invasive/poorer esthetics) in 47%, and more destructive/disruption or loss of esthetic subunits in 9% [78].
Radiotherapy
Radiotherapy is indicated in primary inoperable tumors, patients with positive margins when further surgery is not possible, or as adjuvant therapy after re-resection in recurrent DFSP [23••, 75••].
Recurrence rate for patients with positive margins was found to be 14 and 0% in patients with negative margins [79••]; thus, radiation is indicated in positive-margin patients where further surgery cannot be performed [75••, 80]. Most studies recommend a dose of 55–65 Gy [79••]. The target volume should include the tumor and 3–5-cm margins [23••] (see Table 2).
Radiotherapy is not indicated in tumors completely resected with negative margins. The side effects of radiation include fibrosis, skin graft failures, necrosis, edema, and joint stiffness, and neoadjuvant radiotherapy increases the risk of wound complications [80, 83].
Systemic therapy
Chemotherapy
There is minimal role for conventional chemotherapy and response rates have been poor [4, 30•, 56•].
Imatinib
Imatinib is an FDA-approved treatment for DFSP [84]. Imatinib has been shown to inhibit DFSP cell growth [12]. It has been shown to have clinical activity against DFSP with t(17;22) but lacks activity against t(17;22)-negative DFSP [81]; thus, the detection of the COL1A1-PDGFB fusion is highly recommended using FISH or RT-PCR prior to the start of imatinib therapy [17]. The overall responses (partial response and stable disease) have been reported to be 46, 73, and 90% [81, 82•, 85]. The dosage is 400 mg BID and 400 mg once a day; results suggest 400 mg once a day may be sufficient [82•] (seeTable 2).
In surgically challenging tumors, size reduction with imatinib may allow more conservative surgery [18]. Two months of preoperative imatinib at a 600-mg dose daily showed 20% reduction of median tumor volume in 36% of cases [86•]. Three months showed an overall response of 57% with median tumor shrinkage of 31.5% [87].
Treatment with imatinib results in sclerotic, hypocellular areas that can harbor pockets of viable discontinuous and widespread tumor [88•]. Imatinib resistance has already been described and novel mutations in genes implicated in various signaling pathways have been found [89].
Cutaneous adverse effects of imatinib include maculopapular, lichenoid, psoriasiform eruptions, acute generalized exanthematic pustulosis, and Stevens-Johnson syndrome [90]. Other systemic adverse effects are congestive heart failure, hematologic, and liver toxicities [84].
Sunitinib has been evaluated for patients with imatinib-resistant DFSP with 40% of patients showing partial or complete response [91].
Follow-up
Three- to six-month interval follow-up is recommended for the first 3–5 years and an annual follow-up after that [9, 23••]. Recurrences after 5 years may occur [70, 92]. Clinical follow-up can be complemented with MRI imaging in selected cases.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Taylor R. Sarcomatous tumor resembling in some respects keloid. Arch Dermatol. 1890;8:384–7.
Darier J. Dermatofibromes progressifs et recidivants ou fibrosarcomes de la peu. Ann Dermatol Syphiligr. 1924;5:545–62.
Hoffman E. Uber das knollentreibende fibrosarkom der haut (Dermatofibrosarkoma protuberans). Derm Ztschr. 1925;43:1–28.
Reha J, Katz SC. Dermatofibrosarcoma Protuberans. Surg Clin North Am. 2016;96(5):1031–46.
•• Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg. 2016;42:S24–31. This is the largest population-based study of DFSP derived from a cohort of almost 7,000 patients.
• Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56(6):968–73. Data were obtained from 9 population-based cancer registries of the Surveillance, Epidemiology, and End Results Program for 1973 to 2002.
Nguyen AH, Detty SQ, Gonzaga MI, Huerter C. Clinical features and treatment of dermatofibrosarcoma protuberans affecting the vulva: a literature review. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2017
Wiszniewska J, Roy A, Masand RP. Myxoid dermatofibrosarcoma protuberans of the vulva: case report of a rare variant in an unusual location, with unusual morphologic and immunohistochemical features. Am J Dermatopathol. 2016;38(3):226–30.
Tsai Y-J, Lin P-Y, Chew K-Y, Chiang Y-C. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthetic Surg JPRAS. 2014;67(9):1222–9.
Noujaim J, Thway K, Fisher C, Jones RL. Dermatofibrosarcoma protuberans: from translocation to targeted therapy. Cancer Biol Med. 2015;12(4):375–84.
Sirvent N, Maire G, Pedeutour F. Genetics of dermatofibrosarcoma protuberans family of tumors: from ring chromosomes to tyrosine kinase inhibitor treatment. Genes Chromosomes Cancer. 2003;37(1):1–19.
Greco A, Fusetti L, Villa R, Sozzi G, Minoletti F, Mauri P, et al. Transforming activity of the chimeric sequence formed by the fusion of collagen gene COL1A1 and the platelet derived growth factor b-chain gene in dermatofibrosarcoma protuberans. Oncogene. 1998;17(10):1313–9.
• Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol. 2016;25:64–71. Discusses thoroughly the genetics of DFSP
Nakamura I, Kariya Y, Okada E, Yasuda M, Matori S, Ishikawa O, et al. A novel chromosomal translocation associated with COL1A2 - PDGFB gene fusion in dermatofibrosarcoma protuberans: PDGF expression as a new diagnostic tool. JAMA Dermatol. 2015;151(12):1330.
Kreicher KL, Honda KS, Kurlander DE, Bordeaux JS. Hormone receptor expression in patients with dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2016;75(6):1205–9.
Eilers G, Czaplinski JT, Mayeda M, Bahri N, Tao D, Zhu M, et al. CDKN2A/p16 loss implicates CDK4 as a therapeutic target in imatinib-resistant dermatofibrosarcoma protuberans. Mol Cancer Ther. 2015;14(6):1346–53.
Rutkowski P, Przybył J, Świtaj T. Genetics of rare mesenchymal tumors: implications for targeted treatment in DFSP, ASPS, CCS, GCTB and PEComa. Int J Biochem Cell Biol. 2014;53:466–74.
van der Graaf WTA, Gelderblom H. New systemic therapy options for advanced sarcomas. Curr Treat Options in Oncol. 2012;13(3):306–17.
Deinlein T, Richtig G, Schwab C, Scarfi F, Arzberger E, Wolf I, et al. The use of dermatoscopy in diagnosis and therapy of nonmelanocytic skin cancer: dermatoscopy in nonmelanolytic skin cancer. JDDG J Dtsch Dermatol Ges. 2016;14(2):144–51.
Piccolo V, Staibano S, Coppola N, Russo D, Alessio L, Argenziano G. Dermoscopy of dermatofibrosarcoma protuberans on black skin. J Am Acad Dermatol. 2016;74(6):e119–20.
Venturini M, Zanca A, Manganoni AM, Pavoni L, Gualdi G, Calzavara-Pinton P. In vivo characterization of recurrent dermatofibrosarcoma protuberans by dermoscopy and reflectance confocal microscopy. J Am Acad Dermatol. 2016;75(5):e185–7.
• Bogucki B, Neuhaus I, Hurst EA. Dermatofibrosarcoma protuberans: a review of the literature. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2012;38(4):537–51. Literature review with great review of case series of DFSP treated with WLE vs. MMS
•• Saiag P, Grob J-J, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer Oxf Engl. 1990;51(17):2604–8. A unique collaboration of multi-disciplinary experts from the European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization of Research and Treatment of Cancer (EORTC) was formed to make recommendations on DFSP diagnosis and treatment, based on systematic literature reviews and the experts’ experience
Serra-Guillén C, Llombart B, Nagore E, Guillén C, Requena C, Kindem S, et al. Estudio de los factores histológicos asociados a la infiltración en profundidad en el dermatofibrosarcoma protuberans. Actas Dermo-Sifiliográficas. 2016;107(5):414–20.
•• Liang CA, Jambusaria-Pahlajani A, Karia PS, Elenitsas R, Zhang PD, Schmults CD. A systematic review of outcome data for dermatofibrosarcoma protuberans with and without fibrosarcomatous change. J Am Acad Dermatol. 2014;71(4):781–6. A systematic review of dermatofibrosarcoma protuberans (DFSP) outcomes based on the presence or absence of fibrosarcomatous (FS) 24 reports containing 1422 patients with DFSP and 225 with DFSP-FS are summarized.
Karanian M, Pérot G, Coindre J-M, Chibon F, Pedeutour F, Neuville A. Fluorescence in situ hybridization analysis is a helpful test for the diagnosis of dermatofibrosarcoma protuberans. Mod Pathol. 2015;28(2):230–7.
Patel KU, Szabo SS, Hernandez VS, Prieto VG, Abruzzo LV, Lazar AJF, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39(2):184–93.
• Italiano A, Di Mauro I, Rapp J, Pierron G, Auger N, Alberti L, et al. Clinical effect of molecular methods in sarcoma diagnosis (GENSARC): a prospective, multicentre, observational study. Lancet Oncol. 2016;17(4):532–8. Assessess the clinical effect of systematic implementation of molecular assays to improve sarcoma misdiagnosis. In this multicentre, observational study, patients from 32 centres of the French Sarcoma Group/Reference Network in Pathology of Sarcomas were recruited
Kornik RI, Muchard LK, Teng JM. Dermatofibrosarcoma protuberans in children: an update on the diagnosis and treatment: update on DFSP in children. Pediatr Dermatol. 2012;29(6):707–13.
• Lemm D, Mügge L-O, Mentzel T, Höffken K. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. 2009;135(5):653–65. Review of the literature of case series of DFSP treated with conservative margins vs. WLE vs. MMS vs. radiotherapy and imatinib
Al Barwani AS, Taif S, Al Mazrouai RA, Al Muzahmi KS, Alrawi A. Dermatofibrosarcoma protuberans: insights into a rare soft tissue tumor. J Clin Imaging Sci. 2016;6:16.
Zhang L, Liu Q, Cao Y, Zhong J, Zhang W. Dermatofibrosarcoma protuberans: computed tomography and magnetic resonance imaging findings. Medicine (Baltimore). 2015;94(24):e1001.
Sung TH, Tam AC, Khoo JL. Dermatofibrosarcoma protuberans: a comprehensive review on the spectrum of clinico-radiological presentations. J Med Imaging Radiat Oncol. 2017;61(1):9–17.
Basu S, Goliwale F. 18F-FDG PET/CT prediction of an aggressive clinical course for dermatofibrosarcoma protuberans. J Nucl Med Technol. 2016;44(2):88–9.
Glazer ES, Prieto-Granada C, Zager JS. Current approaches to cutaneous sarcomas: dermatofibrosarcoma protuberans and cutaneous leiomyosarcoma. Curr Probl Cancer. 2015;39(4):248–57.
Amin M, Edge S, Greene F, Byrd D, Brookland R, Washington M, et al. AJCC Cancer Staging Manual. Eighth Edition. 2017.
•• Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152(12):1365. Assesses the prognostic factors of DFSP by examining data for 3686 patients with histologically confirmed cases of DFSP diagnosed between 1972 and 2012 from the 18 US regional registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program
•• Hoesly PM, Lowe GC, Lohse CM, Brewer JD, Lehman JS. Prognostic impact of fibrosarcomatous transformation in dermatofibrosarcoma protuberans: a cohort study. J Am Acad Dermatol. 2015;72(3):419–25. Compares clinical features and biological behavior of DFSP and DFSP-FS
Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88(12):2711–20.
Hayakawa K, Matsumoto S, Ae K, Tanizawa T, Gokita T, Funauchi Y, et al. Risk factors for distant metastasis of dermatofibrosarcoma protuberans. J Orthop Traumatol. 2016;17(3):261–6.
Mahajan BB, Sumir K, Singla M. Metastatic dermatofibrosarcoma protuberans: a rare case report from North India. J Cancer Res Ther. 2015;11(3):670.
Yokoyama Y, Murakami Y, Sasaki M, Morifuji M, Hayashidani Y, Kobayashi T, et al. Pancreatic metastasis of dermatofibrosarcoma protuberans. J Gastroenterol. 2004;39(8):798–800.
Lal P, Goel A, Mandal AK. Dermatofibrosarcoma protuberans of scalp with cervical lymph node metastasis. Sarcoma. 2004;8(1):43–5.
Nakra T, Cook T, Douglas RS, Goldberg RA. Dermatofibrosarcoma protuberans metastatic to the orbit. Arch Ophthalmol Chic Ill 1960. 2004;122(8):1240–1.
• Rutkowski P, Debiec-Rychter M. Current treatment options for dermatofibrosarcoma protuberans. Expert Rev Anticancer Ther. 2015;15(8):901–9. This review summarizes state of the art and perspectives on the DFSP management
Rutgers EJ, Kroon BB, Albus-Lutter CE, Gortzak E. Dermatofibrosarcoma protuberans: treatment and prognosis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 1992;18(3):241–8.
Mark RJ, Bailet JW, Tran LM, Poen J, Fu YS, Calcaterra TC. Dermatofibrosarcoma protuberans of the head and neck: a report of 16 cases. Arch Otolaryngol Head Neck Surg. 1993;119(8):891–6.
Fiore M, Miceli R, Mussi C, Lo Vullo S, Mariani L, Lozza L, et al. Dermatofibrosarcoma protuberans treated at a single institution: a surgical disease with a high cure rate. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(30):7669–75.
Du Bay D, Cimmino V, Lowe L, Johnson TM, Sondak VK. Low recurrence rate after surgery for dermatofibrosarcoma protuberans: a multidisciplinary approach from a single institution. Cancer. 2004;100(5):1008–16.
Fields RC, Hameed M, Qin L-X, Moraco N, Jia X, Maki RG, et al. Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann Surg Oncol. 2011;18(2):328–36.
Ratner D, Thomas CO, Johnson TM, Sondak VK, Hamilton TA, Nelson BR, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37(4):600–13.
•• Loghdey MS, Varma S, Rajpara SM, Al-Rawi H, Perks G, Perkins W. Mohs micrographic surgery for dermatofibrosarcoma protuberans (DFSP): a single-centre series of 76 patients treated by frozen-section Mohs micrographic surgery with a review of the literature. J Plast Reconstr Aesthetic Surg JPRAS. 2014;67(10):1315–21. One of the largest case series of MMS with frozen sections for DFSP
Parker TL, Zitelli JA. Surgical margins for excision of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1995;32(2 Pt 1):233–6.
Arnaud EJ, Perrault M, Revol M, Servant JM, Banzet P. Surgical treatment of dermatofibrosarcoma protuberans. Plast Reconstr Surg. 1997;100(4):884–95.
Stojadinovic A, Karpoff HM, Antonescu CR, Shah JP, Singh B, Spiro RH, et al. Dermatofibrosarcoma protuberans of the head and neck. Ann Surg Oncol. 2000;7(9):696–704.
• Gloster HM. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3 Pt 1):355–74. quiz 375–6. This study retrospectively compared the recurrence rates of DFSP after MMS with those after wide surgical excision; results at the Mayo Clinic and in the world literature were evaluated
• Farma JM, Ammori JB, Zager JS, Marzban SS, Bui MM, Bichakjian CK, et al. Dermatofibrosarcoma protuberans: how wide should we resect? Ann Surg Oncol. 2010;17(8):2112–8. Case series of WLE using en face pathological sectioning shows recurrence rates similar to MMS
Mohs FE. Chemosurgery. Clin Plast Surg. 1980;7(3):349–60.
Breuninger H, Schaumburg-Lever G. Control of excisional margins by conventional histopathological techniques in the treatment of skin tumours. An alternative to Mohs’ technique. J Pathol. 1988;154(2):167–71.
• Hancox JG, Kelley B, Greenway HT. Treatment of dermatofibroma sarcoma protuberans using modified Mohs micrographic surgery: no recurrences and smaller defects. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2008;34(6):780–4. Important series on slow MMS for DFSP with no recurrences
Gloster HM, Harris KR, Roenigk RK. A comparison between Mohs micrographic surgery and wide surgical excision for the treatment of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(1):82–7.
• Paradisi A, Abeni D, Rusciani A, Cigna E, Wolter M, Scuderi N, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34(8):728–36. Very important comparative case series of MMS vs. WLE and review of the literature
• Meguerditchian A-N, Wang J, Lema B, Kraybill WG, Zeitouni NC, Kane JM. Wide excision or Mohs micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol. 2010;33(3):300–3. One of the few comparative case series of WLE vs. MMS
•• Lowe GC, Onajin O, Baum CL, Otley CC, Arpey CJ, Roenigk RK, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2017;43(1):98–106. Publication with the longest follow-up of DFSP treated with MMS
Mullen JT. Dermatofibrosarcoma protuberans: wide local excision versus Mohs micrographic surgery. Surg Oncol Clin N Am. 2016;25(4):827–39.
• Foroozan M, Sei J-F, Amini M, Beauchet A, Saiag P. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148(9):1055–63. Twenty-three nonrandomized trials (4 comparative and 19 noncomparative) were reviewed for recurrence of WLE and MMS
Massey RA, Tok J, Strippoli BA, Szabolcs MJ, Silvers DN, Eliezri YD. A comparison of frozen and paraffin sections in dermatofibrosarcoma protuberans. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1998;24(9):995–8.
Garcia C, Viehman G, Hitchcock M, Clark RE. Dermatofibrosarcoma protuberans treated with Mohs surgery. A case with CD34 immunostaining variability. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1996;22(2):177–9.
•• Serra-Guillén C, Llombart B, Nagore E, Guillén C, Requena C, Traves V, et al. Mohs micrographic surgery in dermatofibrosarcoma protuberans allows tumour clearance with smaller margins and greater preservation of healthy tissue compared with conventional surgery: a study of 74 primary cases. Br J Dermatol. 2015;172(5):1303–7. Specifically addresses the issue of MMS for tissue preservation MMS can achieve tumor clearance with smaller margins and greater preservation of healthy tissue than CS.
Snow SN, Gordon EM, Larson PO, Bagheri MM, Bentz ML, Sable DB. Dermatofibrosarcoma protuberans: a report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer. 2004;101(1):28–38.
Pallure V, Dupin N, Guillot B. Association for recommendations in dermatology. Surgical treatment of Darier-Ferrand dermatofibrosarcoma: a systematic review. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2013;39(10):1417–33.
Loss L, Zeitouni NC. Management of scalp dermatofibrosarcoma protuberans. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2005;31(11 Pt 1):1428–33.
Nouri K, Lodha R, Jimenez G, Robins P. Mohs micrographic surgery for dermatofibrosarcoma protuberans: University of Miami and NYU experience. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2002;28(11):1060–4. discussion 1064
Woo K-J, Bang SI, Mun G-H, Oh KS, Pyon J-K, Lim SY. Long-term outcomes of surgical treatment for dermatofibrosarcoma protuberans according to width of gross resection margin. J Plast Reconstr Aesthet Surg. 2016;69(3):395–401.
•• Bichakjian CK, Olencki T, Aasi S, Alam M, Andersen J, Berg D, et al. Dermatofibrosarcoma protuberans, version 1.2017. J Natl Compr Cancer Netw JNCCN. 2016;12(6):863–8. Current NCCN Guidelines for the treatment of DFSP
Kim BJ, Kim H, Jin US, Minn KW, Chang H. Wide local excision for dermatofibrosarcoma protuberans: a single-center series of 90 patients. Biomed Res Int. 2015;2015:642549.
Wilder F, D’Angelo S, Crago AM. Soft tissue tumors of the trunk: management of local disease in the breast and chest and abdominal walls. J Surg Oncol. 2015;111(5):546–52.
•• Wain RJ, Tehrani H. Reconstructive outcomes of Mohs surgery compared with conventional excision: a 13-month prospective study. J Plast Reconstr Aesthetic Surg JPRAS. 2015;68(7):946–52. Prospective study focused on the reconstructive outcomes of MMS vs. WLE, not only how MMS spares tissue but how this reflects on reconstruction outcomes
•• Chen Y-T, Tu W-T, Lee W-R, Huang YC. The efficacy of adjuvant radiotherapy in dermatofibrosarcoma protuberans: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol JEADV. 2016;30(7):1107–14. Huge meta-analysis, largest on radiotherapy for DFSP
Sun LM, Wang CJ, Huang CC, Leung SW, Chen HC, Fang FM, et al. Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2000;57(2):175–81.
McArthur GA, Demetri GD, van Oosterom A, Heinrich MC, Debiec-Rychter M, Corless CL, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(4):866–73.
• Rutkowski P, Van Glabbeke M, Rankin CJ, Ruka W, Rubin BP, Debiec-Rychter M, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(10):1772–9. One of the key articles of imatinib for DFSP
El-Bared N, Wong P, Wang D. Soft tissue sarcoma and radiation therapy advances, impact on toxicity. Curr Treat Options in Oncol. 2015;16(5):19.
Odueyungbo M, Ratner D. Update on the use and treatment of targeted molecular inhibitors for locally advanced and metastatic non-melanoma skin cancers. Dermatol Surg. 2016;42:S49–56.
Rutkowski P, Dębiec-Rychter M, Nowecki Z, Michej W, Symonides M, Ptaszynski K, et al. Treatment of advanced dermatofibrosarcoma protuberans with imatinib mesylate with or without surgical resection. J Eur Acad Dermatol Venereol JEADV. 2011;25(3):264–70.
• Kérob D, Porcher R, Vérola O, Dalle S, Maubec E, Aubin F, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(12):3288–95. One of the articles that proposes neoadjuvant imatinib
• Ugurel S, Mentzel T, Utikal J, Helmbold P, Mohr P, Pföhler C, et al. Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: a multicenter phase II DeCOG trial with long-term follow-up. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20(2):499–510. One of the key articles of imatinib for DFSP
Clarke LE. Fibrous and fibrohistiocytic neoplasms: an update. Dermatol Clin. 2012;30(4):643–56. vi
Hong JY, Liu X, Mao M, Li M, Choi DI, Kang SW, et al. Genetic aberrations in imatinib-resistant dermatofibrosarcoma protuberans revealed by whole genome sequencing. PLoS One. 2013;8(7):e69752.
Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Pérez N. Adverse skin effects of imatinib, a tyrosine kinase inhibitor. Actas Dermosifiliogr. 2014;105(7):655–62.
Fu Y, Kang H, Zhao H, Hu J, Zhang H, Li X, et al. Sunitinib for patients with locally advanced or distantly metastatic dermatofibrosarcoma protuberans but resistant to imatinib. Int J Clin Exp Med. 2015;8(5):8288–94.
Chang CK, Jacobs IA, Salti GI. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2004;30(3):341–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alvaro E. Acosta and Catalina Santa Vélez declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Acosta, A.E., Vélez, C.S. Dermatofibrosarcoma Protuberans. Curr. Treat. Options in Oncol. 18, 56 (2017). https://doi.org/10.1007/s11864-017-0498-5
Published:
DOI: https://doi.org/10.1007/s11864-017-0498-5