Opinion Statement
Skin cancer is the most common of human cancers and outnumbers all other types of cancer combined in the USA by over threefold. The majority of non-melanoma skin cancers are easily treated with surgery or locally destructive techniques performed under local anesthesia in the cost-effective outpatient setting. However, there is a subset of “high-risk” cases that prove challenging in terms of morbidity, mortality, adjuvant treatment required, as well as overall cost to the health care system. In our opinion, the term “high risk” when applied to skin cancer can mean one of three things: a high-risk tumor with aggressive histologic and/or clinical features with an elevated risk for local recurrence or regional/distant metastasis, a high-risk patient with the ongoing development of multiple skin cancers, and a high-risk patient based on immunosuppression. We have recently proposed classifying NMSC as a chronic disease in a certain subset of patients. Although no consensus definition exists for a chronic disease in medicine, there are three components that are present in most definitions: duration of at least 1 year, need for ongoing medical care, and functional impairment and/or alteration of activities of daily living (ADLs) and quality of life (QOL). Immunosuppression can refer to exogenous (organ or stem cell transplant patients,) or endogenous (HIV, leukemia, lymphoma, genodermatoses with DNA mismatch repair problems or other immunosuppression) causes. These patients are at risk for high-risk tumors and/or the development of multiple tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-melanoma skin cancer (NMSC) has become an epidemic in the USA. Basal cell carcinoma (BCC) is the most common human cancer comprising the majority of the estimated 5 million new cases of NMSC each year [1••, 2••, 3]. Squamous cell carcinoma (SCC) is the second most common cutaneous cancer and possesses an annual incidence of approximately 200,000 to 800,000 cases [1••, 3]. Local treatments such as Mohs micrographic surgery (MMS), wide local excision (WLE), electrodessication and curettage, topical chemotherapy, and photodynamic therapy are sufficient to clear the tumor in the majority of NMSC cases, and systemic therapy is typically not required [3]. However, there is a subset of cutaneous tumors that may go on to be more aggressive or “high risk.” The overall risk of metastasis of SCC to regional nodes or distant areas is approximately 2 to 5 %. BCC may become locally aggressive (laBCC) and destructive, or in certain cases, may become metastatic (mBCC), which is extremely rare with an estimated incidence of 0.0028 to 0.55 % reported in the literature [4, 5].
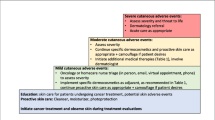
There are both tumor- and patient-related factors that are associated with high-risk skin cancer (Fig. 1). Tumor-related factors that contribute to high-risk skin cancer include recurrent disease, size greater than 2 cm, association with scars, burns, or chronic wounds, histologic features such as poorly differentiated, perineural or angiolymphatic invasion. Tumors in these cases display very aggressive histological and clinical features, resulting in extensive local invasion and rates of nodal or distant metastasis that range from 5 to 45 %. Individual high-risk tumors are recommended to be managed in a multidisciplinary treatment team with treatment algorithms recommended by the National Comprehensive Cancer Network (NCCN) including complete skin and regional nodal examination, imaging as appropriate, excision with wider surgical margins vs. MMS with complete margin assessment, and adjuvant therapies as appropriate.
In addition to tumor-related factors, patients may be high risk in terms of those who develop a large number of cutaneous tumors. These patients are sometimes termed “frequent fliers,” “end stage skin disease,” or “NMSC as a chronic disease” patients [6]. Wehner and colleagues found that after developing a non-first NMSC, an individual’s 10-year risk of developing a subsequent NMSC was 91.2 % [7•]; however, a “high risk” subset of patients will develop a significant number of new skin tumors. By stratifying patients at the highest risk for developing multiple NMSCs, we can increase surveillance and apply specific prophylactic and treatment strategies discussed in this review.
Finally, patient-related factors that indicate “high risk” skin cancer commonly include both endogenous and exogenous forms of immunosuppression. For example, organ transplant recipients are at 65-fold increased risk for SCC [8]. Patients with chronic lymphocytic leukemia develop SCC at increased rates and also have elevated recurrence and mortality rates of 25 and 41 %, respectively [9]. These high-risk skin cancer patients with underlying immunosuppression are at risk for both individual “high risk” lesions as well as the development of increasing numbers of tumors.
This article will provide an update of the last 3 years of the literature regarding treatment of high-risk tumors and skin cancer patients.
Treatment options
Diet and lifestyle
-
Photoprotection
-
Ultraviolet protection factor (UPF) is used for photoprotective clothing, is analogous to SPF, and is measured using a spectrophotometer to assess levels of UVA and UVB transmission. Fabrics that perform best are tightly woven fabrics, dark colors, wool, and polyester [10]. In addition to photoprotective clothing, wide-brimmed hats and sunglasses that block both UVA and UVB are useful for preventing photodamage.
-
Amount and frequency are the main determinants of protection for sunscreen use. Patients should understand that sun protection factors (SPF) are derived from international standards of using approximately 2 mg/cm2 applied every 2 to 3 h of exposure. This is loosely translated to the “teaspoon rule” which advises a teaspoon to face, ears, and neck, a teaspoon for each upper limb and two teaspoons each to the back, abdomen/chest, and lower extremity, or roughly 12 teaspoons per application [11, 12]. Patients taking phototoxic or photosensitive medications or phototoxic procedures like laser and chemical peels should choose creams with an SPF of 50+. Caution is advised when using physical filters on traumatized skin as no safety data exists yet for this application [13]. Mineral or physical blocking sunscreens have improved spectrum coverage over products with chemical blocking mechanisms.
-
-
Diet
-
Supplementation with specific antioxidants is not recommended. Randomized control trials of beta-carotene, selenium, vitamin A, E, and C have not showed any benefit, and in some case, increased risk of NMSC [14, Level of evidence IA].
-
Nicotinamide (vitamin B3) has demonstrated significant benefit in a phase 3, double-blinded, randomized controlled trial. At 500 mg orally twice daily for 1 year, there was a 23 % reduction in new NMSCs and 13 % reduction in actinic keratoses [15•, Level of evidense [IB]. There were no differences in clinically significant events between groups. This is not to be confused with nicotinic acid.
-
Pharmaceuticals
Cost is reported as average wholesale price (AWP) per unit and does not reflect actual transaction prices, discounts, or rebates. AWP is the suggested price determined by the marketing firm and is supplied for all packages (Table 1).
Platins (cisplatin, carboplatin)
Cisplatin has been in use for over 35 years and is the standard of care for various forms of cancer, including advanced and metastatic SCC. The agent is cytotoxic and acts by introducing crosslinks into DNA, thereby interfering with mitosis and cell division. The platins are inexpensive and often serve as a vital component of combination chemotherapy regimens used in the treatment of many solid tumors. Interactions with cidofovir and amphotericin cause an increase in serious nephrotoxicity and ototoxicity as well as many others. Platins also reduce efficacy of vaccines including influenza. Nausea, vomiting, nephrotoxicity, ototoxicity, and myelosupression are the most common adverse effects. Dose reduction is needed with a CrCl 10–50 mL/min and is contraindicated in patients with CrCl <10 mL/min. Cost is $0.36 to 0.48 for cisplatin and $0.10 to 0.39 for carboplatin per milligram [16].
Primary and adjuvant
In a retrospective review of eight cases of metastatic SCC, five received cisplatin (60–90 mg/m2/day, day 1) and adriamycin (20–40 mg/m2/day, day 1 or 2), two received cisplatin (10–15 mg/m2/day, days 1–5) and epirubicin (10–15 mg/m2/day, days 1–5), and one received carboplatin (200–400 mg/m2/day, day 1) and adriamycin (20–40 mg/m2/day, day 1 or 2). Overall, the response rate was 37.5 %. This includes two complete and durable responses, one with lung metastasis on cisplatin and adriamycin and one with lymph node metastasis on cisplatin and epirubicin. There was grade 3 and 4 neutropenia, thrombocytopenia, and anorexia [17, Level of evidence III].
Capecitabine
Capecitabine is the prodrug of the inactive form of 5-FU and is converted via thymidine phosphorylase to irreversible inhibit thymidylate synthase as an antimetabolite. It is currently approved for breast and colon cancer. It interacts with many medications but most seriously with warfarin and tofacitinib. The most common adverse effects include nausea and gastrointestinal symptoms (nausea, vomiting, and diarrhea). This medication is contraindicated in renal impairment <30 mL/min. Cost is $0.08 to 0.09 per milligram or $40.00 to 45.00 500 mg tablet [16].
Prevention
In a case series of 10 SOTRs, capecitiabine 500–1500 mg/m2 divided into two daily doses (on days 1 through 14 in a 21 day cycle) achieved a mean reduction in monthly SCCs of 68.1 ± 29.8 % after 12 months of treatment and 53.4 ± 43.1 % after 24 months of treatment. Grades 3 and 4 events occurred in 70 % of patients with most common being gastrointestinal symptoms, fatigue, and gout [18, Level of evidence III].
Hedgehog inhibitors (vismodegib, sonidegib, itraconazole)
Basal cell carcinomas commonly are activated by the Hh signaling pathway. Smoothened (SMO) is a transmembrane protein involved in Hedgehog (Hh) signaling. SMO inhibitors are a new class of drugs for the treatment of advanced BCC that include laBCC and mBCC. Vismodegib was first in class, followed by sonidegib with several additional Hh inhibitors currently in development. There are no required dose adjustments for patients with renal or hepatic impairment. Only one medication, ivacaftor is listed as a serious drug interaction with this class. The most common adverse effects are muscle spasms, alopecia, and dysguesia. Vismodegib’s cost is $2.81 per milligram or $422.57 per 150 mg pill. Sonidegib’s cost is $2.01 per milligram or $402.40 per 200 mg pill [16].
Itraconazole also acts as a SMO inhibitor but has a mechanism distinct from others in this class [19]. This medication is a potent CYP4503A4 inhibitor and thus has many contraindicated medications. Congestive heart failure is also a condition that would preclude treatment. The most common adverse effects are rash, nausea, and edema. Itraconazole’s cost is $0.09 to 0.15 per milligram or $9.27 to 15.72 per 100 mg pill [16].
Itraconazole
Primary treatment
In an open-label phase 2 trial of itraconazole for basal cell carcinomas, dosing of itraconazole was either 100 mg twice daily for an average of 2.3 months or 200 mg twice per day for 30 days. The overall tumor size was decreased by 24 %, cell proliferation by 45 %, and activated hedgehog pathway activity by 65 %. This was trialed in 19 patients with 90 BCC tumors treated, with two adverse events of fatigue and congestive heart failure [20•, Level of evidence IIA].
Sonidegib
Primary treatment
In an ongoing multicenter randomized, double-blinded phase 2 trial, patients received either 200 or 800 mg of sonidegib. At the lower dose, similar efficacy was observed with a decrease from 60 to 32 % in adverse events leading to dose reduction or interruption. The 200 and 800 mg group achieved an objective response of 36 and 39 %, respectively [21•, Level of evidence IB]. These adverse events consisted of muscle spams, dyesgeusia, alopecia, nausea, fatigue, and increased creatine kinase.
In an open-label study, nine patients with advanced BCC that was previously resistant to treatment with vismodegib received 800 mg of sonidegib daily for a median of 6 weeks. No patients improved and five with identifiable mutations in the Hedgehog pathway had progressive disease. This demonstrates that resistance to one SMO inhibitor may experience tumor progression on other members of the class. There were two grade 3 events of altered mental status and rhabdomyolysis [22, Level of evidence III].
Vismodegib
Primary treatment
In an open-label, 2-cohort, multicenter study designed to assess safety and efficacy of vismodegib in patients with limited options, patients received 150 mg daily until tumor progression or intolerable toxicity. One hundred nineteen patients received a median of 5.5 months of treatment, with objective response in 46 % of laBCC and 30 % in mBCC and complete response in 10 and 5 %, respectively. In this study, 97 % of patients experienced a treatment emergent adverse event. In general, these occurred within 2 months of treatment and were mostly grades 1 and 2 [23•, Level of evidence IIB].
A preplanned interim analysis of an international open-label trial assessing vismodegib in the setting of routine clinical practice revealed an objective response in 66 % of laBCC and 38 % of mBCC. Five hundred patients with 1 year of follow-up received 150 mg of vismodegib for a median of 36 weeks. However, adverse events were experience in 98 % of patients with 80 % needing to discontinue treatment. Serious adverse reactions occurred in 22 % of patients and of the 31 patients that died, 21 were the result of adverse events [24, Level of evidence IIB].
Neoaduvant
An open-label trial to assess change in target tumor surgical defect area, pre- and post-vismodegib demonstrated a 27 % reduction in area from baseline. Eleven of the 15 patients were able to complete at least 3 months of daily treatment with 150 mg daily. If patients could not complete 3 months, no benefit was seen. Of the 13 target tumors selected for surgery, only one recurred 17 months post-MMS [25•, Level of evidence III].
In an open-label, 3-cohort, multicenter study designed to assess safety and efficacy of vismodegib in patients with operable BCC, patients received 150 mg daily followed by excision and MMS. Cohort 1 received 3 months of vismodegib; cohort 2 received 3 months of vismodegib followed by 6 months of observation; and cohort 3 received vismodegib 2 months on/1 month off/2 months on. Complete histologic clearance occurred in 42, 16, and 44 %, respectively. Treatment was discontinued in only 7 % of patients [26, Level of evidence III].
Epidermal growth factor receptor inhibitors
Epidermal growth factor receptor (EGFR) plays a crucial role in signal transduction pathways that regulate key cellular functions and used for SCC. Cetuximab is a recombinant monoclonal antibody that competitively inhibits EGFR by binding to the extracellular domain. This induces internalization and downregulation of intracellular signals. Monitoring of electrolytes for hypomagnesemia, hypocalcemia, and hypokalemia is recommended. There is also a black box warning for the possibility of severe infusion reactions that can be fatal. The most common adverse effects are acneiform rash, fatigue, hypomagnesemia, and malaise. Cost is $6.55 per milligram or $1310.40 per 200 mg vial [16].
Erlotinib is a tyrosine kinase inhibitor (TKI) that targets the EGFR tyrosine kinase, preventing the formation of phosphotyrosine after homodimerization of the receptor. It has decreased bioavailability with any medications that effect pH of GI tract. It also is seriously affected by any other medications that alter CYP3A4 metabolism. Concurrent cigarette smoking requires dose escalation. Most common adverse effects are rash, anorexia, diarrhea, and fatigue. Cost is $1.93 per milligram or $289.82 per 150 mg pill [16].
Gefitinib is also an EGFR TKI, but has no recent studies published for use in non-melanoma skin cancer.
Cetuximab
Neoaduvant and primary treatment
In a open-label, single site, 2-cohort study, patients with locally advanced SCC considered to be inoperable (AJCC stage II and III) were pretreated with 1 cycle of cetuximab 400 mg m2 every 3 weeks with smaller subsequent weekly doses of 250 mg m2, combined with cisplatin 100 mg m2 or carboplatin, plus 5-FU 1000 mg m2 daily for 4 days (25 patients). If platin and/or 5 FU was contraindicated, patients received cetuximab alone (9 patients). Of patients, 92 % on combination therapy became amenable to surgery and 78 % achieved complete remission. In a mean duration of 30-month follow-up, 4 of 23 patients relapsed. While 70 % of patients developed grade 1–2 typical cetuximab induced folliculitis, only one developed grade 3 [27•, Level of evidence IIB].
Adjuvant
Among 22 patients at a single institution with “very high risk SCC” (tumors displaying lymphovascular, perineural, parotid, periorbital, cartilaginous, or bony invasion; in-transit metastasis; or regional or distant metastasis), those receiving adjuvant cetuximab had increased CR and decreased disease progression. Specifically, six patients who received surgery + cetuximab had 50 % CR compared to 36 and 25 %, respectively, for those with surgery + radiation (n = 11) and surgery alone (n = 4). A single patient who received surgery + radiation + cetuximab had a CR [2••, Level of evidence III].
Erlotinib
Neoadjuvant and adjuvant
In a single-arm, open-label, phase 1 study, 15 patients (80 % with recurrence, 13 with T4, 7 with N0, and 10 with selective neck dissections) with head and neck cutaneous SCC received erlotinib 150 mg daily for 14 days before wide local excision and lymphatic dissection. Within 8 weeks of resection, patients were started concurrently on erlotinib 150 mg daily and 60–66 Gy for 6 weeks. Disease free survival at 1 year was 73 and 60 % at 2 years. Most common grade 3 events were dermatitis and mucositis [28, Level of evidence III].
Vitamin A analogs
Vitamin A analogs or retinoids are thought to reduce NMSC via promotion of cell differentiation, growth regulation in hyperproliferative epithelia, and downregulation of proto-oncogenes by binding nuclear receptors [29]. While there is no role of vitamin A analogs in the treatment of high-risk NMSC, they have been used prophylactically in immunosuppressed individuals and in patients developing multiple tumors. Benefit of acetretin use has been seen as early as 6 months in the SOTR population [30]. The tetracycline class of antibiotics, methotrexate, and ethanol are contraindicated with this medication. Acitretin decreases the effectiveness of oral conceptive medication as well. The most common side effects are mucositis, alopecia, hypertriglyceridemia, and skin peeling. Cost is $1.54 to 3.12 per milligram or $31.20 for a 10 mg pill and $38.41 for a 25 mg pill [16].
Acetretin
Prevention
In a prospective, randomized, double-blind, placebo controlled trial, patients without a transplant but considered high risk due history of previous skin cancers were given 25 mg of acetretin 5 days a week for 2 years. There was a significant trend that favored use; however, likely due to sample size, an overt statistical benefit was not seen. Of patients, 46 % in the acetretin arm never developed another NMSC vs 26 % in the placebo arm. Mucositis, skin peeling, and alopecia were the most common adverse effects reported; however, these were mostly grades 1 and 2 [31, Level of evidence IA].
Topicals
Actinic keratoses (AK) are a common lesion in the high-risk patient. Field treatment may halt progression of AKs and development of squamous cell carcinoma. Available agents for topical treatment include fluorouracil, imiquimod, ingenol mebutate, and diclofenac. Contraindications are few and mainly include prior reported hypersensitivity. Common adverse effects would be limited to skin irritation, edema, and hypersensitivity. The cost of fluorouracil is $10.43 per gram of 5 % cream. The cost of imiquimod is $367.64 per gram of 5 % cream (250 mg of cream per packet). The cost of ingenol mebutate is $992.76 per gram of 0.05 % gel (1 packet is 0.47 g) or $661.84 per gram of 0.015 % gel [16].
Fluorouracil
Prevention
A prospective, randomized, double-blind, 2-cohort trial examining the long-term efficacy of one 4-week treatment of twice daily 5-fluoruracil 5 % cream to face and ears yielded a significant difference in AK clearance rates at 6 months of 38 vs 17 %, drug vs control, respectively. There was also decreased AK development at 2-year follow-up [32, Level of evidence IB].
In a case series, four SOTR patients were treated in a sequential fashion of light curettage of hypertrophic AKs, with 5 days of twice daily 5-fluoruracil 5 % cream to face, and photodynamic therapy (PDT) on day 6 with a 1-h pretreatment. Patients were able to tolerate regimen with clearance of 1 to 6 months [33, Level of evidence III].
Successful treatment outcomes require a high level of patient compliance. We typically repeat 5-fluoruracil cream treatment as needed every 1 to 3 years, and as frequently as every 4–6 months, but only after clinical evaluation of the field to be treated. There is some concern that use can alter the contiguous nature of certain tumors which could decrease the efficacy of MMS. Hyperkeratotic skin or areas resistant to any improvement may benefit from pretreatment with 3 to 4 weeks of tretinoin 0.1 % cream, urea cream, or Am-Lactin 12 % cream. In addition, areas of severe photodamage and hyperkeratoses can be treated with occlusion “chemowraps.” Our protocol for 5FU chemo wraps entails weekly visits and topical application followed by occlusion with zinc oxide and crepe bandages for 4–8 weeks as tolerated. Treatment hiatus is allowed as needed for 1 week. There is also some exciting evidence not yet published from Demehri et al. demonstrating a synergistic benefit of combining twice daily 5-fluoruracil 5 % cream with twice daily calcipotriene 0.005 % cream.
Imiquimod
Prevention
In a case series, two non-SOTR patients with field AKs over the face and scalp received sequential treatment of PDT followed 2 weeks later by imiquimod 5 % cream three times a week for 8 weeks. At 7 and 11-month follow-up, neither patient had any AK recurrence [34, Level of evidence III].
Ingenol mebutate
Prevention
In a randomized, single-site, 3-cohort study, 24 patients with 4–8 AKs in a contiguous 25 cm2 on the face received either sequential PDT two times separated by 4 weeks, sequential PDT followed 2 weeks later by 3 days of ingenol mebutate 0.015 % gel, or igenol mebutate alone. All modalities reduced AKs by greater than 86 % and a non-significant local skin reaction score trend favored PDT and sequential PDT and igenol mebutate over ingenol mebutate alone [35, Level of evidence IIB].
Interventional procedures
Chemical peels
-
Can be used in a fashion similar to topical 5-fluoruracil or imiquimod as field therapy and prevention for patients at high risk for multiple skin cancers
Standard procedure: medium depth peels, typically trichloroacetic acid (TCA) 20–30 % with or without Jessner’s solution are used to treat larger areas of precancer and actinic damage.
Contraindictions: not recommended for skin types V and VI.
Complications: the most common complications with chemical peeling is hyper or hypopigmentation. Erythema is expected after chemical peeling and the duration is dependent on the depth of the chemical peel.
Special Points: typically used for arms and scalp areas. Easy to perform with single-day application and can be repeated.
Photodynamic therapy
Standard procedure: photodynamic therapy (PDT) is a treatment using a photosensitizing agent (5-aminolevulinic acid, methylaminolevulinate) applied to the skin followed by activation with a light source (blue light, red light, laser light, or ambient daylight). Improved delivery and penetration of the medication can be aided by curettage or laser-assisted delivery. There are several different protocols reported in the literature.
Contraindictions: patients with sensitivity at 400–450 nm, porphyria, allergies to porphyrins, known sensitivity to photosensitizing agent.
Complications: excessive phototoxic reactions, pigmentary abnormalities, hypersensitivity reactions.
Special points: PDT should be used as a preventive measure in high-risk patients for treatment of actinic keratoses and is not a standard primary treatment for high-risk individual skin cancers.
Prevention
A randomized clinical trial of 16 patients with 542 AKs combination ablative fractional laser (AFL) and daylight-mediated PDT for AKs in SOTRs revealed improved complete response (CR) rates of 74 % compared to 46 % after daylight PDT alone, 50 % after conventional PDT, and 5 % after AFL alone [36, Level of evidence IB].
Additionally, benefit from PDT is highly dependent on the immediate preprocedure skin preparation. Pretreatment of hyperkeratotic areas with tretinoin 0.1 % cream for a month prior to the procedure is also helpful. We incubate with ALA for at least 1 h and will repeat as often as every 6 to 12 months in patients with extensive photo damage. Other protocols such as daylight PDT and no incubation PDT with extended light exposure have been reported to have no to minimal discomfort with similar results. We have not used these at our practice as results are still early in terms of permanence of effect. Extremities can be more difficult to treat evenly due to patient positioning, limb caliber, and body hair. Therefore, we tend to recommend topical field therapies in these areas. Occlusion with saran wrap does increase cellular uptake of ALA and can offset the effect of light source spacing. We also do not use PDT on the scalp unless the patient has a significant amount of alopecia, >90 % of treatment area.
Laser treatment
Standard procedure: laser-assisted delivery of topical preventive therapies or photodynamic therapy may be used for improved penetration and efficacy of the medication, particularly in hyperkeratotic actinic keratoses. Ablative lasers (C02, Erb:YAG) have been reported for use in low-risk superficial NMSCs; however, this is not considered standard of care and is not recommended in high-risk patients or tumors.
Contraindictions: ablative lasers are typically not recommended for skin types IV, V, and VI.
Complications: prolonged erythema, hyper/hypopigmentation, infection, herpes reactivation, scarring, koebnerization, contact dermatitis.
Primary treatment
Two cases of superficial and nodular BCC were treated using 3D mapping with reflectance confocal microscopy (RCM) for guided laser ablation with an Erbium:YAG [37, Level of evidence IV].
Primary treatment
A single-blinded 18-month clinical trial of nodular BCCs ablated using a diode laser under ultrasound control. Half were treated 3 weeks later with fractional carbon dioxide laser followed by ALA-PDT, the other half were treated using curettage (control). Of tumors, 93 % (52/56) pretreated with the carbon dioxide laser responded to ALA-PDT compared to 80 % (45/56) in the control group [38, Level of evidence IB].
Surgery
Surgical excision
Standard procedure: excision with postoperative margin assessment is not the preferred treatment but can be used as primary therapy for high-risk skin cancers as determined by tumor and patient-related factors. Standard vertical section processing allows the pathologist to only examine representative areas of the peripheral and deep margins. Standard “bread-loaf” techniques take vertical sections at 2–4 mm intervals allowing examination of less than 0.01 % of the surface area of the specimen [39]. It has been estimated that this technique is at best 44 % sensitive in detecting residual tumor at the surgical margin on re-excision [40, Level of evidence IV].
Standard margins for low-risk BCCs are 4 mm. 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend wider surgical margins with linear or delayed repair or Mohs surgery (see below) for high-risk BCCs. Consensus recommendations for wider surgical margins have not been clearly defined although certain subtypes, such as morpheaform, have been shown to require 13–15 mm margins to achieve 95 % clearance [41, Level of evidence IV].
For low-risk SCCs excision with 4-mm margins is recommended to achieve a 95 % chance of clearance [42]. Wider surgical margins or Mohs surgery or resection with complete margin assessment (see below) are recommended for high-risk SCCs. Although there is no consensus recommendation for larger margins, the Interdisciplinary European guidelines for management of invasive squamous cell carcinoma were recently published and recommended 10-mm margins for high-risk SCCs [43].
Contraindictions: inability to tolerate surgery under local anesthesia.
Complications: serious complications are rare; more common complications include infection, hematoma/seroma formation, wound dehiscence, necrosis, scarring, nerve damage, and cosmetic asymmetry.
Special Points: NCCN states that excision with Mohs and comprehensive complete margin assessment is the preferred technique for high-risk BCC and SCC.
Mohs micrographic surgery
Standard procedure: Mohs micrographic surgery (MMS) is the gold standard for removing high-risk and recurrent cutaneous tumors and involves surgical extirpation under local anesthesia with complete histologic margin assessment allowing for high cure rates and tissue conservation. Appropriate use criteria were recently developed for most types of cutaneous tumors and include high-risk BCC and SCC tumors as well as other rare tumors at high risk for metastasis [44].
Contraindictions: inability to tolerate surgery under local anesthesia.
Complications: serious complications are rare; more common complications are similar between Mohs surgery and standard surgical excision including infection, hematoma/seroma formation, wound dehiscence, necrosis, scarring, nerve damage, and cosmetic asymmetry.
Primary
A randomized trial of MMS versus surgical excision (SE) for high-risk facial BCC (diameter at least 1 cm, H-zone location, or aggressive histologic subtype) was completed with 10 years of follow-up. The 10-year recurrence rates were 4.4 % for MMS and 12.2 % for SE for treatment of primary tumors. For recurrent tumors, the 10-year recurrence rates were 3.9 % for MMS and 13.5 % for SE [45••, Level of evidence IB].
Primary
A Cochrane database systematic review was performed to evaluate the effectiveness, cost, and complication rates of MMS versus surgical excision for periocular BCC; however, no randomized trials were found to be eligible for inclusion in the review. There remains a paucity of data for treatment of high-risk periocular lesions [46].
Radiation
Standard procedure: radiation can be used as a primary or adjuvant therapy for high-risk skin cancer. Primary radiation therapy is typically reserved for patients who are not appropriate surgical candidates, particularly the elderly or those with multiple medical comorbidities as the cure rate is less than both surgical excision and Mohs micrographic surgery. In addition, cure rates with primary radiation reduce with increasing size and thickness of the tumor [47]. Adjuvant radiation may be considered in patients with extensive or large caliber perineural invasion, with regional metastasis or high-risk for regional metastasis [48]. The typical radiation regimen requires daily treatment for 3–6 weeks with delivery of external beam photons (75–150 kV orthovoltage) or electrons (6 to 12 MeV). Electronic brachytherapy (EBT) using surface applicators has also been reported for low-risk lesions and will not be covered here.
Contraindictions: collagen vascular diseases, inherited hypersensitivity syndromes, recurrent tumors, younger age (relative), certain anatomic sites (below the knee, hands) where healing will likely be poor.
Complications: radiation dermatitis and telangiectasias, alopecia, long-term risk of second malignancy in treatment field, off-target side effects and damage.
Special points: recurrent lesions have significantly lower cure rates. Immunosuppressed patients may have decreased response rates compared to those with intact immune systems.
Primary
A review was performed of BCCs treated with radiotherapy from 1984 to 2013. Thirteen papers were included in the final analysis, 11 are cohort studies and two were randomized trials utilizing radiotherapy for BCC. The overall cure rates ranged from 79.2 to 100 % (2 year cure rates ranged from 86.6 to 100 %; 5-year cure rates were 79.2 to 95.8 %) This study did not specifically include high-risk lesions [49, Level of evidence IIA].
Other treatments
-
Electronic skin surface brachytherapy is being evaluated for the treatment of patients with non-melanoma skin cancer (typically not recommended in high-risk patients or tumors).
Emerging therapies
-
There are several Hedgehog inhibitors in development for locally advanced and metastatic BCC
-
There is an ongoing multicenter clinical trial evaluating vismodegib in the neoadjuvant setting followed by surgery for operative BCCs.
-
An oncolytic virus, Tilimogene laherparepvec (often called “T-VEC”), is being evaluated for non-operable or metastatic cutaneous SCC
-
There is an ongoing Phase II study of radiation therapy with vismodegib for advanced head and neck basal cell carcinoma
-
There are ongoing studies of topical itraconazole application for basal cell carcinoma
-
Buparlisib, a pan-PI3K inhibitor is being investigated in combination with sonidegib for locally advanced and metastatic BCC.
-
Erlotinib, an EGFR tyrosine kinase inhibitor, for the treatment of recurrent or metastatic SCC
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, Hwang WT, Gelfand JM, Whalen FM, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA dermatol. 2013;149(4):402–10 In a retrospective cohort study, a proposed alternative staging system to the AJCC stratifies T2 tumors and highlights a sub grouping, T2b, that is responsible most poor outcomes.
O'Bryan K, Sherman W, Niedt GW, Taback B, Manolidis S, Wang A, et al. An evolving paradigm for the workup and management of high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2013;69(4):595–602.e1 A retrospective case review of 27 patients in an effort to establish an aggressive protocol for the management of high risk and “very” high risk SCC. Very high risk SCC may have improved outcome cetuximab, radaition, and surgical resection.
Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA dermatol. 2015;151(10):1081–6.
Ozgediz D, Smith EB, Zheng J, Otero J, Tabatabai ZL, Corvera CU. Basal cell carcinoma does metastasize. Dermatol Online J. 2008;14(8):5.
Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149(5):615–6.
Sutton A, Crew A, Wysong A. Redefinition of skin cancer as a chronic disease. JAMA Dermatol. 2015;1-2.
Wehner MR, Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Chren MM. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol. 2015;151(4):382–8 A prospective observational study of 1,284 patients with a mean of 5.7 years of follow up that established many aspects of risk of NMSC development.
Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40(2 Pt 1):177–86.
Frierson Jr HF, Deutsch BD, Levine PA. Clinicopathologic features of cutaneous squamous cell carcinomas of the head and neck in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hum Pathol. 1988;19(12):1397–402.
Davis S, Capjack L, Kerr N, Fedosejevs R. Clothing as protection from ultraviolet radiation: which fabric is most effective? Int J Dermatol. 1997;36(5):374–9.
Isedeh P, Osterwalder U, Lim HW. Teaspoon rule revisited: proper amount of sunscreen application. Photodermatol Photoimmunol Photomed. 2013;29(1):55–6.
Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138(6):838–9.
Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61(4):685–92.
Chang YJ, Myung SK, Chung ST, Kim Y, Lee EH, Jeon YJ, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatol (Basel, Switzerland). 2011;223(1):36–44.
Chen AC, Martin AJ, Choy B, Fernandez-Penas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618–26 Nicotinamide in a phase 3, double blinded, randomized controlled trial, dosed at 500 mg orally twice daily for one year created a 23% reduction in new NMSCs and 13% reduction in actinic keratoses.
RED BOOK Online: Truven Health Analytics; 2016 [02/15/2016]. Available from: www.micromedexsolutions.com.
Nakamura K, Okuyama R, Saida T, Uhara H. Platinum and anthracycline therapy for advanced cutaneous squamous cell carcinoma. Int J Clin Oncol. 2013;18(3):506–9.
Endrizzi B, Ahmed RL, Ray T, Dudek A, Lee P. Capecitabine to reduce nonmelanoma skin carcinoma burden in solid organ transplant recipients. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al]. 2013;39(4):634–45.
Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–99.
Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(8):745–51 In an open label phase 2 trial of itraconazole for BCC, overall tumor size was decreased by 24%, cell proliferation by 45%, and activated hedgehog pathway activity by 65%.
Migden MR, Guminski A, Gutzmer R, Dirix L, KD L, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–28 In an ongoing multicenter randomized, double blinded phase 2 trial, patients received either 200 mg or 800 mg of sonidegib. The 200 mg and 800 mg group achieved an objective response of 36% and 39% respectively.
Danial C, Sarin KY, Oro AE, Chang AL. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015.
Chang AL, Solomon JA, Hainsworth JD, Goldberg L, McKenna E, Day BM, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60–9 In an open label, 2-cohort, multicenter study of vismodegib, patients received 150 mg daily until tumor progression or intolerable toxicity. 119 patients received a median of 5.5 months of treatment, with objective response in 46% of laBCC and 30% in mBCC.
Basset-Seguin N, Hauschild A, Grob JJ, Kunstfeld R, Dreno B, Mortier L, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16(6):729–36.
Ally MS, Aasi S, Wysong A, Teng C, Anderson E, Bailey-Healy I, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71(5):904–11.e1 An open label trial to assess change in target tumor surgical defect area, pre and post vismodegib demonstrated a 27% reduction in area from baseline.
Sofen H, Gross KG, Goldberg LH, Sharata H, Hamilton TK, Egbert B, et al. A phase II, multicenter, open-label, 3-cohort trial evaluating the efficacy and safety of vismodegib in operable basal cell carcinoma. J Am Acad Dermatol. 2015;73(1):99–105.e1.
Reigneau M, Robert C, Routier E, Mamelle G, Moya-Plana A, Tomasic G, et al. Efficacy of neoadjuvant cetuximab alone or with platinum salt for the treatment of unresectable advanced nonmetastatic cutaneous squamous cell carcinomas. British J Dermatol. 2015;173(2):527–34 In an open label, single site, 2-cohort study, patients with locally advanced SCC considered to be inoperable were pretreated with one cycle of cetuximab, cisplatin, or carboplatin, plus 5-FU. 92% of patients on combination therapy became amenable to surgery and 78% achieved complete remission.
Heath CH, Deep NL, Nabell L, Carroll WR, Desmond R, Clemons L, et al. Phase 1 study of erlotinib plus radiation therapy in patients with advanced cutaneous squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(5):1275–81.
Desai A, Kartono F, Del Rosso JQ. Systemic retinoid therapy: a status report on optimal use and safety of long-term therapy. Dermatol Clin. 2007;25(2):185–93 vi.
Bavinck JN, Tieben LM, Van der Woude FJ, Tegzess AM, Hermans J, ter Schegget J, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol Off J Am Soc Clin Oncol. 1995;13(8):1933–8.
Kadakia KC, Barton DL, Loprinzi CL, Sloan JA, Otley CC, Diekmann BB, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (north central cancer treatment group study 969251). Cancer. 2012;118(8):2128–37.
Pomerantz H, Hogan D, Eilers D, Swetter SM, Chen SC, Jacob SE, et al. Long-term efficacy of topical fluorouracil cream, 5 %, for treating actinic keratosis: a randomized clinical trial. JAMA dermatology. 2015;151(9):952–60.
Jambusaria-Pahlajani A, Ortman S, Schmults CD, Liang C. Sequential curettage, 5-fluorouracil, and photodynamic therapy for field cancerization of the scalp and face in solid organ transplant recipients. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al]. 2016;42(Suppl 1):S66–72.
Held L, Eigentler TK, Leiter U, Garbe C, Berneburg MJ. Effective combination of photodynamic therapy and imiquimod 5 % cream in the treatment of actinic keratoses: three cases. Biomed Res Int. 2013;2013:102698.
Berman B, Nestor MS, Newburger J, Park H, Swenson N. Treatment of facial actinic keratoses with aminolevulinic acid photodynamic therapy (ALA-PDT) or ingenol mebutate 0.015 % gel with and without prior treatment with ALA-PDT. J drugs Dermatol JDD. 2014;13(11):1353–6.
Togsverd-Bo K, Lei U, Erlendsson AM, Taudorf EH, Philipsen PA, Wulf HC, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. British J Dermatol. 2015;172(2):467–74.
Chen CS, Sierra H, Cordova M, Rajadhyaksha M. Confocal microscopy-guided laser ablation for superficial and early nodular basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA dermatol. 2014;150(9):994–8.
Lippert J, Smucler R, Vlk M. Fractional carbon dioxide laser improves nodular basal cell carcinoma treatment with photodynamic therapy with methyl 5-aminolevulinate. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al]. 2013;39(8):1202–8.
Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73(3):492–7.
Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53(3):469–74.
Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatologic Surg Oncol. 1991;17(7):574–8.
Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27(2 Pt 1):241–8.
Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer (Oxford, England: 1990). 2015;51(14):1989–2007.
Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al]. 2012;38(10):1582–603.
van Loo E, Mosterd K, Krekels GA, Roozeboom MH, Ostertag JU, Dirksen CD, et al. Surgical excision versus Mohs' micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. European journal of cancer (Oxford, England: 1990). 2014;50(17):3011–20 A randomized trial of MMS versus surgical excision (SE) for high risk facial BCC. The 10-year recurrence rates were 4.4% for MMS and 12.2% for SE for treatment of primary tumors.
Narayanan K, Hadid OH, Barnes EA. Mohs micrographic surgery versus surgical excision for periocular basal cell carcinoma. Cochrane database Syst Rev. 2014;12:Cd007041.
Kwan W, Wilson D, Moravan V. Radiotherapy for locally advanced basal cell and squamous cell carcinomas of the skin. Int J Radiat Oncol Biol Phys. 2004;60(2):406–11.
Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthetic Dermatol. 2010;3(4):39–48.
Cho M, Gordon L, Rembielak A, Woo TC. Utility of radiotherapy for treatment of basal cell carcinoma: a review. British J Dermatol. 2014;171(5):968–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
James W. Behan, Adam Sutton, and Ashley Wysong declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Behan, J.W., Sutton, A. & Wysong, A. Management of Skin Cancer in the High-Risk Patient. Curr. Treat. Options in Oncol. 17, 60 (2016). https://doi.org/10.1007/s11864-016-0435-z
Published:
DOI: https://doi.org/10.1007/s11864-016-0435-z