Opinion statement
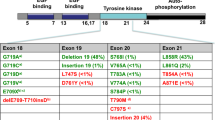
Epidermal growth factor receptor (EGFR) mutations have been detected in approximately 10 % of North American patients diagnosed with non-small cell lung cancer (NSCLC). Approximately 90 % of these mutations are exon 19 deletions or exon 21 L858R point mutations. First- and second-generation EGFR tyrosine kinase inhibitors (TKIs) are approved as first-line therapy based on clinical trials demonstrating superior response rates, progression free survival (PFS), and overall survival (OS) compared to chemotherapy in patients with EGFR mutation-positive NSCLC treated with an EGFR TKI prior to chemotherapy. However, the majority of patients treated with an EGFR TKI develop resistance to therapy within about 12 months, approximately 50 % of patients due to a second site mutation, the T790M mutation occurring within exon 20. At the time of progression, the EGFR TKI is most commonly discontinued and a different systemic therapy is initiated. However, oncogene addiction persists and recent exciting data with third-generation EGFR TKIs suggests that acquired resistance may be surmountable. The newest EGFR TKIs have shown activity against EGFR-mutant NSCLC after progression on first-generation TKIs, including those with T90M, while sparing wild-type EGFR and hence appear to be both well tolerated and efficacious. At this time, it appears that third-generation EGFR TKIs are effective following first-generation therapy, and determining the most appropriate sequence to maximize overall survival is a matter of ongoing investigation. As the arsenal of active agents in EGFR mutant NSCLC grows, future research into potential combinations, optimal timing, and resistance mechanisms of these new treatments, as well as their possible role in the adjuvant, post-chemoradiation, and neoadjuvant settings holds great promise for this group of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple guideline panels recommend that all patients with newly diagnosed non-small cell lung cancer (NSCLC) should undergo molecular testing to determine whether they have genetic alterations that would make them candidates for targeted therapy. The epidermal growth factor receptor (EGFR) belongs to a family or receptor tyrosine kinases (RTKS) that include EGFR/ERBB1, HER2/ERBB2, HER3/ERBB3, and HER4/ERBB4. When stimulated, the transmembrane receptors trigger a cascade of intracellular signaling that affects cellular proliferation and apoptosis. To date, the EGFR inhibitors used in the treatment of NSCLC fall into two main categories: monoclonal antibodies to the extracellular domain of the EGFR (e.g., cetuximab and panitumumab) or small molecules that inhibit the intracellular tyrosine kinase inhibitor (TKI) domain by interfering with autophosphorylation by adenosine. Sensitizing EGFR mutations are found in approximately 10 % of Caucasian patients with NSCLC and up to 50 % of Asian patients [1]. Approximately 90 % of these mutations are exon 19 deletions or exon 21 L858R point mutations [2, 3]. EGFR TKIs are approved as first-line therapy in patients with advanced NSCLC harboring the EGFR mutation. Unfortunately, all patients with EGFR mutant tumors treated with EGFR TKIs eventually develop acquired resistance. Disease progression usually occurs around 12 months. Approximately 50 % of patients with acquired resistance have tumors that harbor a second site mutation, most commonly the T790M mutation occurring within exon 20 [4–8]. At the time of progression, the EGFR TKI is generally discontinued prior to initiating subsequent therapy. This paper reviews progress in the last decade in the treatment of EGFR mutation-positive NSCLC patients.
First-generation EGFR inhibitors
Erlotinib was the first EGFR TKI to receive FDA approval for the treatment of NSCLC patients based on a randomized phase III trial comparing erlotinib to placebo in an unselected patient population who had progressed following first- or second-line chemotherapy. In this trial, patients were randomized in a 2:1 ratio to single-agent erlotinib or placebo. There was a significant improvement in OS for patients receiving erlotinib compared to placebo (6.7 vs. 4.7 months). There was also a significant improvement in 1-year survival (31 vs. 22 %) as well as RR (8.9 vs. <1 %) and the time to deterioration of tumor-related symptoms of pain, cough, and dyspnea [3]. A trial of similar design failed to demonstrate a survival advantage with gefitinib compared to placebo, although the trends in time to progression and overall survival favored the gefitinib arm [9] leading to approval of gefitinib in other countries. Notably, the start of the aforementioned placebo-controlled phase III trials predates the discovery of somatic mutations in the EGFR gene that appear to be associated with clinical responses to EGFR TKIs. However, certain clinical features were associated with response including gender (female), smoking status, Asian ethnicity, and adenocarcinoma histology.
Based on this data, the Iressa Pan Asian Study (IPASS) compared first-line gefitinib to carboplatin and paclitaxel in patients with stage IV non-small cell lung cancer. This trial was designed to enroll patients who were clinically more likely to respond to an EGFR TKI, and tumor samples were analyzed retrospectively for the presence or absence of an EGFR mutation. This was the first trial to demonstrate an improvement in response rate and 12-month progression-free rate in patients with EGFR mutation-positive NSCLC treated with an EGFR TKI compared to chemotherapy (71.2 vs. 47.1 % and 25 vs. 7 %, hazard ratio (HR) for progression or death 0.74, respectively). Several trials comparing gefitinib and erlotinib to chemotherapy in EGFR mutation-positive NSCLC patients have demonstrated similar results with progression-free survival (PFS) ranging from 9.2 to 13.1 months and no significant improvement in OS; the lack of benefit in OS is thought to be due to high rates of crossover to the EGFR TKI after progression with chemotherapy [10–14].
Combinations of targeted therapies have also been explored with EGFR TKIs. A recent phase II trial compared erlotinib to the combination of erlotinib and bevacizumab, a monoclonal antibody to VEGF [15]. This trial demonstrated a superior PFS for combination therapy compared to erlotinib alone although with higher toxicities in the combination treatment group, specifically hypertension and proteinuria.
Icotinib is an oral, small-molecule EGFR TKI which has exhibited anti-tumor activity against EGFR-mutated NSCLC in preclinical studies [21]. It has a shorter half-life (6–8 h) than gefitinib or erlotinib [16, 17]. A phase III double-blind non-inferiority trial (ICOGEN) compared icotinib versus gefitinib in an unselected Chinese patient population with advanced NSCLC who had received at least one line of platinum-based chemotherapy [18]. Patients were randomized to either icotinib (125 mg three times daily) or gefitinib (250 mg daily). Common adverse events with icotinib included rash (41 %) and diarrhea (22 %) typical of EGFR TKIs. There was a nonsignificant improvement in PFS with icotinib compared to gefitinib, at 4.6 vs. 3.4 months, respectively, which met the study’s endpoint for non-inferiority and led to its approval in China.
Given the modest and non-overlapping toxicities observed with EGFR TKIs compared to chemotherapy, four large randomized trials compared chemotherapy with or without erlotinib or gefitinib in unselected patient populations and failed to demonstrate an improvement in RR, PFS, or OS [19–22]. Preclinical studies have suggested that treatment with an EGFR TKI arrests cells in G1 phase, preventing chemotherapy, which acts in the S or G2/M phase, from exerting cytotoxic effects. The First-line Asian Sequential Tarceva and Chemotherapy Trial (FASTACT) was a phase II trial that evaluated chemotherapy (day 1 and 8) intercalated with erlotinib (day 15 to 28) in an unselected patient population with advanced NSCLC [23]. This trial demonstrated a significant improvement in PFS for patients treated with intercalated therapy and prompted a second trial, FASTACT 2, a phase III trial including patients from the same patient population. This trial also demonstrated a superior PFS (7.6 vs. 6.0 months) and OS (18.3 vs. 15.2 months) for combination therapy. Of the 451 patients enrolled, tumor samples for EGFR mutation testing were available in 241 patients, 97 (40 %) of whom harbored an EGFR mutation. A subset analysis suggested the benefit of intercalated therapy was confined only to patients with EGFR mutation-positive NSCLC [24].
Prior studies of EGFR TKIs in the adjuvant setting have not shown a benefit in overall survival with their use, although limitations with trial design should be noted. NCI-C BR19 was a phase III trial evaluating adjuvant gefitinib vs. placebo in patients with stage IB, II, or IIIA resected NSCLC. The trial was halted early after enrollment of 503 patients as no benefit was seen in the gefitinib arm as compared to placebo. EGFR mutation status was later determined in 71 % of specimens; of the 359 tumors tested, only 15 (4 %) were found to have EGFR mutations [25]. The RADIANT trial compared adjuvant erlotinib to placebo in patients with resected stage I-IIIA NSCLC. Of the 973 patients enrolled, 72 % were EGFR positive by FISH and 16.5 % had documented EGFR mutations, deletion 19 or L858R. While there was no improvement in disease-free survival (DFS) with erlotinib compared to placebo (median DFS 50.5 vs. 48.2 month, respectively), there was a trend towards improved DFS with erlotinib in a subset analysis of patients who were EGFR mutation positive (DFS of 46.4 months with erlotinib and 28.5 months with placebo; HR = 0.61; 95 % CI = 0.38–0.98, p = 0.039) [26]. Due to hierarchical testing procedures, this difference was not considered statistically significant. There was also no improvement in OS for patients treated with erlotinib regardless of molecular status, an important consideration for adjuvant trials, where the goal of therapy is cure. The SELECT trial was a nonrandomized phase II trial that enrolled only patients with resected stage IA-IIIA EGFR-mutant NSCLC. Patients were treated with erlotinib 150 mg daily for 2 years after completing standard adjuvant chemotherapy and/or radiotherapy. The disease free survival rate of 90 % at 2 years (97 % stage 1, 73 % stage 2, and 92 % stage 3) was considered superior to historical genotype-matched controls; most recurrences happened over 12 months after stopping treatment [27]. ALCHEMIST is a phase III trial initiated in 2014 with the goal of exploring the role of molecularly targeted therapy in the adjuvant setting; unlike previous adjuvant trials, all patients will undergo centralized molecular testing prior to enrollment [28]. Resected tumor specimens will be tested for both ALK rearrangements and EGFR mutations; patients harboring one of these genetic alterations will then be referred into either the ALCHEMIST-ALK or ALCHEMIST-EGFR trials, where they will receive crizotinib or erlotinib vs. placebo, respectively, after completing standard adjuvant chemotherapy.
Second-generation EGFR inhibitors
Second-generation EGFR TKIs (e.g., HKI-272 (neratinib), BIBW-2992 (afatinib), and PF-00299804 (dacomitinib)) are more potent than gefitinib and erlotinib against EGFR T790M [6, 29]. However, because they inhibit drug-sensitive mutants at lower doses than they inhibit the T790M mutant, they still select for T790M-harboring clones in models of acquired resistance in vitro [29].
Afatinib is an irreversible ErbB-family blocker. A randomized phase IIb/III trial (LUX-1 trial) compared afatinib to placebo in patients who had been treated with an EGFR TKI (erlotinib or gefitinib) for at least 12 weeks prior and had disease progression [30]. This study did not meet its primary endpoint of improved OS in patients treated with afatinib compared to placebo (10.8 vs. 12.0 months, HR = 1.09, 95 % CI = 0.86–1.35). There were 29 (7 %) responses observed in afatinib-treated patients compared to 0.5 % for patients receiving placebo (p < 0.01). The presence of a known EGFR mutation was not required and over half of patients were on a first line EGFR TKI for less than 48 weeks prior to entry onto study. Of the 585 patients enrolled, 141 patients had tissue available for EGFR mutation testing of which 96 were EGFR mutation positive. In a subset analysis of patients with EGFR mutation-positive lung cancer, PFS was longer for patients treated with afatinib compared to placebo (3.3 vs. 1.0 months, p = 0.009). This difference was not observed in patients who were EGFR mutation negative. LUX-LUNG 8 was a randomized, phase III trial comparing afatinib and erlotinib in patients with advanced squamous NSCLC who progressed on platinum-based chemotherapy. Median PFS and disease control rate were higher in the afatinib arm compared with erlotinib at 2.4 vs. 1.9 months (p = 0.0427) and 45.7 vs. 36.8 % (p = 0.020), respectively [31].
The LUX-2 trial evaluated afatinib as first-line therapy in EGFR mutation-positive NSCLC who had progression on up to one prior chemotherapy regimen and were EGFR-TKI naïve [32]. One hundred and twenty-nine patients were treated at two different doses of afatinib. The primary endpoint was response rate. Ninety-nine patients received a starting dose of 50 mg daily and 30 patients a starting dose of 40 mg daily. Forty-eight percent (61/129) of the patients received afatinib as a first-line therapy, and 62 % (68/129) patients had received at least one prior line of chemotherapy. Overall, a response was noted in 61 % of patients. Patients with L858R mutations and deletions on exon 19 were the most sensitive to afatinib with 66 % of those patients demonstrating a response in comparison to 39 % of patients harboring other types of EGFR mutations. Two patients had a complete response to therapy. Response rates were similar for patients taking 40 mg daily (60 %) and 50 mg daily (62 %). Ninety percent of patients receiving afatinib as a second-line therapy received the higher dose (50 mg daily). The median OS was 24 months with median PFS of 14 months. Patients with deletion 19 mutations had a PFS of 16.1 months and patients with L8585R had a shorter PFS of 13.7 months. Grade 3 side effects were significantly higher in patients taking 50 mg daily (diarrhea 22 % and rash 28 %) in comparison to those patients taking 40 mg daily (7 % experienced grade 3 diarrhea and rash). Therefore, efficacy was maintained at a lower dose (40 mg/daily) while decreasing the frequency of grade 3 toxicities. Two large randomized phase III trials, LUX-3 and LUX-6, compared afatinib to standard first-line chemotherapy cisplatin and gemcitabine (LUX-3) or cisplatin and pemetrexed (LUX-6) in NSCLC patients with advanced stage disease, positive for the EGFR mutations [33, 34]. Both trials reported a significant improvement in PFS with no significant improvement in OS. Recently, a combined analysis of both trials demonstrated a striking improvement in OS in patients with the deletion 19 mutation treated with first-line afatinib compared to chemotherapy (LUX-Lung 3—HR = 0.54, 95 % CI = 0.36–0.79, p = 0.0015; LUX-Lung 6—HR = 0.64, 95 % CI = 0.44–0.94, p = 0.023) [35•]. This benefit was not seen in patients who were L858R positive. LUX-Lung 7, a second head-to-head trial evaluating afatinib versus gefitinib as a first-line treatment in EGFR mutation-positive NSCLC patients, is currently ongoing (NCT01466660).
Neratinib is an irreversible, small molecule inhibitor of both EGFR (HER1) and HER2 which was found to have preclinical activity against EGFR-mutated NSCLC, including cell lines harboring a T790M resistance mutation [36]. A phase I study of neratinib in 72 patients with advanced solid tumors, 21 % of whom had NSCLC, determined the maximum tolerated dose to be 320 mg daily, with grade 3 diarrhea as the dose-limiting toxicity [37]. A subsequent phase II trial of 167 previously treated patients with advanced NSCLC, 91 of whom had an EGFR mutation, demonstrated disappointing results with an ORR of 3 % in patients with EGFR-mutated NSCLC, and no responses in patients with wild-type EGFR [38]. Of note, the dose administered in this trial was reduced to 240 mg daily due to a high incidence of grade 3 diarrhea.
Dacomitinib (PF299804) is an irreversible pan-HER inhibitor targeting EGFR (HER1), HER2, and HER4 tyrosine kinases that was shown to have preclinical activity in both gefitinib-sensitive and gefitinib-resistant NSCLC models [39]. A phase I study of 121 patients, 57 of which had NSCLC, determined a maximum tolerated dose of 45 mg, with dose-limiting stomatitis and skin toxicity observed [40]. This dose has since been evaluated in a phase III trial of patients with advanced NSCLC who had progressed on 1 to 3 prior lines of chemotherapy as well as a reversible EGFR TKI; notably, patients with both wild-type and mutant EGFR tumors were included [41]. Four hundred eighty patients were randomized to either dacomitinib or placebo in a 2:1 ratio. While no overall survival advantage was seen (median OS 6.83 months with dacomitinib vs. 6.31 months with placebo), there was a small improvement in median PFS with dacomitinib compared to placebo (2.66 vs. 1.38 months; HR = 0.66, 95 % CI = 0.55–0.79, p < 0.001). Improvement in median PFS was highest among patients with a documented EGFR mutation (3.52 months, 95 % CI = 2.53–3.68), but this did not translate into an overall survival advantage for this group. A phase II study of dacomitinib in treatment-naïve patients with advanced NSCLC and either EGFR mutations or clinical characteristics associated with EGFR mutations found an ORR of 53 % and median PFS of 11.5 months [42]. Common treatment-related adverse events included diarrhea (93 %), acneiform rash (78 %), dry skin (44 %), and stomatitis (40 %). Dacomitinib has also been compared to erlotinib in a phase III trial (ARCHER 1009) in an unselected group of heavily pretreated patients with NSCLC who were EGFR TKI-naïve; no benefit in overall or progression-free survival with dacomitinib over erlotinib was observed [43].
Treatment on progression
Unfortunately, all patients with EGFR mutant tumors treated with EGFR TKIs will eventually develop progressive disease, a concept referred to clinically as acquired resistance. Acquired resistance to EGFR TKIs typically occurs around 12 months after initiation of therapy. The most common mechanism of acquired resistance to gefitinib/erlotinib—occurring in approximately 50 % of cases—is the T790M second-site mutation within EGFR exon 20 [44, 45]. This “gatekeeper” mutation, which involves a threonine-to-methionine substitution in exon 20, increases the affinity of the mutant EGFR receptor for ATP, thereby competitively inhibiting the binding ability of reversible TKIs. Other mechanisms of resistance include EGFR gene amplification, activation of bypass signaling pathways, and histologic transformation to small cell lung cancer [46–48]. Progression in so-called “sanctuary sites” such as the central nervous system are also observed, and thought to be due to poor drug penetration of these areas. At the time of progression, there is no standard of care treatment regimen and the choice of therapy is determined by the number of sites of progression and location of disease progression [49].
Multiple treatment strategies have been employed to attempt to delay or overcome acquired resistance to EGFR TKIs. These strategies include later generation EGFR inhibitors, rationale combinations of targeted small molecule inhibitors and/or monoclonal antibodies, and the addition of traditional cytotoxic chemotherapy to EGFR TKI therapy at the time of progression. A retrospective study of 70 patients with EGFR mutant NSCLC and acquired resistance to an EGFR TKI suggested an improvement in response rate for combination chemotherapy with an EGFR TKI compared to chemotherapy alone (41 vs. 18 %) [50]. This was further explored in the IMPRESS trial, a randomized phase III study comparing cisplatin and pemetrexed for 6 cycles with or without gefitinib in patients with acquired resistance to gefitinib [51]. This trial failed to demonstrate a significant improvement in PFS with no improvement in OS for patients treated with an EGFR TKI and chemotherapy compared to chemotherapy alone, suggesting that the TKI should be discontinued in patients who are starting chemotherapy at the time of progression. The ASPIRATION trial was a phase II trial in which treatment-naïve patients with EGFR-mutated NSCLC were treated with erlotinib 150 mg daily, and then allowed to continue erlotinib beyond RECIST-defined progression at the discretion of the investigator, provided certain criteria were met. These criteria included slow PD (>6 months of partial response/stable disease), asymptomatic minimal PD, or a new brain metastasis that responded to a local therapy. The median interval between PD1 and subsequent progression was 3.7 months, demonstrating that erlotinib continuation is possible in selected patients, albeit for a limited period of time [52].
Third-generation EGFR inhibitors
The most recently developed EGFR TKIs were designed to maximize selectivity for mutant forms of EGFR, including the T90M resistance mutation, and spare wild-type EGFR. Because of the reduced affinity for wild-type EGFR, toxicity profiles with these agents appear to be more tolerable, with lower incidences of the dermatologic and gastrointestinal toxicities classically seen with earlier generation EGFR TKIs.
AZD9291 is a potent, irreversible TKI that selectively targets EGFR-activating mutations, including the T790M resistance mutation, while having a low affinity for wild-type EGFR [53]. A phase I trial of 253 patients with EGFR-mutated NSCLC who had progressed on a first-generation EGFR TKI, 62 % of which had T790M mutations, found an ORR of 51 %, and disease control rate (DCR; complete response, partial response, or stable disease) of 84 % [54••]. Response rates were higher if a T790M mutation was present, with ORR and DCR of 61 and 95 %, respectively, compared to 21 and 61 % amongst patients without a T790M mutation. Initial data reported a median PFS of 9.6 months among patients with a T790M mutation, 2.8 months if the T790M was absent. Adverse events including diarrhea, rash, nausea, and decreased appetite increased at doses of 160 mg daily or higher, suggesting that at a higher drug concentration inhibition of wild-type EGFR may occur. Further evaluation of AZD9291 at a dose of 80 mg daily is ongoing as first-line therapy (NCT02296125), advanced line therapy for patients with acquired resistance due to T790M (NCT02094261 and NCT 02151981) as well as combination therapy (NCT02143466). At this lower dose, the incidence of rash and diarrhea appears to be significantly lower than what has been reported traditionally with first- and second-generation EGFR TKIs [54••].
Rociletinib (CO-1686) is a second small-molecule irreversible EGFR TKI which is selective for EGFR-activating mutations, including T790M, while sparing wild-type EGFR [55]. A phase I/II study of 130 patients with EGFR-mutated NSCLC who had progressed on prior EGFR TKI therapy, 57 % of whom had a T790M mutation reported promising response rates, particularly among patients with a T790M mutation [56••]. Although the study was begun with a free-base form of Rociletinib, a hydrogen bromide salt was later added for an improved pharmacokinetic profile, and the therapeutic dose of 900 mg twice daily was chosen using the HBr form. This resulted in an ORR of 59 % and DCR of 93 % among patients who were T790M positive, and ORR 29 % and DCR of 59 % among patients who were T790M negative. The median PFS at time of analysis was 13.1 and 5.6 months for patients with and without T790M mutations, respectively. Rociletinib was well-tolerated, with hyperglycemia and QT interval prolongation being the predominant grade 3 adverse events. Hyperglycemia was typically managed with metformin, and it is thought that this effect is caused by inhibition of the type I insulin-like growth factor receptor (IGF-IR), as well as insulin receptor kinases. Unlike first- and second-generation EGFR TKIs, the constellation of rash, paronychia, stomatitis, and diarrhea was generally absent, with grade 1 rash observed in 1 patient and grade 1–2 diarrhea in only 20 % of patients. Further studies of Rociletinib as first-line therapy (NCT02186301 and NCT02147990), as well as in patients who have acquired resistance (NCT02322281 are currently underway.
Three other third-generation EGFR TKIs, ASP8273, EGF816, and HM61713, are currently in phase 1/2 studies. ASP8273 is a small molecule TKI that is selective for mutated EGFR, and specifically targets the T790M mutation. Thirty-one Japanese patients with acquired resistance to first-generation TKIs have been enrolled in the dose escalation portion of the study, with doses ranging from 25 to 600 mg daily [57]. The preliminary ORR of 78 % was comparable to other third-generation TKIs, and the most common adverse events were diarrhea, nausea, and vomiting. EGF816 is a covalent, mutant-selective EGFR inhibitor that targets several activating EGFR mutations including the T790M resistance mutation, while sparing wild-type EGFR [58]. Early results of a phase I/II study enrolling patients with confirmed T790M mutations reported a preliminary ORR of 54.5 % and a disease control rate of 86.4 %. The toxicity profile was typical of EGFR TKI therapies and included rash, diarrhea, and stomatitis. HM61713 is another third-generation EGFR TKI that was developed to selectively target mutant EGFR. A phase 1/2 trial in 173 Korean patients who had progressed on prior EGFR TKI therapy evaluated doses of 75 to 800 mg daily, and a dose of 300 mg daily was chosen for the expansion phase [59]. Preliminary findings reported an ORR of 58.8 % and DCR of 97.1 % in 34 patients with centrally confirmed T790M mutations. A phase II study of HM61713 in the first-line setting in patients with EGFR-mutated NSCLC is currently underway (NCT02444819).
Conclusion
The story of EGFR-mutated NSCLC is one of the most gratifying in medical oncology, in which understanding of the biology of the disease has been intricately linked with the development of newer and increasingly more effective therapies. The advent of highly selective EGFR TKIs that also appear to be efficacious in patients with the T790M mutation adds an exciting new therapeutic option for patients with EGFR-mutant NSCLC. The improved toxicity profile of these agents is also promising, particularly for patients who may have been poorly tolerant of first-generation TKIs. While the primary endpoint of these studies is PFS, an important secondary endpoint is OS. Current data suggests that it is safe and efficacious to give a third-generation EGFR TKI to patients with acquired resistance to first- or second-generation inhibitors. However, oral treatment options for patients who progress on a third-generation EGFR TKI are currently unknown. Whether early use of these newer agents will preclude the development of T790M mutations and delay overall time to progression, as compared to first-generation TKIs, remains to be seen. Novel combination strategies targeting different pathways and mechanisms of acquired resistance are still needed to continue to improve outcomes for this patient population. The role of these agents in the adjuvant setting or in patients with locally advanced disease as neoadjuvant therapy or following definitive chemoradiation therapy continues to be explored.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hirsch FR, Bunn Jr PA. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10:432–3.
Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21 Suppl 2:S16–22.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. The New England journal of medicine. 2005;353:123–32.
Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2005;352:786–92.
Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73.
Bean J, Riely GJ, Balak M, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7519–25.
Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1169–80.
Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6494–501.
Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527–37.
Janne PA, Wang XF, Socinski MA. Randomized phase II trial of erlotinib (E) alone or in combination with carboplatin/paclitaxel (CP) in never or light former smokers with advanced lung adenocarcinoma: CALGB 30406. J Clin Oncol (Meeting Abstracts). 2010;28:7503.
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England journal of medicine. 2010;362:2380–8.
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–57.
Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67.
Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. The Lancet Oncology. 2014;15:1236–44.
Yang G, Yao Y, Zhou J, Zhao Q. Effects of icotinib, a novel epidermal growth factor receptor tyrosine kinase inhibitor, in EGFR-mutated non-small cell lung cancer. Oncology reports. 2012;27:2066–72.
Zhao Q, Shentu J, Xu N, et al. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung cancer. 2011;73:195–202.
Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. The Lancet Oncology. 2013;14:953–61.
Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1545–52.
Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5892–9.
Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:777–84.
Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:785–94.
Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5080–7.
Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. The Lancet Oncology. 2013;14:777–86.
Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3320–6.
Kelly K. ANK, Eberhardt W.E.E., O’Brien M.E.R., Spigel D.R., Crino L., Tsai C-M., Kim J-H., Cho E.K., Szczesna W., Burghuber O., Hoffman P.C., Keshavjee S., Orlov S., Serwatowski P., Wang J., Foley M.A., Horan J.D., Park J.W., Shepherd F.A. A randomized, double-blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection with or without adjuvant chemotherapy in patients (pts) with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC): RADIANT results. In: American Society of Clinical Oncology; 2014; Chicago, IL; 2014.
Pennell N.A. NJW, Chaft J.E., Azzoli C.G., et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. In: American Society of Clinical Oncology Annual Meeting; 2014; Chicago, IL: J Clin Oncol; 2014.
Gerber DE, Oxnard GR, Govindan R. ALCHEMIST: bringing genomic discovery and targeted therapies to early-stage lung cancer. Clinical pharmacology and therapeutics. 2015;97:447–50.
Godin-Heymann N, Ulkus L, Brannigan BW, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–9.
Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. The Lancet Oncology. 2012;13:528–38.
Goss G. FE, Cobo M., et al. A randomized, open-label, phase III trial of afatinib vs erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung following first-line platinum-based chemotherapy: LUX-Lung 8. In: ESMO; 2014 September 27th, 2014; Madrid, Spain; 2014.
Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. The Lancet Oncology. 2012;13:539–48.
Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. The Lancet Oncology. 2014;15:213–22.
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3327–34.
Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. The Lancet Oncology. 2015;16:141–51. This combined analysis of LUX-Lung 3 (afatinib vs. pemetrexed-cisplatin) and LUX-Lung 6 (afatinib vs. gemcitabine-cisplatin) in the first-line setting stratified patients by EGFR mutation (exon 19 deletion, L858R mutation, or other). While no overall survival benefit was seen in either trial individual, pre-planned combined analysis of patients with exon 19 deletions found improvement in overall survival favoring afatinib, suggesting that response to EGFR TKI therapy may depend upon the type of EGFR mutation present.
Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7665–70.
Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2552–8.
Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3076–83.
Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer research. 2007;67:11924–32.
Janne PA, Boss DS, Camidge DR, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1131–9.
Ellis PM, Shepherd FA, Millward M, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. The Lancet Oncology. 2014;15:1379–88.
Janne PA, Ou SH, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. The lancet oncology. 2014;15:1433–41.
Ramalingam SS, Janne PA, Mok T, et al. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. The lancet oncology. 2014;15:1369–78.
Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2005;352:786–92.
Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73.
Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine 2011;3:75ra26.
Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2240–7.
Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:2895–9.
Gandara DR, Li T, Lara PN, et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clinical lung cancer. 2014;15:1–6.
Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with Erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. The oncologist. 2013;18:1214–20.
Mok TS WY, Nakagawa K, Kim S, Yang J, Ahn M, Wang J, Yang JC, Lu Y, Atagi S, Ponce S, Shi X, Webster A, Jiang H, Soria J. Gefitinib/chemotherapy vs chemotherapy in epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC) after progression on first-line gefitinib: the phase III, randomised IMPRESS study. In: European Society of Medical Oncology Annual Meeting; 2014: Annals of Oncology; 2014.
Park K. AM, Yu C., Kim S., Lin M., Sriuranpong V., Tsai C., Lee J., Kang J., Perez-Moreno P., Button P., Gregory D., Mok T.S.K. ASPIRATION: first-line erlotinib (E) until and beyond RECIST progression (PD) in Asian patients (pts) with EGFR mutation-positive (mut+) NSCLC. In: European Society of Medical Oncology Annual Meeting; 2014; Madrid, Spain; 2014.
Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer discovery. 2014;4:1046–61.
Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. The New England journal of medicine. 2015;372:1689–99. This Phase 1/2 study of AZD9291 in patients with EGFR-mutated NSCLC progressive after a first-generation EGFR TKI showed that this agent was both active, with ORR 51%, and well tolerated with few of the dermatologic and gastrointestinal toxicities associated with first generation EGFR TKIs. Response rates were higher in patients with the T790M resistance mutation than in those without.
Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer discovery. 2013;3:1404–15.
Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. The New England journal of medicine. 2015;372:1700–9. This Phase 1/2 study of Rociletinib in patients with EGFR-mutated NSCLC associated with the T790M resistance mutation demonstrated significant activity and a promising response rate of 59% in this previously treated patient population, with the primary toxicity being hyperglycemia. The response rate was higher in patients harboring the T790M resistance mutation than in those with T790M-negative NSCLC.
Murakami H NH, Shimizu T, et al. Antitumour activity of ASP8273, an irreversible mutant selective EGFR-TKI, in NSCLC patients with tumours harbouring EGFR activating mutations and T790M resistance mutation. In: 26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2014 November 18–24, 2014; Barcelona, Spain: Europ J Cancer; 2014. p. 198.
Tan D. S-W. ST, Leighi N.B., et al. First-in-human phase I study of EGF816, a third generation, mutant-selective EGFR tyrosine kinase inhibitor, in advanced non-small cell lung cancer (NSCLC) harboring T790M. In: American Society of Clinical Oncology (ASCO) Annual Meeting; 2015; Chicago, IL: J Clin Oncol; 2015.
Park K. LJ-S, Lee K.H., Kim J-H et al. Updated safety and efficacy results from phase I/II study of HM61713 in patients (pts) with EGFR mutation positive non-small cell lung cancer (NSCLC) who failed previous EGFR-tyrosine kinase inhibitor (TKI). In: American Society of Clinical Oncology Annual Meeting; 2015; Chicago, IL: J Clin Oncol; 2015.
Compliance with Ethics Guidelines
Conflict of Interest
Emily Castellanos declares that she has no conflict of interest.
Leora Horn has received compensation from Genentech and Merck for service on advisory boards, has served on an unpaid advisory board for Bristol-Myers Squibb, as an unpaid consultant for Xcovery, and as an unpaid steering committee member for Bayer.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
About this article
Cite this article
Castellanos, E.H., Horn, L. Generations of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: Perils and Progress. Curr. Treat. Options in Oncol. 16, 51 (2015). https://doi.org/10.1007/s11864-015-0365-1
Published:
DOI: https://doi.org/10.1007/s11864-015-0365-1