Abstract
Background
The study aimed to determine the variability in the stages of diabetic nephropathy by examining specific biochemical functions associated with the target organ. As a result, various biochemical parameters were assessed in all of the groups under investigation.
Material and methods
These parameters encompassed soluble α-Klotho and serum insulin, which were determined through ELISA. Additionally, spectrophotometric methods were employed to assess other parameters such as blood levels of urea in all groups. Instead of using HPLC method, HbA1c levels were determined. Blood and urine samples were obtained from a total of 90 participants, who were aged between 37 and 70 years. A total of 70 patients were categorized into three groups according to their ACR. The first group consisted of patients with an ACR value of less than 30 mg/g. The second group included patients with an ACR value ranging from 30 to 300 mg/g. The third group comprised patients with an ACR value greater than 300 mg. Additionally, the study also involved 20 healthy individuals.
Result
The serum soluble α-Klotho in the patient group was significantly lower than that of the healthy subjects. There were strong negative correlations between serum soluble α-Klotho and both ACR and HOMA-IR. The AUC value was excellent, measuring at 0.93 with a p < 0.0001.
Conclusions
Soluble α-Klotho levels in the sera of diabetic patients were shown to be lower and significantly linked to patients with diabetic nephropathy. This implies that klotho levels may be influenced by ACR in addition to playing a significant role in insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Klotho gene has been identified as a gene that inhibits the aging process and promotes longevity when its expression is enhanced, but accelerates aging when it is disrupted [1]. This gene is primarily expressed as a transmembrane protein in the distal convoluted tubules of the kidney and choroid plexus in the brain. Additionally, it is expressed in various endocrine organs, including the pituitary, pancreas, parathyroid gland, adipocytes, and vascular endothelial cells [2]. Secreted Klotho and membrane Klotho are known for their capacity to regulate the activity of various glycoproteins found on cell surfaces. These glycoproteins include ion channels and growth factor receptors, such as insulin and insulin-like growth factor 1 receptor [3, 4]. It has been reported that individuals with chronic kidney disease exhibit lower levels of α-klotho in both plasma and urine during the early stages of the disease. These levels further decline as the disease progresses to more advanced phases [1, 5]. The precise role of α-klotho in the development of diabetic nephropathy is uncertain. Nonetheless, our recent cross-sectional study demonstrated that diabetic patients with relatively preserved renal function (GFR ≥ 60 ml/min/1.73 m2) exhibited significantly elevated levels of plasma and soluble α-klotho in urine when compared to individuals without any known health conditions [6]. In addition, the α-klotho levels in plasma decreased proportionally to urinary albumin excretion, whilst the α-klotho levels in urine remained stable with urinary albumin excretion increased. However, it is still unclear whether the levels of α-klotho in plasma and urine occur prior to the decrease in glomerular filtration rate (GFR) and albuminuria during the initial phases of diabetic nephropathy [7].
Materials and methods
Diabetic patients with CKD
Seventy diabetic patients with nephropathy, whose ages ranged from 37 to 70 years, were categorized into three groups according to their microalbumin value (ACR). The first group (G1) consisted of patients with an ACR level below 30 mg/g, indicating normoalbuminuria. The second group (G2) included patients with an ACR level between 30 and 300 mg/g, indicating microalbuminuria. The third group (G3) comprised patients with an ACR level exceeding 300 mg, indicating macroalbuminuria.
Healthy subjects
The study involved twenty healthy control participants who were selected to match the patients in terms of age (37–70 years) and gender (male and female). The average age of the control group was 60.85 ± 1.14 years. The controls were chosen according to specific criteria set by the physicians.
Sample collection
Ten milliliters of blood was obtained from each patient and control group. The blood samples were obtained between 8.00 and 11.00 A.M. following a fasting period of 12–15 h. Each blood sample was divided into two portions. The first portion was treated with ethylene diamine tetra-acetic acid (EDTA) (1.5 mg/ml) to measure HbA1c within a timeframe of less than 3 h. The second portion was used to obtain serum by transferring the sample into a regular tube and allowing it to clot at room temperature (22 °C). Subsequently, the serum was extracted by centrifuging the sample at 3000 r.p.m.
Anthropometries measurements
Including age, weight, and height. The body mass index (BMI) is determined using a formula that contains the basic equation of weight divided by the square of height.
Assessment of the homeostasis model assessment (HOMA-IR)
Various methods were employed to measure insulin resistance (IR) in the study, with the most commonly used approach being the calculation of homeostasis model assessment (HOMA). This calculation involved the use of fasting insulin (U/ml) and glucose (mg/dl), as shown in the following equation. Insulin resistance is a critical topic to investigate since it affects the balance of various metabolic [8]:
Determination of glomerular filtration rate (eGFR)
Glomerular filtration rate (GFR) was calculated using the modification of Diet in Renal Disease (MDRD) study formula and expressed in milliliters per minute per 1.73 m2 [9]. The MDRD equation is widely used in the estimation of GFR. It incorporates factors such as age, gender, and race to account for muscle mass. Weight is not necessary in this equation as the result is adjusted to a standardized body surface area of 1.73 m2, which represents the average surface area of adults [10].
Determination albumin creatinine ratio (ACR)
Albumin creatinine ratio was estimated by dividing the value of urine microalbumin in (mg/L) to urine creatinine (mg/dl), as shown below:
Microalbumin in urine was estimated by the MALB method used on Dimension® clinical chemistry system, in vitro diagnostic test for quantitative measurement of microalbumin (mg/L) in human urine by particle-enhanced turbidimetric inhibition immunoassay (PETINIA). Urine creatinine (mg/dl) was measured by Flex reagent cartridge using the CREA method used on the Dimension® clinical chemistry system and analyzer an in vitro diagnostic test for the quantitative determination of creatinine in urine.
Clinical laboratory analysis of groups
An ELISA plate reader was used to determine the quantities of soluble α-Klotho (Human Alpha Klotho kit, Ybiont, China) and Insulin (Demeditec Diagnostic GmbH, Germany) (ROCH Cobas Integra 400 Plus from Human GmbH, Germany). The serum fasting blood sugar, blood urea, serum creatinine, and lipid profile tests were assessed using an automated chemical analyzer (COBSe 411, Germany).
Statistical analysis
The statistical analysis was conducted using SPSS. The numerical variables with normally distributed data were analyzed by calculating the mean and standard deviation, while categorical variables were analyzed by determining the frequency and percentage. To examine potential significant differences between the numerical variables, independent t-tests and ANOVA tests were utilized. A significance level of 0.05 was employed in this study. The Pearson correlation and t-test were utilized to determine the significance of the correlation between the two quantitative variables. A receiver operating characteristic (ROC) curve method was used to assess the utility of soluble α-Klotho as a diagnostic marker or disease screening tool.
Result
Anthropometries and biochemical parameters of patient groups and healthy subjects
The biochemical results among study groups are given in Table 1. No significant differences were detected among the renal function tests, metabolic parameters (including, FSG, HbA1c, HOMA-IR), and BMI in any of the study groups. The insulin levels were significantly higher in patient groups compared with the control group. In addition, in the diabetic groups, there were significant differences observed in insulin levels (p = 0.001) (Table 1).
Serum soluble α-klotho in patient groups and healthy subjects
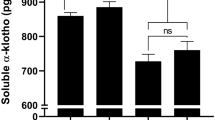
The serum soluble α-Klotho levels in healthy subjects and patients with diabetic nephropathy are presented in Fig. 1. The soluble α-Klotho levels for the control group were 1.54 ± 0.07 ng/ml. Although it was lower in patients with normoalbuminuria (0.737 ± 0.03 ng/ml) and microalbuminuria (0.539 ± 0.06 ng/ml), the differences in soluble α-Klotho levels between the control and two early-stage groups were not significant (p = 0.07 and p = 0.05, respectively) (Fig. 1). However, we found a statistically significant difference in the levels of microalbuminuria between patients (0.362 ± 0.02 ng/ml) and the healthy group (p = 0.007) (Fig. 1).
Simple linear regression
Table 2 shows the correlation coefficient of α-Klotho level with each of age, duration of diabetes, FBS, insulin, HOMO-IR, HbA1C, S.Cr, urea, ACR, and GFR in diabetic patients with nephropathy. The findings from the correlation coefficient analysis indicated a significant negative correlation (P < 0.01) between α-Klotho and age (r = − 0.311, P = 0.003), S.Cr (r = − 0.768, P = 0.001), blood urea (r = − 0.701, P = 0.001), FBS (r = − 0.442, P = 0.001), insulin (r = − 0.488, P = 0.001), HOMO-IR (r = − 0.717, P = 0.001), and HbA1C (r = − 0.616, P = 0.001). The study demonstrates a negative correlation (P < 0.05) between α-Klotho and ACR (r = − 0.270, P = 0.011) as well as duration (r = − 0.282, P = 0.018). In addition, a strong positive correlation (P < 0.01) is observed between α-Klotho and eGFR (r = 0.862, P = 0.001).
Multiple regression analysis
To determine the impact of a set of explanatory variables on the baseline serum Klotho level, a multiple linear regression model was employed. All potential explanatory variables were initially included in the model. The equation resulting from this analysis can be found in Figs. 2 and 3, while Table 3 demonstrates a positive correlation between several factors, including age (measured in years), gender (male versus female), index, BMI, baseline HbA1c%, and baseline FBS, with the average serum α-Klotho level. However, none of these variables demonstrated statistical significance. Conversely, only two variables, namely, ACR (r = 0.510, p = 0.000) and HOMA-IR (r = − 0.149, p = 0.04), independently exerted an influence on Klotho levels. The resulting equation was found to be statistically significant (P = 0.001), accounting for 89% of the observed variability in the response variable.
Receiver operator characters curve analysis (ROC)
The analysis of the ROC curve categorizes the parameters based on their placement within the ROC area and determines whether this placement holds significance. In this study, the ROC curve was used to distinguish between the T2DM with different stages of nephropathy and the control group. Based on the ROC curve analysis, the optimal threshold for soluble α-Klotho is determined to have a sensitivity of 98.0% and a specificity of 100.0% as a marker. Analysis reveals the area under the curve (AUC) of ROC in a value of 0.993 for soluble α-Klotho as shown in Table 4 and Fig. 4.
Discussion
Persistently elevated blood glucose levels have the potential to harm various kidney cell types, leading to the advancement of kidney failure. Consequently, it becomes challenging to identify the hazards linked to early-stage kidney disease, highlighting the difficulties in successfully preventing diabetic nephropathy in individuals diagnosed with type 2 diabetes mellitus [11]
Our study findings align with previous research that has observed elevated fasting blood sugar levels in individuals with diabetes and chronic kidney disease (CKD) compared to healthy individuals without any medical conditions [12]. Multiple studies have provided evidence of a direct correlation between elevated serum creatinine levels and an accompanying increase in serum urea levels [13, 14]. During our study, we discovered that as diabetes progressed, there were increased levels of urea and creatinine. This resulted in glomerular injury, a reduction in glomerular filtration capacity, and the buildup of metabolic byproducts. Among these by-products, urea, and creatinine are acknowledged as noteworthy indicators of alterations in renal function [15].Therefore, sustained hyperglycemia can cause irreparable damage to the kidneys.
The results of our study showed a notable decrease in serum levels of s-Klotho compared to the control group used as a reference. Furthermore, we investigated the relationship between serum Klotho levels and both ACR and insulin resistance in individuals with diabetic nephropathy. Additionally, the average values observed in our study for individuals with diabetic nephropathy and those in a healthy control group align with the results reported in previous literature that utilized the same assay method [16,17,18]. The decrease in serum levels, coupled with the progression of renal damage, is likely attributed to a reduction in renal synthesis of Klotho. If renal Klotho production remained stable, reduced excretion due to renal damage would result in higher serum levels. Conversely, if renal excretion were to remain normal, a predictable decrease in serum levels would not be expected. Furthermore, the likelihood of increased renal excretion appears highly improbable, as it contradicts available clinical evidence [1] and experimental data indicating reduced urinary s-Klotho in CKD [19].
The statistical analysis demonstrated a significant negative correlation between soluble alpha klotho and each of the variables creatinine, glucose, urea, HbA1c, ACR, and age. Conversely, a positive correlation was observed between soluble alpha klotho and insulin as well as eGFR according to the data presented in Table 2. This finding aligns with studies [20, 21]. After transferring the variables to the multiple regression model, we observed that the relationship between Klotho and other parameters was no longer significant. It was found that Klotho primarily correlated with ACR. Our findings align with previous studies, further substantiating the observed correlation. In our study, we found that ACR was the strongest predictor of s-Klotho levels, which confirms the hypothesis we have previously discussed [1, 19].
Additionally, the multiple regression model suggests a connection between Klotho and insulin resistance, which aligns with outcomes from other studies [22, 23]. These studies have indicated that Klotho levels can influence insulin metabolism by inhibiting tyrosine phosphorylation of insulin and IGF1 receptors, as well as enhancing glucose-induced insulin secretion through the upregulation of TRPV2 membrane retention. Considering the apparent deficiency of Klotho observed in CKD stages, it is plausible to hypothesize that low Klotho levels could lead to excessive insulin release, resulting in a state of insulin resistance.
ROC analysis for serum α-Klotho demonstrated an AUC value of 0.993, with a sensitivity of 98.0% and a specificity of 100% at a cutoff value of 0.82. The findings of this study suggest that serum α-Klotho can be a valuable tool for differentiating individuals with diabetic nephropathy from those without the condition. These findings align with Drew et al. [24], although Seiler et al. [25] did not support the hypothesis of reduced plasma levels of secreted Klotho in CKD. It should be noted that this discrepancy in hypothesis may be attributed to Seiler et al.’s omission of measuring α-Klotho in healthy controls or stage I CKD patients. The ROC analysis for α-Klotho at a cutoff value of ≤ 9.9 yielded a sensitivity of 92%, a specificity of 100%, a positive predictive value of 100%, a negative predictive value of 88.2%, and an accuracy of 97% in discriminating between individuals with CKD and those without. The area under the curve for α-Klotho was 0.970.
Conclusions
Lower levels of soluble α-Klotho were observed in the blood samples of diabetic patients, and these reduced levels were found to be significantly associated with diabetic nephropathy patients. This suggests that the levels of klotho may be influenced by ACR also play crucial roles in insulin resistance.
References
Akimoto T, Yoshizawa H, Watanabe Y et al (2012) Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol 13(1):1–9
Donate-Correa J, Mora-Fernández C, Martínez-Sanz R et al (2013) Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol 165(1):179–183
Hu MC, Kuro-o M, Moe OW (2012) Secreted klotho and chronic kidney disease. Adv Exp Med Biol 728:126–57. https://doi.org/10.1007/978-1-4614-0887-1_9. PMID: 22396167; PMCID: PMC3780390
Hu MC, Shi M, Zhang J et al (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22(1):124–136
Kim SS, Song SH, Kim IJ et al (2014) Nonalbuminuric proteinuria as a biomarker for tubular damage in early development of nephropathy with type 2 diabetic patients. Diabetes Metab Res Rev 30(8):736–741
Kim HR, Nam BY, Kim DW et al (2013) Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis 61(6):899–909
Lee EY, Kim SS, Lee JS et al (2014) Soluble α-klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS ONE 9(8):e102984
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Levey AS, Bosch JP, Lewis JB et al (1999) Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Int Med 130(6):461–70
Stevens PE, Levin A (2013) Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Int Med 158(11):825–30
Fioretto P, Mauer M (2007) Histopathology of diabetic nephropathy. In Seminars in Nephrology (Vol. 27, No. 2, pp. 195–207). WB Saunders
Santha K, Mahendran BK, Perumal KK et al (2017) Renal resistive index and its association with insulin resistance in patients with Type 2 diabetes mellitus. IOSR J Dental Medical Sci 16:51–57
Kim YJ, Hwang SW, Lee T et al (2023) Association between urinary albumin creatinine ratio and cardiovascular disease. PLoS One 18(3):e0283083. https://doi.org/10.1371/journal.pone.0283083. PMID: 36943853; PMCID: PMC10030008
Al-Jameil N, Khan FA, Arjumand S et al (2014) Dyslipidemia and its correlation with type 2 diabetic patients at different stages of proteinuria. Biomed Res 25(3):327–331
Deepa K, Manjunatha G, Oinam S et al (2011) Serum urea, creatinine in relation to fasting plasma glucose levels in type 2 diabetes. Int J Pharm Bio Sci 1(1):279–283
Pavik I, Jaeger P, Ebner L et al (2013) Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28(2):352–359
Yokoyama K, Imura A, Ohkido I et al (2012) Serum soluble α-klotho in hemodialysis patients. Clin Nephrol 77(5):347–351
Nowak A, Friedrich B, Artunc F et al (2014) Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PloS One 9(7):e100688
Rotondi S, Pasquali M, Tartaglione L et al (2015) Soluble α - klotho serum levels in chronic kidney disease. Int J Endocrinol 2015:872193. https://doi.org/10.1155/2015/872193. Epub 2015 Mar 19. PMID: 25873958; PMCID: PMC4383388
Wang Q, Su W, Shen Z, Wang R (2018) Correlation between soluble α-Klotho and renal function in patients with chronic kidney disease: a review and meta-analysis. Biomed Res Int 12:2018
Seo MY, Yang J, Lee JY et al (2015) Renal Klotho expression in patients with acute kidney injury is associated with the severity of the injury. Korean J Intern Med 30(4):489
Kurosu H, Yamamoto M, Clark JD et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309(5742):1829–1833
Lin Y, Sun Z (2012) Antiaging gene Klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 in MIN6 β-cells. Endocrinology 153(7):3029–3039
Drew DA, Katz R, Kritchevsky S et al (2017) Association between soluble Klotho and change in kidney function: the health aging and body composition study. J Am Soc Nephrol 28(6):1859–1866
Seiler S, Wen M, Roth HJ et al (2013) Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 83(1):121–128
Author information
Authors and Affiliations
Contributions
The authors contributed to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lefta, R.F., Hassan, E.A. Serum soluble α-Klotho levels in patients with diabetic nephropathy. Ir J Med Sci 193, 725–731 (2024). https://doi.org/10.1007/s11845-023-03502-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03502-7