Abstract
Background
Aerobic exercise training contributes to improvement of cardiopulmonary capacity, mobility, neurological function, and quality of life.
Aims
To investigate the effects of arm crank ergometer training on aerobic capacity, quality of life, and Parkinson’s disease (PD)-related disability
Methods
Seventeen patients with PD were recruited to study. Assessments were performed at baseline and at the end of an 8-week arm crank ergometer (ACE) training program (3 days/week; 1 h per session, 50–70% VO2peak) with patients acting as their own control. Outcome measures included aerobic capacity assessment, 6-min walk test (6MWT), timed up and go test (TUG), Unified Parkinson’s Disease Rating Scale (UPDRS), Parkinson’s Disease Questionnaire-39 (PDQ-39), Beck Depression Index (BDI), the Falls Efficacy Scale (FES), and Montreal Cognitive Assessment (MoCA).
Results
At the end of the study, an increase of 30.49% in aerobic capacity was observed. Statistically significant improvements were found for the 6MWT (p = 0.001), TUG test (p = 0.001), UPDRS total score (p = 0.002), quality of life assessed with PDQ-39 (p = 0.006), BDI (p = 0.001), and FES scores (p = 0.002) after an 8-week ACE training. No significant effect on MoCA was found (p = 0.264).
Conclusion
An 8-week ACE training led to significant improvement in aerobic capacity, physical performance, and PD-related disabilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease, usually characterized by the significant motor deficits (tremor, bradykinesia, postural stability, rigidity, gait disorders) that cause to an impairment in physical, psychological, and social functions and emotional status of patients [1, 2]. The disability in motor characteristics of PD and chronic inactivity can lead to increased risk of secondary health problem such as cardiovascular deconditioning in patients with PD [3].
Patients experience progressive disorders despite pharmacological treatment and deep brain stimulation. Studies have indicated that aerobic exercise training is commonly considered to have therapeutic effects on cardiopulmonary and musculoskeletal systems [4, 5]. Aerobic exercise programs are very important for patients with PD to maximize functional capacity, physical functioning, and quality of life [1]. Additionally, exercise training is not only intended to optimize functional capacity, but also avoid secondary problems [2]. It has also beneficial effect on risk of falls and dementia [6, 7]. Previous studies have suggested that aerobic exercise training with treadmill may contribute to improvement of cardiopulmonary fitness, mobility, neurological function, and quality of life in patients with PD [8,9,10]. However, aerobic training with treadmill may not always be the most proper method because of the high rate of falls and postural instability in this patient group. With regard to be safe form of exercise therapy, cycle ergometry training has been recommended to improve cardiopulmonary fitness in patients with neurological disorders [11, 12]. Ridgel et al. [13] demonstrated significant improvements in motor functions after 8 weeks of bicycle training. However, Lauhoff et al. [10] reported that a 6-week exercise program with cycle ergometry did not significantly affect exercise capacity, but improved functional status, balance, and disease-related inability.
Upper extremity training, such as arm crank ergometry (ACE), is indicated to be successful at enhancing central and peripheral fitness markers, and may transfer it to lower extremity due to the intensity of exercise [14, 15]. Pogliahgi et al. [16] demonstrated that ACE and cycle training bring out similar cross transfer effects. ACE has been shown to be a well-tolerated alternative form of exercise training to improve walking distance in patients with peripheral arterial disease [17, 18]. Recent studies have also suggested that ACE training improves aerobic capacity and seated balance in patients with spinal cord injury [19, 20]. Furthermore, ACE training has been indicated to lead to positive impact on walking ability and postural balance in stroke patients [21]. However, to the best of our knowledge, no studies have investigated the effects of ACE training in patients with PD. As such, the aim of our study was to investigate the effects of an 8-week ACE training on aerobic capacity, quality of life, and PD-related disability. We hypothesized that an 8-week ACE training program would lead to significant improvements in aerobic capacity, as well improve quality of life and PD-related disability, when compared to baseline period.
Material and methods
Participants
It was found appropriate to work with at least 12 people with 80% power and 5% type 1 error with an effect size of 0.8 (one-tail) between baseline and after training program evaluation. Calculation was done in G*Power 3.1 program.
We initially invited 21 patients to the first screening. After explaining the study protocol, 4 patients refused to participate to study, while 17 volunteered to be screened in this process. All patients were asked to fill a Physical Activity Readiness Questionnaire (PAR-Q), which measured the readiness of the subject to engage in a physical activity [22].
Inclusion criteria were a diagnosis of idiopathic PD by a movement disorders specialist and a disease severity ranging between stage 1 and 3 as measured by modified Hoehn and Yahr rating scale [23]; stable medication use; and near standard range of motion of upper extremity for be able to use ACE. Exclusion criteria included having received a physical therapy and rehabilitation program in the last 6 months; exercising regularly for the last 6 months; those with additional chronic metabolic, neuromuscular, musculoskeletal, or cardiopulmonary system diseases; and those using drugs for these. Ethical approval was obtained from the local ethics committee before the commencement of study. Written informed consent was obtained from all patients.
Procedure
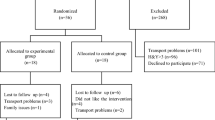
Evaluations were performed at baseline and after an 8-week ACE training program with patients acting as their own control (Fig. 1). All assessments were performed by the same investigator, while the patients were “on” and at the same time in the same order to minimize the effect of medication on measurement data. No modifications were done to any patient’s medication throughout to the trial. In order to account for the “on/off” state, patients were often advised to take their medicine at the same time every day. During the study, the patients did not undergo any other physiotherapy program. None of patients participated in any exercise groups or had been recommended a home exercise program. Furthermore, they were told to maintain their usual diet and physical activities.
Outcome measurements
Demographic data recorded at baseline included age, body composition, presence of comorbidities, disease duration, current medical treatment, and smoking status. The modified Hoehn and Yahr scale were used to evaluate disease severity [23]. Motor impairment was assessed by using Unified Parkinson’s Disease Rating Scale (UPDRS) [24]. The UPDRS was evaluated by the same experienced movement disorders neurologist in order to prevent the confusing effects of interrater variability.
Primary outcomes
Cardiorespiratory fitness evaluation
The exercise test was performed after a good night’s sleep and at 3 h of fasting. Subjects were instructed to refrain from vigorous exercise, caffeine, tobacco, and alcohol on the day before and on the test day. All patients underwent an assessment of exercise capacity with an arm crank ergometer (ACE) (Monark 831 E; Monark Exercise AB, Varberg, Sweden). During the exercise test, respiratory gas exchanges were collected by a calibrated system according to manufacturer’s instruction (Quark CPET, COSMED, Rome, Italy). Respiratory variables were collected using a 20-s rolling average throughout the test. After 2-min warm-up period with no resistance, the test began with a workload of 30 W by a constant rate of 50–60 rpm. The intensity of test was increased by 10 W at 2-min intervals until subject reached 2 or 3 of the following terminate criteria: volitional fatigue; achieve to ± 10 beats of maximum heart rate according to “220-age” formula; and respiratory exchange ratio (RER) > 1.0 [3, 25]. Rating of perceived exertion (RPE) was recorded at the end of each stage and at the end of test. The RPE was obtained using the 6–20-point Borg’s scale [26]. Heart rate (HR) was recorded via by a transmitter belt during the test (Wireless HR Monitor, COSMED). Peak oxygen uptake (VO2peak: ml/kg/min), peak HR (bpm: beat per minute), and peak RER were defined as the average of the highest value achieved during the last two 20 s.
Physical performance tests
The 6-min walk test (6MWT) was used to measure functional status and exercise tolerance [27]. Patients were asked to walk with their preferred walking speed as far as they could over a 6-min period along a 30-m hallway. Total distance was recorded as meters.
Patients were also asked to complete the timed up and go test (TUG) at a self-paced speed [28]. They were told to stand up from a chair without the use of their hands, walk 3 m as quickly as possible, turn back 1800, and sit down to chair again. Total duration was recorded as seconds.
Secondary outcomes
Parkinson’s disease-specific health-related quality of life was assessed with the Parkinson’s Disease Questionnaire-39 (PDQ-39) [29]. The questionnaire consists of 39 items providing the measurement of 8 dimensions. These are mobility (10), activities of daily living (6), emotional well-being (6), stigma (4), social support (3), cognition (4), communication (3), and bodily discomfort (3). As a result, the total score is found by adding all scores. Low score in total indicates better quality of life.
Beck Depression Index (BDI) was used to evaluate the levels of depression in patients with PD. The purpose of the index, which is frequently used in psychiatric research, is not to diagnose depression, but to objectively evaluate the severity of depressive symptoms on numerical values. The lowest score that can be obtained from the scale is 0, and the highest score is 63. A score of 17 or above in the BDI indicates that there is a risk of depression in the individual [30]. Levels of depression scores were categorized as follows: 0–9 points, no or minimal depression; 10–16 points, mild depression; and 17–63 points, cutoff point and over [31].
The Falls Efficacy Scale (FES) was used to investigate the fear of falling. All patients were asked to rate their confidence during ten daily activities on a scale numbered between 1 and 10. A total score of greater than 70 indicates that the person has a fear of falling [32].
Cognitive impairment of patients with PD was assessed with Montreal Cognitive Assessment (MoCA). The total score obtained can vary between 0 and 30. The cutoff point for cognitive impairment is 21. Patients who perform below 21 points are considered to have cognitive impairment [33].
Arm crank ergometer training
ACE training was conducted at 50–70% of measured VO2peak [34]. Exercise intensity was re-determined by performing an intermediate cardiorespiratory fitness test in the 4th week. The intervention consisted of 60-min ACE training, 3 times per week for 8 weeks (Monday, Wednesday, Friday). All training sessions were performed at the same time of day. The total 60-min training session consisted of a 4-min warm-up, a 52-min intermittent ACE training (10-min exercise, 4-min rest), and a 4-min recovery period.
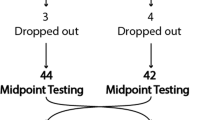
Training intensity was progressively increased weekly. Initial work load was corresponding at 50% of measured VO2peak during week 1, 60% during week 2, and 70% during weeks 3–4. Intensity was reorganized after intermediate test performed at the end of 4th week. The training program was continued with a work load corresponding at 60% of new VO2peak during weeks 5–6 and 70% during weeks 7–8 (Fig. 2). Patients who participated at least 80% of ACE training program were included in statistical analysis.
Statistical analyses
Categorical variable was summarized as count and percentage. Continuous demographic variables were presented as mean ± standard deviation. Shapiro Wilk test was used to determine whether the variables were suitable for normal distribution. In the comparison of two dependent groups, paired sample t test and Wilcoxon test were used depending to the distribution assumption, and group statistics were summarized as mean ± standard deviation and median [min.-max]. p < 0.05 was accepted as statistical significance level.
Results
Seventeen patients agreed to participate in the study. One patient became unwell before baseline evaluations and 4 of them failed to complete the ACE training program, leaving 13 patients (male/female: 9/4) included in statistical analysis. Demographic characteristics of participants are presented in Table 1. Mean disease diagnosis duration was 5.23 ± 3.11 years. While the rate of severity of Parkinson disease was 11:2 (stage 2/stage 1) at baseline evaluation, it is changed as 8:5 according to the Hoehn and Yahr. The 8-week ACE training program was well-tolerated by all patients, and no exercise related adverse events observed during this period.
Primary outcomes
A mean increase of 30.49% occurred in the aerobic capacity (VO2peak) after an 8-week ACE training program. No statistically difference was found in the RER and HRpeak (p = 0.06 and p = 0.61, respectively). The RPE was not changed after training program (p = 0.83). The ACE training had a positive effect on physical performance tests. The distance during 6MWT increased 53.31 m, and duration of TUG test was decreased 1.74 s after training period (Table 2).
Secondary outcomes
The motor impairment that evaluated with UPDRS score was significantly decreased after an 8-week ACE training (p = 0.002). The mean BDI score was 20.84 at baseline, and it significantly decreased to 16.07 (p = 0.001) after an 8-week ACE training. Similarly, the mean FES scores significantly decreased after 8 weeks (p = 0.002). The ACE training program had also a statistically significant positive improvement on quality of life assessed with PDQ-39 compared with baseline measurement (p = 0.006) (Fig. 3). The mean MoCA score was not significantly changed after training program (p = 0.264) (Table 3).
Discussion
This study aimed to investigate the effect of an 8-week ACE training program in patients with PD. The ACE training had significant beneficial effects on aerobic capacity, physical performance, quality of life, and PD-related disabilities as hypothesized.
Physical exercise programs have been demonstrated to have positive effects on gait and mobility in PD subjects [8, 13, 35]. Among the different kind of exercise training programs, aerobic exercise has been shown to be very crucial for patients with PD to be independent in their daily life activities and to prevent PD-related disabilities. Aerobic exercise training has also been reported to have a beneficial non-pharmacological intervention to encourage, through improved plasticity in motor-related structures, not only physical fitness in PD, but also stronger motor learning ability useful in daily activities [36]. Although the mechanism of aerobic exercise in PD still remains unclear, it has been asserted that exercise-dependent plasticity after aerobic exercise acts on the brain in a similar pathway like dopaminergic derived therapy using the similar manner to generate symptomatic relief [35]. Several studies provide evidence for the favorable effects of aerobic exercise training with treadmill and cycle ergometer on patients with various neurological disorders, including PD [8,9,10, 25, 36, 37]. However, a meta-analysis conducted by Goodwin et al. [1] reveals the lack of consensus on optimal exercise prescription for patients with PD disease. Kurtais et al. [38] indicated that treadmill training led to significant improvements in functional task and physical fitness in PD patients. Pohl et al. [39] reported that exercise program with treadmill might be more beneficial than overground gait training. Similarly, incremental-speed dependent treadmill training reported to cause an improvement in mobility, reduce postural instability, and also fear of falling [40]. On the other hand, a 6-week cycle ergometer training program did not lead to significant improvement in exercise tolerance; however it had significantly beneficial effect on balance, functional ability, and PD-related disability [10]. In a study carried out by Shulman et al. [37], the efficiency of 3 types of exercises (high-intensity treadmill training: 70–80% of heart rate reserve; low-intensity treadmill training: 40–50% of heart rate reserve; stretching and resistance exercise) was compared. They concluded that although the most improvement was seen in lower intensity treadmill training, all 3 types of exercises increased the 6-min walk distance, and exercise capacity was improved with both treadmill training programs [37]. The intensity of exercise program to prescribe exercise for enhancing cardiorespiratory capacity should be based on subject’s aerobic capacity.
ACE training has been largely studied in healthy older people [41] and also in different patient groups such as stroke patients [21], peripheral arterial disease with claudication [14, 17, 18, 42], and spinal cord injury [19, 34]. It has been demonstrated that ACE training leads to improvements in balance and walking ability in stroke patients [21]. Pain-free walking distance, walking capacity, physical fitness, and cardiorespiratory function improved similarly after arm and treadmill exercise training in patients with peripheral arterial disease-induced claudication [14, 42]. This is conducive of similar enhanced walking capacity and physical fitness-related cardiopulmonary functions in response to any type of exercise training (treadmill or ACE) in patients with peripheral arterial disease [42]. Arm crank ergometer spin training improved balance and aerobic capacity in patients with spinal cord injury [19]. Akkurt et al. [34] reported that 12 weeks of ACE training resulted with an increase of 39.6% in aerobic capacity in patients with spinal cord injury. Similarly, we found a significant increase of 30.49% in aerobic capacity of our patients after an 8-week ACE training. Although Parkinson disease affects the musculature of the limbs and spine, areas are not affected equally [3]. Therefore, upper limb exercise training should not be ignored in this patient group. Upper body muscle group exercise training tends to result in a generalized systematic training impact that may increase the exercise capacity of muscle groups that do not engage in the training effort [42]. While upper limb training increases the development of force generation and muscle endurance which trained, intensity of exercise appears to be a main factor for the fitness of upper extremities and transition to lower limb fitness [14].
Studies have demonstrated that improvements in exercise capacity following treadmill or cycle training led to significant effects on 6MWT distance and TUG time in patients with PD [10, 25, 37]. The 4-week treadmill walking training (initial speed of 2 km/h and was increased by 0.5 km/h every 3 days) had positive impacts on walking performance (TUG and 6MWT) in PD patients [25]. However, the positive development of TUG and 6MWT after ACE training was an interesting finding of our study. In fact, the 6MWT distance was significantly increased by 53.31 m, and TUG duration was decreased 1.74 s after an 8-week ACE training. ACE training and cycling training had similar positive effects on TUG performance in healthy older people [41]. Kaupp et al. [21] has shown that ACE is an effective training mode, and it improved both TUG and duration of 10-m walk in stroke patients. The connatural neural and mechanical connection between the active arms and legs during locomotion could be exploited by ACE training [43]. Consequently, it is indicated that ACE training may have been trigger the interlimb connection which is necessary for coordination of rhythmic walking [21]. Training of the upper extremity is also indicated to be effective at improving central and peripheral fitness parameters and might transfer to lower limb fitness [14]. Therefore, training with ACE improved the exercise capacity which affects the physical performance of our patients.
Muscular rigidity seen in PD first of all affects the proximal musculature such as shoulders and neck and then may spread to the facial and limb muscles which explains decreased arm swing in this population (3). It has concluded that restricted arm swing in PD patients may affect the postural control of trunk and dynamic balance [44]. Milosevic et al. [45] reported that time of the TUG was significantly faster when arms were used freely than limited arms movements in older subjects. Although methodological restrictions prevent us from providing direct adaptive mechanisms for the enhanced functional efficiency of the lower body after ACE training, it is clear that arm movements contribute to the generation of upper body torques. In particular, upper body activity tends to assist ankle and hip movements by raising the center of mass up above the support base for balance control [46]. Fear of falling is believed to be documented more frequently in patients with Parkinson’s disease due to the altered postural control and increased frequency of falls reported in this population [40]. A fear of future falls may lead to restraint of daily living activities. The present study revealed that FES scores were significantly decreased after ACE training. The restricted mobility may cause to cerebrovascular pathologies, joint degeneration, skeletal muscle weakness, and loss of tendon flexibility [40]. Physical activity facilitates functional motor improvements, cardiovascular fitness, and musculoskeletal conditioning and can avoid or postpone secondary complications [47].
Since such changes were associated with disabilities and quality of life, significant improvement in the PDQ-39 questionnaire and PD-related disability were obtained after an 8-week ACE training in our patient group. This study also found an 8-week ACE aerobic training program to have both statistically and clinically significant positive impact on depression symptoms of patients. While the baseline evaluation indicated to the moderate to severe depression, it changed to the mild depression after aerobic training program. Similar to our results, 6 weeks of treadmill training improved quality of life in patients with PD [8, 47]. Depression is a common symptom in PD and is associated with increased disease-related disability. Furthermore, it is very important factor which affects the quality of life in patients with PD. Aerobic exercise training programs lead to an improvement in quality of life by increasing physical function and reducing depressive symptoms in patients with PD [48]. Therefore, non-pharmacological therapies, such as exercise programs, should be a choice of treat the depressive symptoms.
Although both motor impairment and disease severity were regressed after 8 weeks of aerobic training with ACE, only UPDRS score was statistically significant. These finding are supported by previous studies that pointed out the positive effect of aerobic training on disease severity and motor impairment in patient with PD [10, 13]. Baatile et al. [49] indicated a 30% decrease in UPDRS score after 8 weeks of gait training, but only 6 patients were included to be analyzed. However, it has been reported that stretching and resistance exercises lead to more improvement in UPDRS score compared to gait trainings in 2 different intensities, which suggests that UPDRS score is more related with muscle strengthening [37]. Similarly, Duchesne et al. [9] stated that, since all their patients were in early stage on account of disease severity, a 3-month aerobic exercise training with cycle did not contribute to significant chance in UPDRS scores.
The major limitation is the relatively small sample size, and patients acted as their own control in this study. Future researches on these aspects should take these limitations into account, and a randomized, controlled study with a larger population needs to be performed.
Conclusion
An 8-week ACE aerobic training leads to a significant improvement in aerobic capacity, physical functions, and PD-related disabilities. Aerobic exercise programs that include the upper extremity as well as the lower extremity should be added to the exercise prescription of Parkinson’s patients. As a conclusion, ACE training program could be used as an important alternative mode of exercise to improve exercise and functional capacity especially among patients with balance problems and also in patients with impaired mobilization.
References
Goodwin VA, Richards SH, Taylor RS et al (2008) The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 23:631–640. https://doi.org/10.1002/mds.21922
Cugusi L, Solla P, Zedda F et al (2014) Effects of an adapted physical activity program on motor and non-motor functions and quality of life in patients with Parkinson’s disease. NeuroRehabilitation 35:789–794. https://doi.org/10.3233/NRE-141162
Protas EJ, Stanley RK, Jankovic J et al (1996) Cardiovascular and metabolic responses to upper- and lower-extremity exercise in men with idiopathic Parkinson’s disease. Phys Ther 76(1):34–40. https://doi.org/10.1093/ptj/76.1.34
Lokk J (2000) The effects of mountain exercise in Parkinsonian persons - a preliminary study. Arch Gerontol Geriatr 31(1):19–25. https://doi.org/10.1016/S0167-4943(00)00062-5
Bergen JL, Toole T, Lii E et al (2002) Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson’s disease patients. NeuroRehabilitation 17(2):161–168. https://doi.org/10.3233/NRE-2002-17209
Protas EJ, Mitchell K, Williams A (2005) Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation 20(3):183–190. https://doi.org/10.3233/NRE-2005-20305
Abrantes AM, Fredman JH, Brown RA et al (2012) Physical activity and neuropsychiatric symptoms of Parkinson disease. J Geriatr Psychiatry Neurol 25(3):138–145. https://doi.org/10.1177/0891988712455237
Herman T, Giladi N, Gruendlinger L et al (2007) Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil 88(9):1154–1158. https://doi.org/10.1016/j.apmr.2007.05.015
Duchesne C, Lungu O, Nadeau A et al (2015) Enhancing both motor and cognitive functioning in Parkinson’s disease: aerobic exercise as a rehabilitative intervention. Brain Cogn 99:68–77. https://doi.org/10.1016/j.bandc.2015.07.005
Lauhoff P, Murphy N, Doherty C et al (2013) A controlled clinical trial investigating the effects of cycle ergometry training on exercise tolerance, balance and quality of life in patients with Parkinson’s disease. Disabil Rehabil 35(5):382–387. https://doi.org/10.3109/09638288.2012.694962
Lennon O, Carey A, Gaffney N et al (2008)A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil 22:125–133. https://doi.org/10.1177/0269215507081580
Rampello A, Franceschini M, Piepoli M et al (2007) Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther 87:545–555. https://doi.org/10.2522/ptj.20060085
Ridgel AL, Vitek JL, Alberts JL (2009) Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 23(6):600–608. https://doi.org/10.1177/1545968308328726
Tordi N, Belli A, Mougin F et al (2001) Specific and transfer effects induced by arm or leg training. Int J Sports Med 22:517–524. https://doi.org/10.1055/s-2001-17608
Earnest CP (2008) Exercise interval training: an improved stimulus for improving the physiology of pre-diabetes. Med Hypotheses 71:752–761. https://doi.org/10.1016/j.mehy.2008.06.024
Pogliaghi S, Terziotti P, Cevese A et al (2006) Adaptations to endurance training in the healthy elderly: arm cranking versus leg cycling. Eur J Appl Physiol 97(6):723–731. https://doi.org/10.1007/s00421-006-0229-2
Tew G, Nawaz S, Zwierska I et al (2009) Limb-specific and cross-transfer effects of arm-crank exercise training in patients with symptomatic peripheral arterial disease. Clin Sci 117(12):405–413. https://doi.org/10.1042/CS20080688
Zwierska I, Walker RD, Choksy SA et al (2005) Upper-vs lower-limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. J Vasc Surg 42(6):1122–1130. https://doi.org/10.1016/j.jvs.2005.08.021
Williams AMM, Chisholm AE, Lynn A et al (2020) Arm crank ergometer “spin” training improves seated balance and aerobic capacity in people with spinal cord injury. Scand J Med Sci Sports 30(2):361–369. https://doi.org/10.1111/sms.13580
Harnish C, Sabo R, Daniels J, Caruso D (2017) The effects of two weeks of arm crank sprint interval training in men with chronic spinal cord injury. Int J Sports Exerc Med 3:56–59. https://doi.org/10.23937/2469-5718/1510059
Kaupp C, Pearcey GE, Klarner T et al (2018) Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke-the arms can give legs a helping hand in rehabilitation. J Neurophysiol 119(3):1095–1112. https://doi.org/10.1152/jn.00570.2017
Thomas S, Reading J, Shephard RJ (1992) Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci 17(4):338–345
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Akbostanci MC, Bayram E, Yilmaz V et al (2017) Turkish standardization of movement disorders society unified Parkinson’s disease rating scale and unified dyskinesia rating scale. Mov Disord Clin Pract 16;5(1):54–59. https://doi.org/10.1002/mdc3.12556
Pelosin E, Faelli E, Lofrano F et al (2009) Effects of treadmill training on walking economy in Parkinson’s disease: a pilot study. Neurol Sci 30(6):499–504. https://doi.org/10.1007/s10072-009-0141-8
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381. https://doi.org/10.1249/00005768-198205000-00012
Burini D, Farabollini B, Iacucci S et al (2006) A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Eura Medicophys 42:231–238
Podsiadlo D, Richardson S (1991) The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39(2):142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x
Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4:241–248. https://doi.org/10.1007/BF02260863
Beck AT, Steer RA, Ball R et al (1996) Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess 67(3):588–597. https://doi.org/10.1207/s15327752jpa6703_13
Mollaoglu H, Ucok K, Kaplan A et al (2012) Association analyses of depression, anxiety, and physical fitness parameters in Turkish obese adults. J Back Musculoskelet Rehabil 25(4):253–260. https://doi.org/10.3233/BMR-2012-0333
Tinetti ME, Richman D, Powell L (1990) Falls efficacy as a measure of fear of falling. J Gerontol 45(6):239–243. https://doi.org/10.1093/geronj/45.6.P239
Ozdilek B, Kenangil G (2014) Validation of the Turkish version of the Montreal cognitive assessment scale (MoCA-TR) in patients with Parkinson’s disease. Clin Neuropsychol 28(2):333–343. https://doi.org/10.1080/13854046.2014.881554
Akkurt H, Karapolat HU, Kirazli Y et al (2017) The effects of upper extremity aerobic exercise in patients with spinal cord injury: a randomized controlled study. Eur J Phys Rehabil Med 53(2):219–227. https://doi.org/10.23736/S1973-9087.16.03804-1
Beall EB, Lowe MJ, Alberts JL et al (2013) The effect of forced-exercise therapy for Parkinson’s disease on motor cortex functional connectivity. Brain Connectivity 3(2):190–198. https://doi.org/10.1089/brain.2012.0104
Duchesne C, Gheysen F, Bore A et al (2016) Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals. Neuroimage Clin 14(12):559–569. https://doi.org/10.1016/j.nicl.2016.09.011
Shulman LM, Katzel LI, Ivey FM et al (2013) Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol 70(2):183–190. https://doi.org/10.1001/jamaneurol.2013.646
Kurtais Y, Kutlay S, Tur BS et al (2008) Does treadmill training improve lower-extremity tasks in Parkinson disease? A randomized controlled trial. Clin J Sport Med 18(3):289–291. https://doi.org/10.1097/JSM.0b013e318170626d
Pohl M, Rockstroh G, Rückriem S et al (2003) Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson’s disease. Arch Phys Med Rehabil 84:1760–1766. https://doi.org/10.1016/S0003-9993(03)00433-7
Cakit BD, Saracoglu M, Genc H et al (2007) The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson’s disease. Clin Rehabil 21(8):698–705. https://doi.org/10.1177/0269215507077269
Hill M, Oxford S, Duncan M et al (2018) Arm-crank training improves postural stability and physical functioning in older people. Exp Gerontol 113:218–227. https://doi.org/10.1016/j.exger.2018.10.007
Bronas UG, Treat-Jacobson D, Leon AS (2011) Comparison of the effect of upper body-ergometry aerobic training vs treadmill training on central cardiorespiratory improvement and walking distance in patients with claudication. J Vasc Surg 53(6):1557–1564. https://doi.org/10.1016/j.jvs.2011.01.077
Zehr EP, Balter JE, Ferris DP et al (2007) Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J Physiol 582(1):209–227. https://doi.org/10.1113/jphysiol.2007.133843
Siragy T, Nantel J (2020) Absent arm swing and dual tasking decreases trunk postural control and dynamic balance in people with Parkinson’s disease. Front Neurol 11(4):1–10. https://doi.org/10.3389/fneur.2020.00213
Milosevic M, McConville KMV, Masani K (2011) Arm movement improves performance in clinical balance and mobility tests. Gait Posture 33(3):507–509. https://doi.org/10.1016/j.gaitpost.2010.12.005
Bostrom KJ, Dirksen T, Zentgraf K et al (2018) The contribution of upper body movements to dynamic balance regulation during challenged locomotion. Front Hum Neurosci 12:8. https://doi.org/10.3389/fnhum.2018.00008
Filippin NT, da Costa PH, Mattioli R (2010) Effects of treadmill-walking training with additional body load on quality of life in subjects with Parkinson’s disease. Rev Bras Fisioter 14(4):344–350. https://doi.org/10.1590/s1413-35552010005000016
Wu PL, Lee M, Huang TT (2017) Effectiveness of physical activity on patients with depression and Parkinson’s disease: a systematic review. PLoS One 27;12(7):e0181515. https://doi.org/10.1371/journal.pone.0181515
Baatile J, Langbein WE, Weaver F et al (2000) Effect of exercise on perceived quality of life of individuals with Parkinson’s disease. J Rehabil Res Dev 37:529–534
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Figen Dağ], [Özlem Bölgen Çimen], and [Okan Doğu]. The first draft of the manuscript was written by [Figen Dağ], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local Ethics Committee of Clinical Research (Protocol Number: 2018–130).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflicts interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dağ, F., Çimen, Ö.B. & Doğu, O. The effects of arm crank training on aerobic capacity, physical performance, quality of life, and health-related disability in patients with Parkinson’s disease. Ir J Med Sci 191, 1341–1348 (2022). https://doi.org/10.1007/s11845-021-02772-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02772-3