Abstract
Background
Rubella is caused by the rubella virus, a single-stranded RNA virus of the Togaviridae family. The most severe complications of rubella in adult women occur during pregnancy when infection can lead to miscarriage, stillbirth or congenital rubella syndrome. Antenatal rubella susceptibility screening is no longer performed in England, Scotland or Wales but continues in Northern Ireland.
Aim
The aims of this seroprevalence study were to (1) determine amongst women presenting for antenatal care the percentage of women who are rubella susceptible, rubella immune and those with equivocal rubella antibody levels by year of birth and (2) to consider how rubella vaccination resources can best be utilised.
Methods
A retrospective study was performed analysing all antenatal rubella IgG antibody tests performed between January 2015 and June 2017 inclusive (n = 19,000; excluding duplicate tests). All antenatal women were included regardless of the country of origin and age.
Results
From our analysis, 88.7% (n = 16,868) women had plasma concentrations of anti-rubella IgG > 10 IU/ml. 7.3% of women (n = 1403) had rubella IgG levels between 5 and 9.99 IU/ml, and 2.8% (n = 729) had IgG levels < 5 IU/ml. A decline in rubella immunity in younger women was evident.
Conclusions
This study has identified an increase in women who are rubella susceptible and women with equivocal rubella antibody levels. International evidence suggests that rubella serology is unreliable and antenatal screening does not confer any benefit to women during their current pregnancy. Consideration should be given to re-direct resources currently utilised for antenatal screening to facilitate the vaccination of pre-pregnancy and postpartum women and also to opportunistically offer vaccination to all women of childbearing age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rubella is caused by rubella virus, a single-stranded RNA virus of the Togaviridae family in the genus Rubivirus [1, 2]. The main symptoms of rubella include pyrexia, lymphadenopathy (cervical and posterior auricular) and a self-limiting maculopapular rash, which may be preceded by anorexia, mild catarrhal symptoms and malaise. Approximately 50% of rubella infections are subclinical [3]. Complications of rubella are uncommon and include post-infectious encephalomyelitis, meningoencephalitis and thrombocytopenia [1]. The incubation period for rubella averages 14–18 days (range 12–23 days). In adults and post-pubertal women, rubella infection can present as arthritis/arthralgia [3]. The most severe complications of rubella in adult women occur during pregnancy when infection can lead to stillbirth, miscarriage or congenital rubella syndrome (CRS) resulting in severe congenital anomalies in multiple organs [1]. The rate of vertical transmission and CRS is high when maternal infection occurs in the first 10 weeks of pregnancy and decreases thereafter [2].
The public health control of rubella and CRS is dependent on ensuring high levels of coverage with a rubella-containing vaccine within the population. Rubella vaccination is safe but contraindicated during pregnancy, because it contains live, attenuated viruses that pose a theoretical risk to the foetus [4, 5]. It has been estimated that in order to achieve complete elimination of CRS, the percentage of women of childbearing age susceptible to rubella infection should be < 5% [6]. After one dose of the measles, mumps and rubella (MMR) vaccine, 97–99% of women will be rubella immune, typically after 10–15 days [7, 8]. In Ireland, prior to the introduction of the combined MMR vaccine, a single rubella vaccine was offered to pre-pubertal girls in 1971. The combined MMR vaccine was then introduced in 1988 as a one-dose vaccine, followed by a change to a two-dose vaccine in 1992. In total, 106 cases of CRS were recorded in Ireland between 1975 and 1990 with two further cases between 1991 and 2000 [9]. The World Health Organisation (WHO) declared Ireland rubella free in April 2016, with the last confirmed acute rubella case notified in 2009 and a probable case (with exposure in another country) notified in 2014 [10].
Antenatal screening for rubella susceptibility in England stopped in April 2016. This was then followed by Scotland in June 2016 and Wales in October 2016. Testing is ongoing in Northern Ireland to date [11]. The WHO declared rubella as eliminated in the UK in 2012. Reasons cited by Public Health England for the cessation of antenatal rubella susceptibility screening included the rarity of rubella infection in pregnancy, to afford midwives more antenatal time with expectant mothers for clinical duties other than phlebotomy, the potential for inaccurate results from the current rubella-susceptibility screening test using serology and crucially that screening for rubella in pregnancy does not give any protection to the unborn baby in that pregnancy and that the test may falsely reassure some women that they are not susceptible to rubella infection if they have equivocal rubella IgG antibody results [12]. Antenatal rubella susceptibility testing continues in Ireland to date. The aims of this seroprevalence study were to determine amongst women presenting for antenatal care the percentage of women presenting for antenatal maternity care who are rubella susceptible, rubella immune or have equivocal rubella antibody levels and to consider how rubella vaccination resources can best be utilised.
Methods
A retrospective seroprevalence study was performed. All plasma rubella IgG antibody (anti-rubella IgG) results from women who had phlebotomy performed in our maternity hospital in Dublin, between January 2015 and June 2017 inclusive (n = 25,264) were collated into a dataset. From this total figure of all rubella testing performed, rubella IgG antibody results from women presenting at their antenatal booking appointment only, in both the public and private clinics, were extracted (n = 19,000; excluding duplicate tests over the study period). All antenatal women were included regardless of the country of origin and age. In-house testing is performed using Abbott Architect i2000. The distribution of anti-rubella IgG titres was assessed as one of three categories utilising international standard cutoff points as per manufacturer instructions: 0.0 to 4.9 IU/ml reported as ‘not detected’ indicating rubella susceptibility, 5.0 to 9.9 IU/ml reported as ‘equivocal’, ≥ 10.0 IU/ml is reported as ‘detected’ indicating rubella immunity. Further epidemiological data on patients within the dataset could not be extracted.
Results
From our analysis, 88.7% (n = 16,868) women had plasma concentrations of anti-rubella IgG > 10 IU/ml. 7.3% of women (n = 1403) had rubella IgG levels between 5 and 9.99 IU/ml, and 2.8% (n = 729) had IgG levels < 5 IU/ml. The high rate of vaccine uptake in the mid-1970s and early 1980s is evident with rubella antibody levels > 10 IU/ml detected in over 90% of women born between 1979 and 1983. Women born between the years 1990–1999 are more susceptible to rubella than women born in the two earlier decades. There is also a steady increase in the number of women with equivocal anti-rubella IgG titres.
Discussion
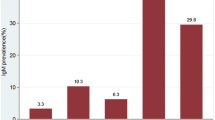
This retrospective rubella seroprevalence study of antenatal women highlights the changing epidemiology of rubella immunity in the Irish population. As illustrated in Fig. 1, plasma concentrations of anti-rubella IgG are highest in those born before 1970, prior to the introduction of vaccination, as although rubella-containing vaccines are highly immunogenic; they produce a lower antibody response than natural infection [13]. Following the introduction of the combined vaccine in the mid-1980s, there is a steady decline in rubella antibody levels. This is similar to a pattern previously described in Canada [14, 15], Spain [16] and Peru [17]. Younger women of childbearing age presenting to our maternity services have lower levels of rubella immunity compared to those born in earlier decades. This susceptible cohort of women with rubella IgG levels < 5 IU/ml, born in the late 80s and 90s, is likely to increase further in the coming years, as due to poor MMR uptake in the late 1990s following the now discredited scare regarding the MMR vaccine [18]. The steady increase in rubella IgG antibody levels that are in the equivocal range (5–9.9 IU/ml) can attribute in part to the absence of circulating wild-type rubella virus in the community. The absence of circulating wild-type rubella virus means that even though a person may have completed their MMR vaccines, the immunity boosting that occurs as a consequence of the immunogenic challenge posed when the a person comes into contact with the rubella virus, leading to a rise in rubella IgG levels, does not occur.
Rubella IgG antibody results from women presenting at their first antenatal booking appointment, in both public and private clinics, between January 2015 and June 2017 inclusive (n = 19,000). In-house testing is performed using Abbott Architect i2000. Interpretative results released: 0.0 to 4.9 IU/ml is reported as not detected, 5.0 to 9.9 IU/ml is reported as equivocal and ≥ 10.0 IU/ml is reported as detected
The current purpose of the antenatal rubella susceptibility testing at the booking appointment is to identify women who would benefit from rubella vaccination via the MMR in the postpartum period. Offering a postpartum rubella vaccination should reduce or eliminate rubella infection in any future pregnancies that the woman may have, thereby preventing CRS. In our current antenatal care system, by the time pregnant women are tested at their booking appointment, either public or private, and receive their rubella result, they are likely to have passed through the first trimester of pregnancy when rubella infection would be most catastrophic for the foetus. For women who are identified as rubella susceptible at screening, they too are usually at low risk of having when delivering a baby with CRS if they subsequently become infected with rubella, as they are often in the second and third trimesters when the risk has substantially decreased [19].
From a public health perspective, a move to ending antenatal rubella susceptibility testing in line with England, Scotland and Wales should be considered. Rubella serology testing is unreliable as an equivocal result may be identified in a woman who has completed the MMR vaccination schedule and rubella antibody IgG levels wane over time leading to increased foetal risk of CRS [20, 21]. As 50% of pregnancies in Ireland are unplanned, resources could be re-directed to general practitioners (GPs), practice nurses and family planning clinics to enable the education of women of childbearing age contemplating pregnancy to get their MMR status checked and also to offer opportunistic rubella antibody testing to all women of childbearing age [22]. Providing phlebotomy and MMR vaccination free of charge as an incentive to increase uptake is essential. Two doses of MMR vaccine should be given a month apart, and women should avoid becoming pregnant for at least a month after the second dose.
Special attention should be paid to encouraging women living in Ireland but born outside Europe to get vaccinated as they are more likely to be rubella seronegative [23]. In 2013, an Irish study using data collected by the National Perinatal Reporting System that reviewed rubella susceptibility found that younger women, first-time mothers and non-EU nationals were most at risk [24]. At antenatal booking if documented evidence of previous MMR vaccination cannot be obtained, this should be noted in the medical records and efforts should be made to vaccinate this high-risk group on the postnatal ward. Education should be provided with an interpreter present if necessary.
Given the current two-dose MMR schedule in Ireland, completing the second dose at the time of the mother’s 6-week check represents a convenient time to vaccinate while causing minimal disruption to a new mother. The engagement of new mothers with the healthcare system during the postnatal period is an opportunistic time to complete the MMR vaccination schedule as catch-up vaccination programmes are notoriously difficult to achieve a complete vaccination schedule. The MMR can be given to breastfeeding women [25].
Conclusions
In conclusion, our rubella seroprevalence study of 19,000 women demonstrates a decline in anti-rubella IgG titres. The majority of antenatal women in this study are rubella immune, but we have identified a rubella susceptible cohort of younger adults now presenting to maternity services. These women are at greater risk of CRS and also pose a potential risk for the re-emergence of rubella in Ireland. Given the similarity of the population of Ireland and England and the safe status of Ireland as rubella eliminated, the reasons cited by Public Health England are applicable here to consider stopping antenatal rubella susceptibility testing and directing resources towards pre-pregnancy and postnatal targeted rubella vaccination programmes and the opportunistic vaccination of all women of childbearing age, in order to protect against rubella sequelae in pregnancy using the MMR. Vaccination must be coupled with a comprehensive education and awareness campaign with particular emphasis on high-risk non-national women.
References
Lambert N, Strebel P, Orenstein W et al (2015) Rubella. Lancet 385(9984):2297–2307. https://doi.org/10.1016/S0140-6736(14)60539-0
White SJ, Boldt KL, Holditch SJ et al (2012) Measles, mumps, and rubella. Clin Obstet Gynecol 55(2):550–559. https://doi.org/10.1097/GRF.0b013e31824df256
Duclos P, Reef (2008) rubella. In: Heymann DL (ed) Control of communicable diseases manual. American Public Health Association, Washington, DC, pp 529–534
Public Health Wales NHS Trust (2017) Antenatal screening Wales. http://www.antenatalscreening.wales.nhs.uk/sitesplus/documents/989/V1e-Rubella%20FAQs%20Professionals%20June%202017.pdf. Accessed 19th Aug 2017
Plotkin SL, Plotkin SA (2004) Rubella vaccine. In: Plotkin S, Orenstein W, Offit P (eds) Vaccines, 4th edn. W.B. Saunders, Philadelphia, pp 707–743
Ministero della Salute. Piano nazionale per l’eliminazione del morbillo e della rosolia congenital 2003–2007. http:salute.gov.it/imgs/C_17_pubblicazioni_730_allegato.pdf. Accessed 15th Aug 2017
The MMR Discussion pack an information guide for health professionals and parents. Published by the Health Boards Executive, 2002. ISBN 0 9542449 1 5
World Health Organisation. Manual for the laboratory diagnosis of measles and rubella virus infection—second edition. http://www.who.int/ihr/elibrary/manual_diagn_lab_mea_rub_en.pdf. Accessed 23rd Aug 2017
Jennings S, Thornton L (1993) The epidemiology of rubella in the Republic of Ireland. Commun Dis Rep 3(8):R115–R117
Department of Health. Immunisation guidelines for Ireland. Chapter 20 Rubella (updated September 6th 2016). http://hse.ie/eng/health/immunisation/hcpinfo/guidelines/immunisationguidelines.html. Accessed 1st Aug 2017
Kowalzik F, Faber J, Knuf M (2017) MMR and MMRV vaccines. Vaccine 27(17):30959–30953. https://doi.org/10.1016/j.vaccine.2017.07.051
Department of Health, Welsh Office, Scottish Office Department of Health, DHSS (Northern Ireland) (1996) Immunisation against infectious disease. HMSO, London
Byrne L, Brant L, Reynolds C et al (2012) Seroprevalence of low rubella IgG antibody levels among antenatal women in England tested by NHS blood and transplant: 2004–2009. Is rubella susceptibility increasing? Vaccine 30(2):161–167. https://doi.org/10.1016/j.vaccine.2011.11.045
Macey JF, Tam T, Lipskie T et al (2011) Rubella elimination, the Canadian experience. J Infect Dis 204(Suppl 2):S585–S592. https://doi.org/10.1093/infdis/jir406
Gilbert NL, Rotondo J, Shapiro J et al (2017) Seroprevalence of rubella antibodies and determinants of susceptibility to rubella in a cohort of pregnant women in Canada, 2008–2011. Vaccine 35(23):3050–3055. https://doi.org/10.1016/j.vaccine.2017.04.057
Vilajeliu A, García-Basteiro AL, Valencia S et al (2015) Rubella susceptibility in pregnant women and results of a postpartum immunization strategy in Catalonia, Spain. Vaccine 33(15):1767–1772. https://doi.org/10.1016/j.vaccine.2015.02.043
Suárez-Ognio L, Adrianzén A, Ortiz A, Martínez C, Whittembury A, Cabezudo E, Oliveira L, Siqueira MM, Castillo-Solórzano C (2007) A rubella serosurvey in postpartum women in the three regions of Peru. Rev Panam Salud Publica 22(2):110–117. https://doi.org/10.1590/S1020-49892007000700005
Taylor B, Miller E, Farrington CP, Petropoulos MC, Favot-Mayaud I, Li J, Waight PA (1999) Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet 353(9169):2026–2029. https://doi.org/10.1016/S0140-6736(99)01239-8
Tookey P. Review of antenatal rubella susceptibility screening and the standard criteria for screening. https://www.google.ie/search?q=13.%09Tookey,+P.+Review+of+antenatal+rubella+susceptibility+screening+and+the+standard+criteria+for+screening.+&ie=utf-8&oe=utf-8&gws_rd=cr&ei=feWbWYu3NMGmwQLYk7nYBw. Accessed 22nd August 2017
Davidkin I, Jokinen S, Broman M et al (2008) Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis 197(7):950–956. https://doi.org/10.1086/528993
Vandermeulen C, Mathieu R, Geert LR et al (2007) Long-term persistence of antibodies after one or two doses of MMR-vaccine. Vaccine 25(37-38):6672–6676
McBride O, Morgan K, McGee H Crisis Pregnancy Programme Report Number 24. Irish contraception and crisis pregnancy study 2010 (ICCP-2010): a survey of the general population
Se B, Vangen S, Holter E et al (2011) Infectious immune status in an obstetric population of Pakistani immigrants in Norway. Scand J Public Health 39(5):464–470. https://doi.org/10.1177/1403494811399653
O'Dwyer V, Bonham S, Mulligan A, O'Connor C, Farah N, Kennelly MM, Turner MJ (2013) Antenatal rubella immunity in Ireland. Ir Med J 106(8):232–235
Australian Government Department of Health. The Australian immunisation handbook 10th edn. Chapter 4.18 Rubella
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
O’Connor, C., Le Blanc, D. & Drew, R.J. Epidemiological changes in rubella IgG antibody levels detected in antenatal women from a retrospective rubella seroprevalence study. Ir J Med Sci 187, 689–692 (2018). https://doi.org/10.1007/s11845-017-1722-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1722-9