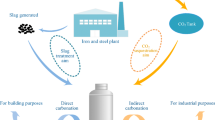
Abstract
Indirect carbonation as an efficient CO2 sequestration strategy has received extensive attention in recent years. This study proposes a two-step leaching indirect carbonation process using NH4Cl and CH3COOH in order to combine the advantages of the two leaching agents to obtain a better experimental outcome. The experimental results show that NH4Cl has a high pH buffering capacity, which can increase the pH of the mixed leachate to an alkaline level. Among the 21 groups of mixed leachate, 17 were alkaline (pH > 7). The mixed leachate has a high Ca2+ ions carbonation rate under low CH3COOH concentration conditions, and the carbonation rate of steel slag with a particle size of < 38 μm can reach 60% when the CH3COOH concentration is 0.1 M. SEM imaging showed that the CaCO3 formed by the reaction exists in a hexagonal calcite crystal form.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dramatic increase in the concentration of CO2 in the atmosphere has caused scientists to act. Forty years ago, in 1980, the concentration of CO2 in the atmosphere was 337 ppm; however, the number has now increased to 419 ppm,1 an increase of 24.3%. As a promising CO2 sequestration measure, carbon capture and storage (CO2 capture and storage, CCS) technology has received increasing attention in recent years. CCS technology includes three parts: CO2 capture, CO2 utilization, and CO2 storage.2 It uses certain technical means (such as chemical absorption, membrane separation, and adsorbent adsorption) to capture CO2 from combustion or gasification processes.3 The captured CO2 is then used in the food and beverage industry, chemical production (such as urea, methanol, etc.), and mineral carbonation,4 or it is pressurized (>100 bar) to be transported to different geological structures or geologically-relevant locations, including deep seas, sea beds, oil and gas fields, saline-alkali aquifers, and other places, for long-term storage.5 The use of CCS technology is considered an important approach for the reduction of the concentration of CO2 in the atmosphere,4 and thus it also plays a very important role in alleviating the ecological and environmental problems caused by the greenhouse effect.
Mineral carbonation is an important method of storage in CCS technology; that is, alkaline earth metal ions (mainly Ca2+ ions) within minerals react with CO2 to form thermodynamically stable carbonate, thereby achieving CO2 sequestration.6 The processes of mineral carbonation are classified as direct carbonation or indirect carbonation according to their technical route. Direct carbonation refers to a process in which mineral feed stock and CO2 react to form carbonate through a one-step reaction. Indirect carbonation includes two steps, a leaching step and a precipitation step; that is, the alkaline earth metal elements in the minerals are extracted with chemical reagents, and then CO2 is introduced into the leachate to react, producing pure carbonate.7 Indirect carbonation has greater advantages than direct carbonation because, firstly, the pH environment required for the leaching reaction and the carbonation reaction is different. An acidic environment is conducive to the leaching reaction, and an alkaline environment is beneficial for the carbonation reaction.6 Indirect carbonation separates the two steps so that they can be carried out in their respective, suitable pH environments; thus, a higher leaching rate and carbonation rate can be obtained.8 Secondly, carbonate generated by direct carbonation will deposit onto the surface of mineral particles,9 hindering further leaching of the alkaline earth metal ions, while the leaching reaction of indirect carbonation does not involve the deposition of carbonate, thus avoiding the reduction of the leaching rate. Finally, indirect carbonation can obtain pure carbonate, which can lead to considerable economic benefits.10,11
Steel slag is a byproduct of the steelmaking process, and is often used as a feed stock for mineral carbonation due to its special physical and chemical properties. Steel slag has a relatively high content of CaO (30–60%),12,13,14 which allows for the provision of more alkaline earth metal elements for the carbonation reaction. At the same time, due to the presence of free CaO, steel slag tends to be alkaline,15,16 which is quite advantageous for the carbonation reaction because CO32− ions are often formed in an alkaline environment.17 In addition, the iron and steel industries that produce steel slag also generate a large amount of CO2, which allows the steel slag to be used as a feed stock of mineral carbonation for nearby carbon sequestration,6 reducing transportation costs.
CH3COOH and NH4Cl are commonly used in the leaching reaction of indirect carbonation to extract Ca2+ ions from steel slag.10,18,19,20,21,22,23,24,25,26,27,28 As an acid, CH3COOH is more acidic than NH4Cl, so its leaching rate for Ca2+ ions is higher. However, the strong acidity also causes the pH of the leachate to be low, which makes the subsequent carbonation reaction difficult because it is hard for CO2 to dissolve in a low pH environment and produce CO32− ions. After the leaching reaction, the leachate contains CH3COO− ions. In the carbonation reaction, the introduction of CO2 will produce H+ ions and CO32− ions in the aqueous phase, and Ca2+ ions will react with CO32− ions to produce CaCO3. Because CH3COOH is a weak acid, the CH3COO− ions will combine with H+ ions to produce CH3COOH again. With the continuous introduction of CO2, the amount of CH3COOH will gradually increase. CH3COOH will dissolve CaCO3 and make the reacted Ca2+ ions dissolve back into the aqueous phase, which leads to a decrease in the carbonation rate.7 Eloneva et al.21 leached Ca2+ ions from steel slag with 1 M CH3COOH, and then carbonated the leachate. In the case of no alkali addition, the carbonation rate of 3% was obtained in the experiment. Bilen et al.20 conducted carbonation reaction of 0.7 M CH3COOH leachate, and their experimental results showed that the precipitation reaction would not occur without the addition of NaOH. For NH4Cl, its acidity comes from the H+ ions produced by the hydrolysis of the NH4+ ions, thus it is slightly lower than that of CH3COOH, and its ability to leach Ca2+ ions is also weak. During the leaching reaction, H+ ions are constantly consumed, which makes the equilibrium of the hydrolysis reaction shift to the right. After the leaching reaction, NH3·H2O is present in the leachate, so it is alkaline. During the carbonation reaction of the NH4Cl leachate, the H+ ions produced by the introduction of CO2 consume NH3·H2O to produce NH4+ ions, which will hydrolyze to produce NH3·H2O. Therefore, in the initial stage of the reaction, the NH4+/NH3·H2O pH buffer system formed in the aqueous phase will keep the pH of the aqueous phase stable for a long time, and this high pH environment is conducive to the carbonation reaction. Therefore, a higher carbonation rate of Ca2+ ions can be obtained from the leachate of NH4Cl. Eloneva et al.27 performed a carbonation precipitation reaction on different concentrations of ammonium salt leachate of steel slag, and the results showed that the carbonation rate of Ca2+ ions was 50–70%. Lee et al.29 carried out the carbonation reaction of 2 M NH4Cl leachate and obtained a carbonation rate of 60% under the optimal experimental conditions.
In view of the characteristics of the two leaching agents, this study proposes a combination of the two reagents to make use of their respective advantages, namely, the high Ca2+ ions leaching rate of CH3COOH and the high Ca2+ ions carbonation rate of NH4Cl, in order to obtain a better experimental outcome.
Materials and Methods
Materials
The steel slag used in the experiment was collected from Masteel (Ma Anshan, China), and three groups of different particle sizes were obtained by crushing, grinding and screening: < 38 μm, 38–75 μm and 75–150 μm. The slag was dried overnight at 105°C to remove moisture, and then stored in sealed bottles for later use. The chemical composition of the three groups of steel slag with different particle sizes was analyzed by x-ray fluorescence, and the results are shown in Table I. The main chemical components of the steel slag are CaO, MgO, SiO2, and Fe2O3. However, the chemical composition of the steel slag varied with the different particle sizes. The CaO content decreased with increasing steel slag particle size, while the Fe2O3 content increased with increasing steel slag particle size.
Glacial CH3COOH (> 99.5 wt.%, AR) was purchased from Saen Chemical Technology (Shanghai), NH4Cl was purchased from Shanghai Aladdin Biochemical Technology (> 99.8 wt.%, GR), and CO2 gas (purity > 99%) was purchased from Beijing Plex Practical Gas.
Method
NH4Cl and CH3COOH were used for the step-by-step leaching experiment, and the experimental flowchart is shown in Fig. 1. In the first step, NH4Cl solution was used to leach the Ca2+ ions in steel slag. After the leaching reaction, the slag obtained in the first step was filtered and reacted with CH3COOH. Finally, the leachates obtained in the first and second steps were mixed, and CO2 was introduced into the mixture for the carbonation reaction. In the experiment, 3 groups of steel slag with particle sizes of < 38 μm, 38–75 μm and 75–150 μm were selected. Seven groups of concentration ratios of NH4Cl and CH3COOH were selected: 2 M, 1.9/0.1 M, 1.8/0.2 M, 1.7/0.3 M, 1.6/0.4 M, 1.5/0.5 M, and 1.4/0.6 M. All the experiments were carried out at room temperature (25 °C).
The First Step of the Leaching Experiment
First, 100 mL of NH4Cl solution with the required concentration was prepared with deionized water, and then the solution was added to a three-neck flask and placed in a water bath. The temperature of the water bath was set at 25°C, and a temperature electrode and pH electrode were inserted into the aqueous phase of the flask. The experiment was stirred by a magnetic rotor, with the rotation speed set at 500 rpm. Subsequently, 5 g of steel slag was weighed, and when the temperature and pH were stable, steel slag was added to the three-neck flask to start the reaction. The reaction time was set to 30 min. After the reaction, the slurry was passed through a PTFE Millipore filter (0.45 μm pore size) to obtain the first leachate and filter residue. A small amount of filtrate was diluted to a certain concentration, and the concentration of Ca2+ ions was measured by inductively coupled plasma–atomic emission spectrometry (ICP-AES).
The leaching rate of the Ca2+ ions in the first leaching reaction (ηCa,1) was calculated by Eq. 1:
where CCa,1 (mg/L) is the concentration of Ca2+ ions in the leachate obtained in the first leaching experiment, V (L) is the volume of the leachate, mslag (g) is the mass of steel slag used in the experiment, xCaO (%) is the mass fraction of CaO in the steel slag, MWCaO (g/mol) is the molar mass of CaO and MWCa (g/mol) is the molar mass of Ca.
The Second Step of the Leaching Experiment
Deionized water was used to prepare 100 mL of CH3COOH solution at the required concentration, and this was added to a three-necked flask and placed in a water bath. A temperature electrode and pH electrode were inserted into the CH3COOH solution to measure the temperature and pH of the solution online, and the rotation speed was still set at 500 rpm. When the temperature and pH stabilized, the filter residue obtained in the first step was added to the flask to start the reaction. After 30 min, the reaction was finished, and the reaction slurry was filtered in the same above-mentioned way to obtain the second leachate and filter residue.
The leaching rate of Ca2+ ions in the second leaching reaction (ηCa,2) is calculated by Eq. 2:
where CCa,2 (mg/L) is the concentration of Ca2+ ions in the leachate obtained by the second-step leaching experiment.
Carbonation Experiment
The leachate obtained from the two-step leaching reaction was mixed in a ratio of 1:1, and the mixed solution was added into a three-neck flask. A temperature electrode and pH electrode were inserted into the mixed leachate. After the temperature and pH were stabilized, CO2 was introduced into the aqueous phase at a flow rate of 0.1 L/min for the carbonation reaction. The reaction time was set to 20 min. After the reaction, the carbonated slurry was filtered by a PTFE Millipore filter to obtain carbonated products and carbonated filtrate. A small amount of carbonated filtrate was diluted, and the concentration of Ca2+ ions was detected by ICP-AES.
The Ca2+ ions carbonation rate (ξCa) was obtained from Eq. 3:
where CCa,3 (mg/L) is the concentration of Ca2+ ions in the carbonated filtrate.
The experimental design is shown in Table II.
Results and Discussion
The pH of the Leaching Reaction
Steel Slag of < 38 μm
The pH variation during leaching process can be referred to in the online supplementary material, where Fig. S1a shows the pH variation of the steel slag leaching with different concentrations of NH4Cl. The pH of the leachate quickly stabilizes within 2 min of the reaction. At the same time, there was little difference in the pH between the NH4Cl leaching processes with different concentrations. After 30 min of reaction time, the pH was in the range of 8.69–8.89, which indicates that the NH4Cl leachate is alkaline. In addition, the pH of the leachate does not completely increase with a decrease in the concentration of NH4Cl. For example, the pH of 2 M NH4Cl was 8.74 after leaching for 30 min, while the pH of the leachate of 1.9 M NH4Cl was 8.69. However, overall, low-concentration NH4Cl leaching resulted in a high-pH leachate. This is because NH4Cl is a strong acid and a weak base salt, and because NH4+ ions will undergo a hydrolysis reaction to generate NH3·H2O, as shown in Eq. 4. The aqueous phases of NH4+ and NH3·H2O coexist during the reaction to form a pH buffer system, so the pH variation is not clear.
The pH of the CH3COOH leaching process is shown in Fig. S1b, from which it can be clearly seen that the pH of the leachate decreases with increasing CH3COOH concentration, and the pH of leachates with slightly differing concentration values differ greatly. For example, when leaching with 0.1 M CH3COOH, the pH of the leachate was 7.54 after 30 min of reaction time, while it was 6.21 at 0.2 M. At the same time, the time it took for the pH of the leachate to stabilize decreased with the increasing CH3COOH concentration. The pH stabilized after 10 min at 0.1 M, and after 2 min at 0.6 M. This is because there are fewer H+ ions that can be ionized under low-concentration CH3COOH leaching conditions, so the reaction takes a long time to reach equilibrium. Under high-concentration CH3COOH leaching conditions, the acid is more abundant than the slag, so the pH stabilizes faster.
The fitting of the pH variation curve of the leaching process found that the pH variation curves of the NH4Cl and CH3COOH leaching processes are in line with the curve \(y \, = \, \frac{{abx^{1 - c} }}{{1 + bx^{1 - c} }}\). That is, in the initial stage of the reaction, the pH increases rapidly with increasing time. After a certain period of time, the pH variation with time decreased, and in the last stage of the reaction, an increase in time did not lead to a variation in pH.
The pH of the mixed leachate of < 38 μm steel slag is shown in Fig. 2. Except for the 1.4/0.6 M group, the pH values of the mixed leachates are all greater than 8, which is alkaline. At the same time, the pH values of the mixed leachate of the 2 M, 1.9/0.1 M, 1.8/0.2 M, 1.7/0.3 M, and 1.6/0.4 M groups are all greater than 8.5. It should be pointed out that the pH values of the mixed leachate of the 1.7/0.3 M and 1.6/0.4 M groups were 5.36 and 4.98, respectively, which were relatively acidic, while the pH values of the mixed leachate were 8.66 and 8.63, respectively, indicating that the NH4Cl had a significant effect on raising the pH of the CH3COOH leachate. For the 1.4/0.6 M group, the pH of the mixed extract was lower, at only 5.44. This is because the pH of the 0.6 M CH3COOH leachate is 4.51, which is low, and the amount of NH4Cl is further reduced, which results in an insufficient alkalinity for the CH3COOH leachate; therefore, the pH of the mixed leachate is low.
Steel Slag of 38–75 μm
The pH variations in the 38–75 μm steel slag leached in NH4Cl and CH3COOH are shown in Fig. S2a and b, respectively. The pH of the NH4Cl leachate at the end of the reaction was not much different from that of the < 38 μm group, which indicated that the particle size had little effect on the pH of the NH4Cl leachate. This phenomenon, and the insignificant effect of NH4Cl concentration on the pH of the leachate in the previous section, suggest that the NH4Cl leachate is not sensitive to changes in the experimental parameters due to the presence of the NH4+/NH3·H2O buffer system. However, for the CH3COOH leachate, the pH of the 38–75 μm group was significantly lower than that of the < 38 μm group. For the 0.1 M CH3COOH leaching, the pH of the leachate of the < 38 μm group was 7.54, while the pH of the 38–75 μm group was 6.37, representing a decrease of 1.17 units. The pH of the leachate under other concentration conditions also decreased to different degrees. This shows that NH4Cl and CH3COOH are two reagents with different properties. NH4Cl is a strong acid and a weak base salt that generates H+ ions through the hydrolysis reaction of NH4+ ions, while CH3COOH is a weak acid that releases H+ ions through an incomplete electrolysis reaction, as shown in Eq. (5), which leads to the difference in pH of their respective leachates.
The pH variations of the 38–75 μm steel slag mixed leachate are shown in Fig. 3. The pH values of the mixed solutions of the 2 M, 1.9/0.1 M, 1.8/0.2 M, and 1.7/0.3 M groups were greater than 8, the pH values of the mixed solutions of the 1.6/0.4 M and 1.5/0.5 M groups were between 7 and 8, and the pH of the 1.4/0.6 M group was less than 7 (6.38). Compared with the < 38 μm group, the pH of the mixture in the 38–75 μm group decreased to a certain extent, but the decrease was not significant. This is also due to the buffering effect of the NH4Cl leachate.
Steel Slag of 75–150 μm
The pH variations of the 75–150 μm steel slag leached in NH4Cl and CH3COOH are shown in Fig. S3. The pH of the NH4Cl leachate was in the range of 8.57–8.74, which was not significantly lower than the results of the first two groups. For the CH3COOH leaching experiment, the pH of the leachate is in the range of 4.39–5.98, and the difference between its pH and the pH of the previous group of particle sizes is smaller than that of the former two groups, which shows that an increase in particle size has a weaker effect on the reaction, and that the relationship between the particle size and the effect on the reaction is not linear.
The pH of the 75–150 μm steel slag mixed leachate is shown in Fig. 4. The pH of the 1.5/0.5 M group dropped below 7 (6.37), the pH of 1.6/0.4 M dropped between 7 and 8 (7.72), and the pH of the other four groups was still greater than 8. It can be seen that the greater the amount of CH3COOH, the greater the drop in pH of the mixed solution.
Ca2+ Ions Leaching Rate
The changes in the Ca2+ ions leaching rate of the three groups of steel slags with different concentrations of NH4Cl and CH3COOH are shown in Fig. 5. The leaching rate of Ca2+ ions decreases with decreasing NH4Cl concentration and increases with increasing CH3COOH concentration. At the same time, as with the pH results, changes in the concentration of NH4Cl have little effect on the leaching rate of the Ca2+ ions, while changes in the concentration of CH3COOH have a more obvious effect on the leaching rate of the ions. For the < 38 μm particle size group, as can be seen from Fig. 5a, the leaching rate of the NH4Cl in the 1.8/0.2 M group was 4.25% lower than that of the 1.9/0.1 M group, while the leaching rate of the CH3COOH increased by 99.60%. At the same time, the Ca2+ ions leaching rate of the NH4Cl is relatively low. When leaching with 2 M NH4Cl, the leaching rates of the three particle size groups of steel slag were 38.65%, 28.77%, and 24.15%, while the leaching rates when leaching with 0.6 M CH3COOH were 31.93%, 29.87%, and 26.45%, respectively. The leaching rates of 0.6 M CH3COOH in the 38–75 μm group and the 75–150 μm group were even higher than that of 2 M NH4Cl, which shows that the leaching ability of CH3COOH is better than that of NH4Cl.
When leaching with 2 M NH4Cl, the leaching rate of the 38–75 μm group was reduced by 25.56% compared with the < 38 μm group, and the 75–150 μm group was reduced by 16.06% compared with the 38–75 μm group, which was similar to the variations in pH, indicating that the relationship between particle size and reaction effect is not linear. The larger the particle size, the smaller the inhibitory effect of increasing particle size on the reaction effect.
In addition, the Ca2+ ions leaching ability of CH3COOH for large particles of steel slag is better than that of NH4Cl. It can be seen from Fig. 5 that, as the particle size increases, the difference between the leaching rates of CH3COOH and NH4Cl gradually decreases. At the concentration of 1.9/0.1 M, the differences in the leaching rates of CH3COOH and NH4Cl in the three groups of particle sizes were 27.47%, 19.02%, and 14.04%, respectively. For the < 38 μm group, the Ca2+ ions leaching rate of all the concentrations of NH4Cl was higher than that of CH3COOH, while in the 38–75 μm group, the leaching rate of CH3COOH in the 1.4/0.6 M group exceeded that of NH4Cl. For the 75–150 μm group, there are 1.5/0.5 M and 1.4/0.6 M groups of CH3COOH with higher leaching rates than NH4Cl. This also shows that the leaching ability of CH3COOH is better than that of NH4Cl, because the specific surface area of large-sized steel slag is small, and the diffusion path of Ca2+ ions from the slag matrix to the surface is longer. Therefore, the leaching reaction of Ca2+ ions does not proceed easily, and the forward reaction necessitates more acidic conditions. The difference in the leaching rate between CH3COOH and NH4Cl decreases with increasing particle size, which indicates that the acidity of CH3COOH is stronger than that of NH4Cl, and its leaching ability for Ca2+ ions is also stronger.
The pH of the Carbonation Reaction
The pH variations during the carbonation reaction of the three groups of steel slag mixed leachate are shown in Fig. 6. Most of the pH variations result in a step shape; in the initial stage of the reaction (3–10 min), the pH drops slowly. Afterward, the pH goes through a rapid decline (approximately 90 s) to a lower level. Finally, the pH drops slowly until it stabilizes.
During the experiment, it was found that, in the initial stage of the reaction, the introduction of CO2 did not cause white precipitation in the leachate. White precipitation began to appear in the solution at about 3 min of reaction, and there was obvious white precipitation in the aqueous phase at approximately 4 min. This is because CO2 needs to be dissolved in the aqueous phase to form carbonic acid after it’s introduced. At the initial stage of the reaction, there are few CO32− ions produced by carbonic acid electrolysis, and the concentration product Q of Ca2+ ions and CO32− ions is less than the solubility product Ksp of CaCO3; therefore, no CaCO3 is produced. The dissolution of CO2 at the beginning of the reaction is the limiting link of the reaction, so the pH of the aqueous phase decreases slowly. As the amount of dissolved CO2 increases, the amount of carbonic acid in the aqueous phase also increases. At this time, carbonic acid continuously ionizes H+ ions, HCO3− ions, and CO32− ions, so that the pH of the aqueous phase drops rapidly. When the Ca2+ ions in the aqueous phase are consumed in large quantities and have a lower concentration, the concentration product Q of Ca2+ ions and CO32− ions also decreases. At this time, the carbonation reaction progresses slowly, and the amount of CO32− ions required for the reaction also decreases. Therefore, the ionization reaction of H2CO3 tends to balance, so the pH drops slowly until it stabilizes.
The above phenomenon occurs when the pH of the mixed solution is greater than 8. For the case of pH < 8, as in the experiments of 1.4/0.6 M in the < 38 μm group and the 1.6/0.4 M, 1.5/0.5 M, and 1.4/0.6 M in the other two particle size groups, the pH first drops rapidly and then stabilizes. This is due to the higher concentration of CH3COOH in these groups of experiments, which resulted in more CH3COO− ions in the leachate. When CO2 is introduced into the aqueous phase, CH3COO− will combine with the H+ ions ionized by H2CO3 to generate CH3COOH, which accelerates the ionization of H2CO3 and makes the pH drop rapidly. At the same time, the low pH makes it hard to generate CO32− ions, so the carbonation reaction proceeds with difficulty, and there is little white precipitate in the aqueous phase. When the combination of CH3COO− ions and H+ ions reaches equilibrium, the ionization reaction of H2CO3 also tends to equilibrium, so the pH slowly stabilizes.
Ca2+ Ions Carbonation Rate
The Ca2+ ions carbonation rate of the three groups of steel slag leachates is shown in Fig. 7. For the < 38 μm group, when leaching with 2 M NH4Cl, the Ca2+ ions carbonation rate was 76.54%. As the concentration of CH3COOH increases and the concentration of NH4Cl decreases, the carbonation rate gradually decreases. The carbonation rate of the 1.9/0.1 M group was 60%, while that of the 1.4/0.6 M group was only 3.05%, and almost no carbonation reaction occurred. The trend in the carbonation rate is not consistent with the trend in the pH of the mixed leachate. Regarding the pH of the mixture, it can be seen from Fig. 2 that, except for the 1.5/0.5 M and 1.4/0.6 M groups, the pH values of the other 5 mixtures were all above 8.6, with no obvious fluctuations. However, the carbonation rate decreased considerably. The carbonation rate of the 1.6/0.4 M group was 23.62%, which was 69.14% lower than that of the 2 M group. This is because, as the concentration of CH3COOH increases, the amount of CH3COO− ions in the leachate also increases. CH3COO− ions will combine with the H+ ions ionized by H2CO3 to form CH3COOH, thus making it difficult for the carbonation reaction to occur. This result shows that the ionic system of the leachate has a significant effect on the carbonation rate, and that its effect is much greater than that of pH. Therefore, in order to control the precipitation reaction during indirect carbonation, attention should be given not only to the pH of the leachate but also to the ionic system of the leachate. Under the same pH condition, the carbonation rate of the leachate of different ionic systems will also have considerable differences.
The carbonation rate of the 38–75 μm group was lower than that of the < 38 μm group. Under the condition of 1.6/0.4 M, the pH values of the leachate of the < 38 μm group and the 38–75 μm group were 8.63 and 7.95, and the carbonation rates were 23.62% and 8.59%, respectively, which indicates that the particle size has a greater impact on the pH of the leachate. At the same time, the pH has a significant effect on the carbonation rate. When the pH drops by 0.68 units, the carbonation rate drops by 63.63%. In addition, the pH of the 1.6/0.4 M group of the 38–75 μm group was lower than that of the 1.5/0.5 M of the < 38 μm group (8.05), but its carbonation rate was higher than that groups (4.79%), which also shows that the effect of the leachate ionic system on the carbonation rate is greater than the effect of pH. When the concentration of CH3COOH in the 1.5/0.5 M group is higher, the amount of CH3COO− ions in the leachate is greater, and the inhibitory effect of CH3COO− ions on the carbonation reaction is more significant, so its carbonation rate is lower. The carbonation rate of the 75–150 μm group was further reduced, and the carbonation rate of the four groups was lower than 10%, including the 1.7/0.3 M group with a pH of 8.14. This is because the large particle size makes the reaction less complete, the amount of CH3COOH that does not participate in the reaction is greater, and its inhibitory effect on the carbonation reaction is more obvious. The carbonation rate under the condition of 1.9/0.1 M concentration was 29.67%, which was 50.55% lower than in the <38 μm group (60%).
Figure 8 shows the x-ray diffraction pattern of the carbonation product. It can be seen that the reaction product is mainly calcite, which indicates the formation of CaCO3. Figure 9 shows the scanning electron microscopy morphology of the CaCO3 formed by the precipitation reaction. The CaCO3 exists in a hexagonal calcite crystal form, with a crystal grain size of approximately 2.5–5 μm. The crystal grains are agglomerated, and the size of the agglomerates is approximately 18–24 μm. The formation of CaCO3 show that the carbonation reaction can take place in the case of multistep leaching using NH4Cl and CH3COOH without the addition of alkali.
Conclusion
This study explored the experimental effects of the indirect carbonation reaction of two-step leaching using NH4Cl and CH3COOH. Among the 21 groups of mixed leachate, 17 groups had an alkaline pH (pH > 7), while the pH of the CH3COOH leachate was acidic except for the 0.1 M group of < 38 μm particle size. This indicates that the NH4Cl leachate has a strong pH buffering capacity and can effectively increase the pH of the mixed leachate, which is favorable for the subsequent precipitation reaction. The pH variations of the mixed leachate are similar to the pH variations of the NH4Cl leachate; that is, the fluctuation is not large, and it is mostly contained between 8 and 9. The mixed leachate of NH4Cl and CH3COOH has a higher Ca2+ ions carbonation rate, because the NH4Cl leachate drives an increase in the pH of the mixed leachate, creating a suitable alkaline environment for the precipitation reaction. However, the trend in the carbonation rate is not completely consistent with the trend in pH; that is, as the concentration of CH3COOH increases, the carbonation rate undergoes a greater decrease. This result shows that the ionic system of the leachate has a significant effect on the carbonation rate, and that its effect is much greater than that of the pH.
This study shows that a two-step leaching reaction that uses NH4Cl and CH3COOH can combine the advantages of the two leaching agents to obtain a higher pH leachate and a higher Ca2+ ions leaching rate. In view of the fact that the Ca2+ ions carbonation rate is greatly affected by the increase of CH3COOH concentration, we will explore in future research how to increase the Ca2+ ions carbonation rate of the leachate with high pH and high CH3COOH concentration to take further advantage of this two-step method.
References
CO2. Earth. “Trends in Atmospheric Carbon Dioxide”(CO2. Earth. 2007), https://www.CO2.earth/. Accessed 2 Sept 2021.
M.E. Boot-Handford, J.C. Abanades, E.J. Anthony, M.J. Blunt, S. Brandani, and N. Mac Dowell, Energy Environ. Sci. 7, 130 (2014).
N. Macdowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, and G. Jackson, Energy Environ. Sci. 3, 129 (2010).
M. Bui, C.S. Adjiman, A. Bardow, E.J. Anthony, A. Boston, and S. Brown, Energy Environ. Sci. 11, 1062 (2018).
K.S. Lackner, Science 300, 1677 (2003).
Y.B. Luo, and D.F. He, Environ. Sci. Pollut. Res. 7, 947 (2021).
Y.B. Luo, and D.F. He, J. Sust. Metall. 28, 49383 (2021).
A. Azdarpour, M. Asadullah, and E. Mohammadian, Chem. Eng. J. 279, 615 (2015).
W.J.J. Huijgen, G.J. Witkamp, and R.N.J. Comans, Environ. Sci. Technol. 39, 9676 (2005).
A. Said, H.P. Mattila, and M. Järvinen, Appl. Energy. 112, 765 (2013).
R. Zevenhoven, D. Legendre, and A. Said, Energy 175, 1121 (2019).
S. Yadav, and A. Mehra, Waste Manag. 64, 348 (2017).
S.H. Kim, S. Jeong, and H. Chung, Waste Manag. 103, 122 (2020).
M. Salimi, and A. Ghorbani, Appl. Clay Sci. 184, 105390 (2020).
P. Suer, J.E. Lindqvist, and M. Arm, Sci. Total Environ. 407, 5110 (2009).
C. Barca, C. Gérente, and D. Meyer, Water Res. 46, 2376 (2012).
Y.T. Yuen, P.N. Sharratt, and B. Jie, Environ. Sci. Pollut. Res. 23, 22309 (2016).
S. Teir, S. Eloneva, and C.J. Fogelholm, Energy 32, 528 (2007).
S. Eloneva, S. Teir, and J. Salminen, Energy 33, 1461 (2008).
M. Bilen, M. Altiner, and M. Yildirim, Part. Sci. Technol. 36, 368 (2018).
S. Eloneva, S. Teir, and S. Eloneva, Ind. Eng. Chem. Res. 47, 7104 (2008).
W.J. Hung, K.I. Lai, and Y.W. Chen, Ind. Eng. Chem. Res. 45, 1722 (2006).
D. Dionysiou, M. Tsianou, and G. Botsaris, Ind. Eng. Chem. Res. 39, 4192 (2000).
W. Bao, H. Li, and Y. Zhang, Ind. Eng. Chem. Res. 49, 2055 (2010).
S. Kodama, T. Nishimoto, and N. Yamamoto, Energy 33, 776 (2008).
Y. Sun, M.S. Yao, and J.P. Zhang, Chem. Eng. J. 173, 437 (2011).
S. Eloneva, S. Teir, and H. Revitzer, Steel Res. Int. 80, 415 (2009).
M. Owais, M. Jarvinen, and P. Taskinen, J. CO2 Util. 31, 1 (2019).
S.M. Lee, S.H. Lee, and S.K. Jeong, J. Ind. Eng. Chem. 53, 233 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51534001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, Y., He, D. Indirect Carbonation by a Two-Step Leaching Process Using Ammonium Chloride and Acetic Acid. JOM 74, 1958–1968 (2022). https://doi.org/10.1007/s11837-022-05217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05217-z