Abstract
Flue gas recirculation (FGR) has been implemented for exhaust gas emissions reduction in iron ore sintering. However, the mechanism of NO x reduction through FGR is still unclear. In this paper, the laboratory pot-grate sintering test showed a 30% reduction in gas flow and 15.51% reduction in NO x emissions achieved with a 30% FGR ratio, and the sinter indexes almost matched those of the conventional process. In the sinter zone, NO-CO catalytic reduction occurs in the range of 500–900°C. When the sinter temperature is 700°C, the highest nitrogen reduction ratio (NRR) achieved is 8%; however, the NO x reduction is inhibited as the post-combustion of CO starts when the temperature increases beyond 700°C. NO x in the flue gas is mainly a product of the fuel combustion in the combustion zone, as the nitrogen conversion rate reaches 50–60%, because the N-containing intermediates exist during the fuel combustion. The existence of NO in the FGR gas inhibits the NO x generation from the fuel combustion, and the NO elimination—through the NO-carbon reaction—is significant in the combustion zone. The NRR in the combustion zone reaches a range of 18–20%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the emission control regulations get stricter, the NO x reduction in the sintering process becomes an important environmental concern owing to its role in the formation of photochemical smog and acid rain.1 The NO x emissions from the iron and steel industry account for approximately 6% of the total emissions from all industry. Meanwhile, NO x emissions from the sintering machine represent approximately 48% of the total emissions from the iron and steel industry.2 , 3 Thus, it is essential to reduce NO x emissions from the sintering machine in order to contribute to the cleaner production of sinter.
There are three methods to reduce the NO x emissions from the sintering plant: source control, process control, and end-of-pipe techniques.4 In the sintering plant, over 90% of NO x comes from the fuel-NO x .5 The application of fuel with low nitrogen content in the sinter mixture is a practical method for reducing NO x emissions,6 but the availability of low-nitrogen fuel is limited. The process control is generally described as adding certain additives, such as carbohydrate, Ca-Fe oxides, ammonia, and modified coke, into the sinter mixture to inhibit NO x generation.7,8, – 9 The efficiency of the process control method is relatively lower than the source control method as there are few appropriate additives to use. The end-of-pipe techniques of flue gas denitration have developed rapidly in recent years. Flue gas denitrationis (DeNO x ) are divided into three categories: wet DeNO x process, semi-dry DeNO x process and dry DeNO x process. The dry DeNO x process dominates in the flue gas denitration techniques, as it has the highest efficiency, which can reach 80–90%.10 , 11 Owing to the high gas flow rate and low NO x concentration of flue gas from the sinter plant, the DeNO x techniques face major problems, such as the high cost of investment/operation and low utilization of by-products.
The flue gas recirculation (FGR) technique was proposed in the twentieth century to reduce exhaust gas emissions and reuse waste heat obtained from the gases recycled into the sintering bed.12 Exhaust gas recirculation can reduce the emissions of NO x and PCDD/Fs owing to their decomposition in the sinter bed. SO x is absorbed or filtered by the sinter layer and the CO is reused as fuel.13,14, – 15 However, the NO x content of exhaust gas increases even though a 20–45% reduction in NO x emissions is achieved when using the FGR technology. The NO x content in the stack should obviously be reduced to comply with the stricter environmental regulations.
This paper attempts to reveal the NO x reduction behavior in the sintering bed during the FGR process, and then proposes the mechanism or operating parameters that contribute to further reduction in the NO x content of exhaust gas in the FGR sintering.
Materials and Methods
Materials
The raw materials used in the sinter pot test include the iron ore blending, fuels and fluxes (dolomite, limestone, quicklime) to produce a sinter of TFe 58.11%, SiO2 4.88%, basicity (R = CaO/SiO2) 1.85%, and MgO 1.60%. The chemical compositions of the raw materials and their mass fractions are given in Table SI. The proximate analysis of coke breeze was investigated on a dry basis. The fixed carbon, ash, and volatile content were 82.72%, 14.36%, and 2.92% respectively. Meanwhile, the nitrogen content was 0.72%.
Methods
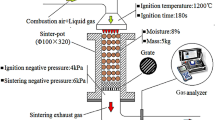
Sinter Pot Tests
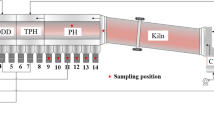
The sinter pot tests were conducted in a laboratorial pot that was 100 mm in diameter and 700 mm in height. The process of the sinter pot tests included ore proportioning, mixing, granulation, ignition, sintering, cooling, sieving, and quality testing of the sinter.16 For simulating the FGR process, a sealed cover was added on top of the sintering pot after ignition, and the FGR gas was introduced onto the surface of the pot through the pipeline. The simulation of the FGR system included a gas-blending system, a steam generator, and a pre-heating furnace. The FGR gas was simulated by mixing air and standard gases (including O2, CO, CO2, NO, and N2), and then steam was introduced into the sintering bed after pre-heating by the vertical furnace. Furthermore, the components and the temperature of the inlet gases were controlled by a gas analyzer. In addition, the properties of the exhaust gas were investigated by another gas analytical instrument (MGA 5; MRU, Germany) during the sintering process.
The components of the FGR gas were calculated based on the principles of the exhaust gas emission rule and mass balance. Table I shows the components and temperature of the FGR gas. As compared to the conventional process, the CO x and steam contents increased, while the O2 content reduced with an increase in the FGR ratio. The O2 content was 15.43%, and the steam content increased to 4.86% when the FGR ratio was 35%. Moreover, the temperature of the FGR gas increased from room temperature to 250°C. The sinter indexes, including vertical sintering velocity (VSV), productivity (P), yield (Y), and tumble index (TI) were investigated after sintering.15
Sinter and Combustion Zone Simulations
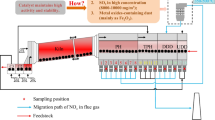
In this paper, the reaction behaviors of NO x in the FGR gas during the sintering process were mainly studied in the sinter and combustion zones, since NO x hardly changed in the sub-layer. The reaction behaviors of NO x in the sinter and combustion zones were investigated in a quartz fixed-bed reactor of a 10 mm diameter and 50 mm height, and the schematic diagram of the sintering apparatus is shown in Fig. S1. The experimental apparatus includes gas atmosphere simulation, heat pattern simulation, and exhaust gas monitoring.
When taking a sinter zone simulation test, 40 g of sinter of 5–8 mm, obtained from the sinter pot test, were charged into the charging cup. The sinter zone temperature was set to the same pre-heat temperature of 500–900°C, as almost no heat was released from the sinter samples. While conducting a combustion zone simulation test, 40 g of the granulated mixture of 5–8 mm was charged into the charging cup. The granulated mixture came from the granulating drum and was dried in advance. The temperatures of the combustion zone varied from 1100°C to 1300°C, which was achieved by setting the pre-heat temperature according to the curve based on a series of experiments on the relationship between the temperature of the sinter bed and the setting temperature of the electric furnace. After charging, a special content of standard gases (O2, CO, CO2, NO, and Ar) was introduced into the quartz tube from the bottom of the apparatus and passed through the charging cup after mixing. Meanwhile, the gas velocity was kept constant at 18 m min−1, which was the same as the sinter pot test. The compositions in the outlet gas were detected by a gas analyzer along with the heating period. The results were expressed in terms of NO x reduction ratio (NRR), which is the mole ratio of the reduced NO to that supplied:
where NRR is the NO x reduction ratio (%), C i is the NO x concentration of the outlet gas (mol m−3), and C 0 is the NO x concentration of the inlet gas (mol m−3).
Results and Discussion
Effect of Flue Gas Recirculation on the Sintering Process
Table II presents the effect of the FGR technique on the sintering process, including the sinter indexes and properties of the exhaust gas. Compared with conventional sintering, the sinter indexes increased when the FGR ratio was <30%, while the sinter indexes started to decrease when the FGR ratio was 35%. The total flow of exhaust gas significantly decreased in the case of the FGR technique. The concentrations of the individual gas compositions of the exhaust gas increased except in the case of O2, while the total gas emissions significantly decreased with the increase in the FGR ratio. Meanwhile, the temperature of the exhaust gas was higher in the FGR technique than the conventional process. Considering the sinter indexes and the exhaust gas reduction, the FGR ratio is no more than 30%.
Effect of Flue Gas Recirculation on the NO x Reduction
Table III presents the effects of the FGR technique on the NO x reduction in the sinter pot test. Compared with the conventional process, the nitrogen conversion rate is reduced gradually with the increase in the FGR ratio owing to the low O2 content in the combustion atmosphere; however, the NRR was not similar to the flow reduction rate, as the NO x content in the emissions increased. A 35% reduction in gas flow was achieved in the case of a 35% FGR ratio. In addition, the nitrogen conversion rate using the FGR technique was 30.15%, which was significantly lower than the 58.09% obtained with the conventional process. However, the NO x content in the exhaust gas increased from 589 mg m−3 to 746 mg m−3, which led to a 17.70% reduction in the NO x emissions. In other words, the specific NO x reduction could reach 374 g NO x per ton of sinter product when the FGR ratio was 35%.
NO x Reduction in the Sinter Zone
During the FGR process, the FGR gas, consisting of O2, CO x , NO x , etc., went through the sinter zone. The NO x content in the FGR gas was reduced because of the NO-CO catalytic reaction occurring in the range of 500–900°C, whereas the post-combustion of CO starts at a temperature >700°C. In order to investigate the NO x reduction behavior in the sinter zone, the mixing gas consisted of 16% O2, 4% CO2, 0.45% CO, and 345 ppm NO, while Ar gas was introduced through the sinter zone at a different temperature. Figure 1 shows the NO x reduction ratio in the sinter zone at a different temperature. NO x and CO contents in the outlet gas changed slightly when the sinter temperature was at 500°C or lower, while NO x and CO significantly decreased between 600°C and 700°C, which means that the NO x reduction ratio in the sinter zone increased. When the sinter temperature was 700°C, the NRR achieved a maximum value of 8%. However, when the sinter temperature continued to rise from 800°C to 900°C, the NO–CO reduction reaction decreased, because the post-combustion of CO was enhanced. In summary, the NO-CO catalytic reaction in the sinter zone proceeded rapidly at 700°C, and the CO in the FGR gas could be combusted completely in the high-temperature (>800°C) sinter zone.
NO x Reduction in the Combustion Zone
The NO x formations in the sintering process originate from the volatile-N and char-N oxidation in the fuel combustion process. However, approximately 70–90% of the nitrogen content of the fuel is separated out in volatiles at a temperature >1000°C. Figure 2 shows the NO x generation behavior during fuel combustion. As described, various nitrogen-containing intermediates are produced during the fuel combustion, which participated in the NO x generation or reduction homogeneously or heterogeneously. NO x generation is conducted by the routes ⑤, ⑥, ⑦ and ⑧, while the existence of NO x in the circulating flue gas inhibited the generation routes, as well as accelerating the NO x reduction routes ①, ②, ③ and ④. Meanwhile, the nitrogen in the fuel was split into nitrogen-containing intermediates (NH i and HCN) more easily under a low O2 partial pressure, which facilitated the NO x reduction. Most importantly, the existence of active reducing agents in the combustion zone, such as CO and coke, could improve the results obtained with route ③ significantly.
In order to emphasize the effect of NO x in the FGR gas on NO x generation in the combustion zone, three combustion zones with different maximum temperatures were set. Figure 3 shows the comparison of NO x reduction ratio in the different combustion zones, with and without 320 ppm of NO gas. With the increase in the temperature of the combustion zone, the NO x content in the outlet gas increased under the air condition. Furthermore, the burning velocity of fuel accelerated. The existence of 320 ppm of NO in the gas atmosphere barely affected the burning velocity of the fuel. However, the total NO x emissions from the fuel combustion decreased significantly, even though the NO x content in the outlet gas increased. When the temperature of the combustion zone varied from 1100°C to 1300°C, the NRR in the combustion zone was in the range of 18–20%. This means that the existence of NO gas in the FGR gas inhibits the NO x generation from fuel combustion, and the NO elimination—through a NO-carbon reaction—is also significant in the combustion zone.
Conclusion
-
1.
The laboratory test results showed that a higher FGR ratio results in a higher reduction in NO x emissions; however, the higher FGR ratio also results in a decrease in sinter indexes owing to the significantly reduced O2 content of the FGR gas. When the FGR ratio is 30%, the yield decreases from 69.24% to 68.20% and the tumble index decreases from 52.70% to 51.75%. Thus, the appropriate FGR ratio of the emission optimised sintering (EOS)-like technique is <30% on the consideration of the sinter indexes.
-
2.
When the FGR gas goes through the sinter zone, the NO x reduction is mainly going in the direction of a NO-CO catalytic reaction with temperatures ranging between 500°C and 900°C. When the sintering temperature reaches 700°C, the NRR could reach 8%; however, the post-combustion of CO starts at a temperature >700°C, which inhibits the NO x reduction.
-
3.
The NO x generation mainly takes place in the combustion zone, and the nitrogen conversion rate reaches a value between 50% and 60%, because the N-containing intermediates exist during the fuel combustion. Besides the NO-CO catalytic reduction and NO oxidation, the NO x reactions in the combustion zone also include the NO-carbon reaction and the CH i /NH i -NO reactions, which are enhanced under a higher temperature or lower O2 partial pressure. When the temperature of the combustion zone varied between 1100°C and 1300°C, the NRR in the combustion zone reached a value in the range of 18–20%.
References
Y. Chen, Z. Guo, and Z. Wang, ISIJ Int. 48, 1517 (2008).
T.Y. Zhu, Technology of Sintering Flue Gas Cleaning (Beijing: Chemical Industry Press, 2009), pp. 1–5.
L.L. Fang (Master Thesis, Shandong University, Jinan, Shandong Province, 2007), pp. 13–14.
Y.G. Chen, Z. Wang, and Z.C. Guo, Acta Sci. Circumst. 28, 1720 (2008).
C.L. Mo, C.S. Teo, I. Hamilton, and J. Morrison, ISIJ Int. 37, 350 (1997).
C.G. Jin, H.G. Su, and L.J. Nam, SO x and NO x reducing method of sintering discharging gas. Korean Patent, Appl. 20000070570 (2002).
K. Morioka, S. Inaba, M. Shimizu, K. Ano, and T. Sugiyama, ISIJ Int. 40, 280 (2000).
X.G. Bi, J.Y. Liao, W. Xiong, G.F. Zhou, and Z.H. Feng, J. Wuhan Univ. Sci. Technol. 31, 449 (2008).
Y.G. Chen, Z. Wang, and Z.C. Guo, Acta Sci. Circumst. 28, 1727 (2008).
M. Gan (Ph.D. Dissertation, Central South University, Changsha, Hunan Province, 2012), pp. 12–15.
T. Terris and B. Hsiaotao, Environ. Sci. Technol. 43, 5049 (2009).
S. Roudier, L.D. Sancho, R. Remus, and M. Aguadomonsonet, JRC-IPTS Working Papers (Luxembourg: Publications Office of the European Union, 2013).
S. Ikehara, Y. Terada, S. Kubo, and J. Sakuragi, Nippon Steel Technical Report, Japan, 70, 55 (1996).
N. Menad, H. Tayibi, F.G. Carcedo, and A. Hernández, J. Clean. Prod. 14, 740 (2006).
X.H. Fan, Z.Y. Yu, M. Gan, W.Q. Li, and Z.Y. Ji, J. Iron Steel Res. Int. 20, 1 (2013).
X. Fan, Z. Yu, M. Gan, X. Chen, T. Jiang, and H. Wen, ISIJ Int. 54, 2541 (2014).
Acknowledgements
This research was financially supported by the outstanding and creative doctor scholarship of Central South University (Grant No. 2013bjjxj015), the 2014 Hunan Provincial Innovation Foundation for Postgraduate (Grant No. CX2014B094) and National Natural Science Foundation of China (Grant No. 51474237).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Z., Fan, X., Gan, M. et al. NO x Reduction in the Iron Ore Sintering Process with Flue Gas Recirculation. JOM 69, 1570–1574 (2017). https://doi.org/10.1007/s11837-017-2268-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2268-z