Abstract
One of the most peculiar biological interactions between pollinators and plants is the use of flowers as sleeping places, but this phenomenon is still poorly understood and it has been proposed to use citizen science in the form of volunteer records to fill the knowledge gaps. In this work, we report for the first time on the use of flowers as sleeping places by five species of Chilean flies of the genus Lasia (Acroceridae) in central Chile. In addition, we seek to determine whether the flower shape and/or color might be good predictors for flies using them as sleeping places. We used standardized records from a long-term citizen science project that exclusively monitors flies. We counted the number of flies that used flowers as a sleeping place and discretized the morphological variables of the flowers to relate both responses and predictors with a generalized linear model. We found that flowers belonging to the genus Alstroemeria, followed by Clarkia and Salpiglossis, were the most used as sleeping places. Our results suggest that zygomorphic flowers (with bilateral symmetry, and usually tubular flowers) are a better predictor than actinomorphic flowers and their color. The use of zygomorphic flowers could represent a better option for flies in adverse environmental conditions or to avoid predators, but the use of flowers as sleeping places could be an as-yet poorly understood way of pollinating plants. Citizen science, although it has some limitations (taxonomic, spatial or temporal biases), has great potential for describing new biological interactions in a changing world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Around 87.5% of angiosperms rely on animals, such as insects, bats, or birds, for their reproduction and seed production. Those animals, in turn, which visit flowers gain pollen, nectar, and other resources as floral rewards (Ollerton et al. 2011; Giannini et al. 2013). At least ~ 350,000—but in all likelihood more than a million – insect species visit flowers for different purposes (Wardhaugh 2015). Paradoxically, little is known about many of these flower-animal interactions even though they have a significant impact on both plant and animal survival and reproduction. These knowledge gaps are known as Eltonian shortfall (Hortal et al. 2015), and many of these biological interactions probably face extinction risk (Rezende et al. 2007; Cardoso et al. 2011). To partially solve these gaps, some authors have proposed capitalizing on secondary information from records obtained during citizen science projects (i.e., projects in which citizens help to gather information on biodiversity or environmental phenomena; Callaghan et al. 2021; Groom et al. 2021). The massive scale of current technological resources such as smartphones with internet availability, social media platforms and high-resolution cameras has notably increased the number of recorded species interactions, such as prey-predator (e.g., Barahona-Segovia and Pañinao-Monsálvez 2020), host-parasitoid (e.g., Doherty et al. 2021), or flower-visitor (e.g., Taylor et al. 2020) and this information could greatly increase our understanding and shape future actions regarding important ecosystem services like food security or conservation biological control.
Although there is a great diversity of arthropod species that visit flowers (Wardhaugh 2015), the best-known interactions are those involving bees and bumblebees and a significant number of citizen science projects are currently focusing on this group of insects in different ecosystems and on different continents (e.g., Bloom and Crowder 2020; Gardiner and Roy 2022; Koffler et al. 2021; Lander 2020). The main resources that flowers deliver to these pollinators are pollen, nectar, and vegetable oils. However, it is well-known that flowers can also be used as sleeping places, although there are only scant records for different ecosystems, mainly of wild bees, in existence (Alves-dos-Santos et al. 2009; Watts et al. 2013; Pinheiro et al. 2017; Sabino et al. 2017). Male bees emerge a few days before females for the mating season and in the meanwhile they use the same flowers for feeding and as sleeping places (Alves-dos-Santos et al. 2009). Flower species can simultaneously host one or several individuals (ca. 30 Eucerinae bee in Iris atropurpurea Baker) and the morphology of the flower suggests the total number of bees that it can accommodate (Alves-dos-Santos et al. 2009; Watts et al. 2013). Paradoxically, other pollinator taxa using flowers as sleeping places, such as flies, have not yet been recorded (Woodcock et al. 2014).
Fly-based citizen science projects are not as common as bee-based ones (Gardiner and Roy 2022). Although several of these investigations are focused exclusively on Syrphidae or Culicidae (see supplementary material for Fig. 3 in Gardiner and Roy 2022), none of them have reported new interactions with flowers or on the use of flowers as sleeping places. ‘Moscas Florícolas de Chile’ is a pioneer project that is using social media (i.e., Facebook, Instagram, Twitter and iNaturalist) to implement a collaboration network between scientists and volunteers throughout Chile to record flies and their interactions with other fly species, flowers, and habitats (Barahona-Segovia et al. 2022a). One of the most striking families for project volunteers is Acroceridae, whose adults visit and pollinate several angiosperm species (Borkent and Schlinger 2008; Botto-Mahan et al. 2011; González et al. 2014, 2019; Borkent et al. 2016). Of particular interest is the Chilean species of Lasia Wiedemann (a total of nine species; González et al. 2018), also commonly known as hummingbird flies, which are characterized by having a large mouthpart that they use to obtain nectar from tubular flowers without necessarily perching on them (Schlinger 1981). Lasia can sustain its flight in the same place like hummingbirds; additionally, it is a very striking metallic color and densely hairy. Although these characteristics differentiate Lasia from other flies, making them an ideal target species for a citizen science project, the interactions of this genus with plants are basically limited to the use of Alstroemeria L. flowers in central Chile (Botto-Mahan et al. 2011; González et al. 2014, 2019). Only one of these studies has attempted to search for relationships between pollinators and the selection of certain floral phenotypes of Alstroemeria in different populations, which found that corolla tube length and nectar guide ratio were important floral traits only for two populations (González et al. 2019).
In this paper, we report for the first time on the use of flowers as sleeping places for hummingbird flies of the genus Lasia in central Chile, using citizen science to detect these new plant–insect interactions. We have also analyzed the number of flies accommodated in each flower, asking the main question: do the flower traits affect the number of hummingbird flies sleeping in them at night? Then, we discuss how certain flower-type traits could impact the incidence of hummingbird flies using flowers as sleeping places, and also the use of citizen science to expand the frontiers of knowledge about plant–fly interactions.
Materials and methods
Citizen science project and dataset
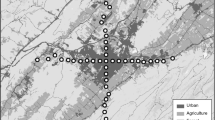
The records of Lasia using flowers as sleeping places were obtained from the citizen science project ‘Moscas Florícolas de Chile’ (https://www.facebook.com/groups/774986852548819/). These records were observed by citizen scientists between the Atacama and Biobío regions, most especially between latitudes 32º and 35º S, an area associated with Mediterranean sclerophyllous forest and shrublands (Fig. 1). Citizen scientists can submit any record (photo or video) of flies present in the sociopolitical territory of Chile and its validation is subject to the provision of three basic data: (a) an original photo; (b) a specific geographic position or coordinates to map the record, and (c) the specific date (day/month/year) of the observation (Barahona-Segovia et al 2022a). When one or more of the above items were missing, we contacted the citizen scientist to obtain them. If this information was not provided, we discarded this occurrence. The validation of the records is based on the published literature on each family of flies. The correct identification at the species level increases with a greater number of photos at different angles of each record, providing a greater set of diagnostic features for each species according to Barahona-Segovia et al. (2022b). So, between 2015 (start of the project) and 2022 (to present), we searched for Lasia records within the project using the Facebook search engine with the words “Lasia” (generic name) AND “mosca colibrí” (Spanish common name) OR “corvina” (specific epithet) OR “metallica” (specific epithet) OR “aenea” (specific epithet) OR “rufovestita” (specific epithet) OR “rufa” (specific epithet) OR “nigritarsis” (specific epithet). We used these keywords to standardize our search by year to include records prior to the start of the project (i.e., 2015), using a protocol in which one person searched this Moscas Florícolas dataset for 1 hr per day, twice a week for 2 months. Additionally, we verified that the records effectively corresponded to specimens sleeping and not feeding on nectar by looking at the photos and communicating directly with the citizen scientists if the specimens had been photographed (1) at sunset, at night, or early in the morning, (2) when the citizen scientist considered the wind chill to be low, (3) when there was a lot of shade in the flowers, or (4) on cloudy or post-rain days according to the information provided by our volunteers. The search was conducted in May and June of each year because Lasia is exclusively active between October and mid-March. With the dataset filtered, we included other useful pieces of information, for example, interactions with flowers, the administrative Chilean region, and a unique link provided by Facebook for each occurrence, which was incorporated in an Excel sheet and was submitted as open access supplementary material (more details in the Figshare https://doi.org/10.6084/m9.figshare.20063450).
From the photographic records received, we discretized two floral traits as possible explanatory variables: flower shape and coloration. Flowers with radial symmetry were classified as actinomorphic, whereas tubular flowers with bilateral symmetry were classified as zygomorphic. In regard to coloration, we separated plants into those with light-colored flowers (e.g., white, yellow, or white as a base) and those with dark-colored flowers (e.g., red, purple, orange). Also, some of the photographic records obtained (see results) showed different flowers occupied by different individuals of Lasia, so we considered such records in the same photo as independent events. Because there is an unbalanced total number of records by Lasia species, in the statistical analyses we considered this variable to be random (see statistical analyses below). In order to be able to distinguish between the Lasia species in the records obtained, we used the original descriptions of integument coloration and hairiness provided by Erichson (1840), Rondani (1863), and Philippi (1865). Thus, we differentiated L. aenea by its coppery-yellowish integument and golden-brownish hairs (Supplementary Fig. S1A); L. corvina by its pilosity and black integument (Fig. 1A, B); L. rufa by its greenish mesonotum and rufous abdomen and reddish pilosity (Fig. S1C); L. metallica by its entirely greenish body and golden-yellow pilosity (Fig. S1B); L. rufovestita because it differs from the rest due to the violaceous coloration of its body and reddish hairiness (Fig. S1D), while L. nigritarsis differed from the rest because of the blue-greenish coloration of its body and grayish hairiness. All those individuals that did not fit morphologically with these traits were classified at the generic level. In addition, the plants that the Lasia used as sleeping places were classified at generic or species level by botanists Diego Alarcón (Universidad de Concepción and Instituto de Ecología y Biodiversidad), Rodrigo Chaura (project volunteer), and Joaquín Sepulveda (project volunteer).
Statistical analyses
To establish differences in the use of flowers as sleeping places, we made a comparison of pairs of plant species for the times that were used with a Chi-square analysis of given probabilities using the software R. We tried to establish potential explanations for the use of certain flower characteristics as sleeping places by the Lasia genus, irrespective of hummingbird fly and flower species, thereby avoiding biases based on these predictors. First, we carried out a pairwise association between autocorrelated characteristics of the flowers through Pearson r correlation analysis using the psych (Revelle 2022) and corrplot (Wei and Simko 2021) packages in R software. Then, to test the effect of the shape (zygomorphic vs actinomorphic) and color (light-colored vs. dark-colored flowers) of the flowers on the number of hummingbird flies found (response variable) in the sleeping places, we performed a General Lineal Model (GLM). Because our data are discrete counts, we fitted a Poisson error distribution and applied an overdispersion test using the AER R package using R software (alpha = 0.22; Kleiber and Zeileis 2008). The shape and color of the flowers were incorporated as categorical fixed predictors, whereas we used the year of each record (1|an), Lasia species (1|sp), plant species (1|sp), and identification of the record (1|ID) as random variables and they were added to each model. Lastly, we avoided multicollinearity by calculating the Variance Inflation Factor (VIF) of each predictor, resulting in models without interactions (VIF < 10). Then, we performed the GLM using MASS, Matrix, and nlme R packages and the models obtained were ranked using the Akaike Information Criterion (AIC), Akaike weight (AICcw) and Akaike delta (ΔAICc) (Burnham and Anderson 1998, 2002) with the MuMIn R package (Barton 2009). Also, we assessed the goodness-of-fit of the selected model by running a likelihood ratio test comparing it to the null model. We performed statistical analyses using R software v.1.3.1093 (R Development Core Team 2022).
Results
Sleeping places and flies
Of the 234 records received, we used only 70 (29.91%) records of five Lasia species (L. aenea, L. corvina, L. metallica, L. rufa, and L. rufovestita) and two morphospecies using flowers as sleeping places, from 52 different citizen scientists. Ninety-three flowers as sleeping places of 15 different plant species were recorded (Table 1). Alstroemeria ligtu var. simsii (Spreng.) Her. Bayer was the main flower species used with 37.63% of records (n = 35; Fig. 2A), followed by the Onagraceae Clarkia tenella (Cav.) F. H. Lewis & M. E. Lewis, with 13.97% of records (n = 13; Fig. 2E), Salpiglossis sinuata Ruiz & Pav. with 12.90% (n = 12; Fig. 2D), Alstroemeria pulchra var. pulchra Sims with 8.60% (n = 8; Fig. 2B), A. ligtu var. ligtu (Spreng.) Her. Bayer with 6.45% (n = 6) and Chloraea bletioides Lindl. with 5.37% (n = 5; Table 1, Fig. 2C). Other flower species used as sleeping places are presented in Table 1. Alstroemeria ligtu var. simsii was the only plant species that showed significant differences in the number of times that it was used as a sleeping site, barely followed by A. pulchra var. pulchra (Table 2). Additionally, C. tenella was significantly different to all the other plant species with the exception A. ligtu var. simsii, A. pulchra var. pulchra and S. sinuata (Table 2).
A Alstroemeria ligtu var. simsii with Lasia corvina, Lagunillas. Photo by Gabriela Carrasco; B Alstroemeria pulchra with L. corvina, Peumo. Photo by Felipe Molina; C Chloraea bletioides with L. corvina transporting polynios, El Ingenio. Photo by Claudio Salas; D Salpiglossis sinuata with Lasia rufovestita grouped, Las Cabras. Photo by Matias Tobar; E several shot of Clarkia tenella with L. aenea and L. metallica, Pencahue. Photo by Mario Antonio; F Verbascum Thapsus with L. aenea, El Volcán. Photo by Tomás Poch and G Nolana sp. with L. metallica, Atacama. Photo by Pedro Vargas
From this selection, 123 individuals were registered as follows: L. corvina was the most frequent species (n = 48; 39.02%) followed by L. aenea (n = 40; 32.52%), L. rufa (n = 16; 13.01%), L. metallica (n = 9 each; 7.31%), L. rufovestita (n = 8; 6.50%), and Lasia sp. (n = 2; 1.62%). Most of L. corvina individuals were recorded in A. ligtu var. simsii (n = 27, 56.25%; Fig. 3A), whereas L. aenea individuals were found to sleep mainly in Alstroemeria species (n = 23, 57.5%; Fig. 3B). Other plant species used as sleeping places by Lasia are found in supplementary material ST1 (and Fig. 2F, G). The 71.43% (n = 50) of the records, only presented one individual hosted (Fig. 2A, B, F, G), 14.28% (n = 10) presented two individuals (Fig. 3A, C), 7.14% (n = 5) presented three individuals (Fig. 3D), 4.28% (n = 3; Fig. 2D) presented four flies and one record presented nine individuals sleeping in the same flower (1.43%; Fig. 3B and Fig. 4). Extraordinarily, 7.14% (n = 5) were recorded sleeping and mating at same time (Fig. 3C), whereas 15.71% (n = 11) presented flies in different flowers in the same photo (Fig. 3C). Lastly, 5.71% (n = 4) presented pollinia of orchids on their thorax (Figs. 2C and 3D).
A Couple of L. corvina mating and sleeping on Alstroemeria ligtu var. simsii. Photo y Andrés Ramírez; B group of Lasia rufa sleeping in Alstroemeria pulchra. Photo by Sebastian Cordero; C group of L. corvina in several flower of A. ligtu var. simsii. Some flowers have couples mating. Photo by Flor de Montaña, and D Lasia rufa mating in Chloraea bletioides and transporting polynios. Photo by Luis Eduardo. White arrows show mating and black arrow show Lasia transporting polynios
Lasia and flower-type interactions
The best-fitted model explained that the use of flowers as sleeping places by hummingbird flies is affected by the shape of the flowers, followed by the null model (Table 3). Hummingbird flies use flowers with a zygomorphic shape (1.5 ± 0.17 individuals/flower) more frequently than those with an actinomorphic one (1.00 ± 0.01 individuals/flower).
Discussion
Sleeping places and flies
Scientific literature has recorded that fly species can use flowers to protect themselves against adverse environmental conditions or facilitate metabolism by taking advantage of the higher temperature of flowers or their heliotropism (Woodcock et al. 2014). Although these behaviors could be similar to the act of sleeping inside flowers, as some native bees do (Alves-dos-Santos et al. 2009; Watts et al. 2013; Sabino et al. 2017), the use of flowers as sleeping places by flies is unprecedented. In this work, we report for the first time on the use of flowers as sleeping places by hummingbird flies of the genus Lasia. Although the richness of flowers used for this purpose was not that high, we found a pattern in the use of flowers that favors some zygomorphic species, such as A. ligtu var. simsii and A. pulchra var. pulchra.
Our results showed that the shape of the flower was the main trait considered for Lasia to use those flowers as a sleeping place. Although most flowers occupied as sleeping places, such as Alstroemeria or Salpiglossis, are zygomorphic, some actinomorphic species like C. tenella may also be used, possibly because these flowers exhibit nyctinasty (i.e., their flowers close at night), as has been recorded for flowers used by wild bees (Pinheiro et al. 2017). Meanwhile, other species with zygomorphic flowers, such as Eccremocarpus or Tropaeolum, have a small flower opening, making it impossible for larger flies, such as L. corvina, to use them as sleeping places. The data we have is not sufficient for us to determine whether flower traits affect whether flies sleep alone or in groups. To respond to additional questions about the relationships between flower traits and the number of sleeping flies accommodated in flowers, we need information about corolla size, flower temperature, or corolla tube length, which cannot be extracted from citizen science data. Moreover, our null model resulted to be the second best-fitted model, which invites us to consider the abovementioned new flower traits and plan systematic samplings to detect other explanations for how they are used as sleeping places.
The use of some flowers as sleeping places could be influenced by the flower phenology (i.e., temporal availability) of certain species on a local scale. There are limitations to studying these interactions with citizen science (Groom et al. 2021), for example spatial biases due to people focusing on areas accessible by roads and recording only in some places in those areas or the systematic sampling of the bloom throughout the flight period of Lasia. These hypotheses should therefore be studied in the field using a systematized design to assess whether the abundance of plant species and their shape leads to a bias that should be considered when studying which flowers Lasia uses as sleeping places.
Lasia is commonly recorded as part of the Alstroemeria flower visitor assemblage and is considered to be an efficient pollinator of these plants (González et al. 2014, 2019; Murúa et al. 2019). The presence or absence, as well as the abundance of Lasia in Alstroemeria populations, could influence floral traits such as floral tube length and nectar guide area (González et al. 2019). The length of the floral tube could be critical for Lasia because it needs better overnight shelter to avoid low temperatures, rain, wind, and predators, as well as an opportunity to mate. This strategy to avoid negative environmental conditions has also been observed in wild bees from the tribe of Tapinotaspidini (Apidae), according to Pinheiro et al. (2017) and Eucera bees (Apidae), according to Sapir et al. (2005). In fact, during the night flowers generate heat via their cell metabolism, creating a warm environment, which is used by pollinators to take refuge from unsuitable environmental conditions (Seymour et al. 1983, 2003; Sapir et al. 2005). However, this behavior has never been registered for flies before this work. On the other hand, L. corvina has low visitation rates compared to the amount of Alstroemeria pollen it carries (Murúa et al. 2019), which suggests that sleeping in Alstroemeria flowers could be a complementary pollination mechanism. This mechanism of pollination has also been observed in night-sheltering solitary male bees in self-incompatibility Oncocyclus irises (Sapir et al. 2005). In fact, some records of Lasia transporting Chilean orchid pollinia suggest an active role in their reproduction, such as has been recorded by several studies into deception mechanisms (e.g., Johnson and Morita 2006; Endara et al. 2010; Gaskett 2011; Figs. 1C and 2D). Unfortunately, other mechanisms for flies using flowers as sleeping places are not studied and further research should seek to understand more precisely what conditions different flowers offer that lead to them being selected as possible sleeping places by Lasia, and whether this behavior influences flower reproduction.
Citizen science and biological interactions
The Intergovernmental Science-policy Platform on Biodiversity and Ecosystem Services (IPBES) has reported significant knowledge gaps regarding pollinators at the global level (IPBES 2016), which also suggests a lack of knowledge of their biological interactions. These biases are more evident between groups of pollinating insects. For example, native bees and bumblebees are more common in citizen science projects than flies (see Gardiner and Roy 2022; Ghilardi-Lopes and Zattara 2022). There is also a significant lack of records in public databases such as GBIF that could facilitate knowledge of the biological interactions in pollinating flies like Syrphidae (Boyd et al. 2022). Other shortfalls in societal knowledge, such as that pertaining to the role of flies in human well-being (public shortfall) or what species of flies citizen scientists observe (scientific knowledge shortfall; Cardoso et al. 2011), increase biases against flies and the potential interactions that they can perform with plants.
The citizen science project ‘Moscas Florícolas de Chile’ began in 2015 and has + 7000 volunteers nationwide, as well as renowned dipterologists studying different families (Barahona-Segovia et al. 2022a). Each year, the project receives between 700 and 1000 records of different fly species in Chile, many of which reveal new biological interactions, especially with plants and their flowers (R. Barahona-Segovia unpublished data). The records of Lasia using sleeping places represent a unique interaction for the whole family, highlighting the power that citizen science can have in advancing scientific knowledge. However, despite the fact that citizen science has a high social connotation due to the participatory nature of its process, it also has limitations and associated biases that can impact the detection of these new biological interactions. For example, not all fly families have a resolved taxonomy, so the identification of some records can be complex. In some cases, to be certain about the identity of a fly (or plant) species a validation process is needed based on the presence of different morphological characters, as in the case of Trichopoda pictipennis (Barahona-Segovia et al. 2022b). In the collection of information for our work, we detected at least two individuals of Lasia that do not match the known taxonomy for the genus, which could limit the knowledge of these flies and their interactions.
Other biological interactions of the project, such as those of giant robber flies preying on invasive Hymenoptera (Barahona-Segovia and Pañinao-Monsálvez 2020), or those where other species of Acroceridae parasitize tarantulas (R. Barahona-Segovia et al., unpublished data), have also been documented from citizen science records over time. These new biological interactions could reveal data that could prove useful in making decisions such as (1) improving the experimental design of research into plant–animal interactions, (2) implementing agricultural management measures to supplement the potential food resources of pollinating flies (e.g. Syrphidae), (3) considering potential uses for flies in conservation biological control (Barahona-Segovia et al. 2022b), (4) detecting specialist fly–plant interactions and potential threats to them, and (5) integrating citizen science as a surveillance mechanism over time into public agricultural and conservation policies.
In conclusion, citizen science can make a significant contribution to the knowledge of new biological interactions in key functional groups for humans as pollinators, including uncharismatic insects like flies. The records of Lasia using flowers as sleeping places are unique on a global scale and open up a range of options for new evolutionary and ecological questions that could in the future be applied to different investigations. Although some biases seem to be insurmountable at the moment, citizen science can describe with greater representativeness the as-yet-unknown richness of biological interactions in highly dynamic landscapes that are constantly changing as a result of human intervention.
Data availability
The dataset generated during and/or analyzed during the current study is available in the Figshare repository https://figshare.com/s/71385c26a69886e7943c
Code availability
Not applicable.
References
Alves-dos-Santos I, Gaglianone MC, Naxara SRC, Engel MS (2009) Male sleeping aggregations of solitary oil-collecting bees in Brazil (Centridini, Tapinotaspidini, and Tetrapediini; Hymenoptera: Apidae). Genet Mol Res 8:515–524
Barahona-Segovia RM, Pañinao-Monsálvez L (2020) Desolation comes from the sky: invasive Hymenoptera species as prey of Chilean giant robber flies (Diptera: Asilidae) through field observations and citizen science. J Asia-Pac Entomol 23:840–844. https://doi.org/10.1016/j.aspen.2020.07.012
Barahona-Segovia RM, Gatica-Barrios P, Barceló M (2022a) Conociendo a las Moscas Florícolas de Chile: un proyecto con y para las personas. In: Ghilardi-Lopes NP, Zattara EE (eds) Ciencia ciudadana y polinizadores nativos de Sudamérica. Editora Cubo, Saõ Carlos, pp 121–124
Barahona-Segovia RM, González CR, Paniñao-Monsálvez L (2022b) Citizen science meet South American tachinids: new records of feather-legged fly Trichopoda (Galactomyia) pictipennis Bigot (Diptera: Tachinidae) from Chile. Neotrop Entomol. https://doi.org/10.1007/s13744-022-00979-2
Barton K (2009) MuMIn: Multi-model inference. R Package Version 0.12.2/r18. http://R-Forge.R-project.org/projects/mumin/. Accessed 21 Dec 2021
Bloom EH, Crowder DW (2020) Promoting data collection in pollinator citizen science projects. Citiz Sci. https://doi.org/10.5334/cstp.217
Borkent CJ, Schlinger EI (2008) Flower-visiting and mating behaviour of Eulonchus sapphirinus (Diptera: Acroceridae). Can Entomol 140:250–256. https://doi.org/10.4039/n07-060
Borkent CJ, Gillung JP, Winterton SL (2016) Jewelled spider flies of North America: a revision and phylogeny of Eulonchus Gerstaecker (Diptera, Acroceridae). ZooKeys 619:103–146. https://doi.org/10.3897/zookeys.619.8249
Botto-Mahan C, Ramírez PA, Gloria Ossa C, Medel R, Ojeda-Camacho M, González AV (2011) Floral herbivory affects female reproductive success and pollinator visitation in the perennial herb Alstroemeria ligtu (Alstroemeriaceae). Int J Plant Sci 172:1130–1136. https://doi.org/10.1086/662029
Boyd RJ, Aizen MA, Barahona-Segovia RM, Flores-Prado L, Fontúrbel FE, Francoy TM, López-Aliste M, Martínez L, Morales CL, Ollerton J, Pescott OL, Powney GD, Saraiva AM, Schmucki R, Zattara EE, Carvell C (2022) Inferring trends in pollinator distributions across the Neotropics from publicly available data remains challenging despite mobilization efforts. Divers Distrib. https://doi.org/10.1111/ddi.13551
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer-Verlag, New York
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretical approach. Springer-Verlag, New York
Callaghan CT, Poore AG, Mesaglio T, Moles AT, Nakagawa S, Roberts C, Rowley JJL, Vergés A, Wilshire JH, Cornwell WK (2021) Three frontiers for the future of biodiversity research using citizen science data. Bioscience 71:55–63. https://doi.org/10.1093/biosci/biaa131
Cardoso P, Erwin TL, Borges PA, New TR (2011) The seven impediments in invertebrate conservation and how to overcome them. Biol Conserv 144:2647–2655. https://doi.org/10.1016/j.biocon.2011.07.024
Doherty JF, Filion A, Bennett J, Bhattarai UR, Chai X, de Angeli DD, Donlon E, Fátima J, Milotic M, Park E, Sabadel AJM, Thomas LJ, Poulin R (2021) The people vs science: can passively crowdsourced internet data shed light on host–parasite interactions? Parasitology 148:1313–1319. https://doi.org/10.1017/S0031182021000962
Endara L, Grimaldi D, Roy B (2010) Lord of the flies: pollination of Dracula orchids. Lankesteriana 10:1–11
Erichson WF (1840) Die Henopier. Eine familie aus der Ordung der Dipteren. In: Entomographien vol. 1, pp. 135–180
Gardiner MM, Roy HE (2022) The role of community science in entomology. Ann Rev Entomol 67:437–456. https://doi.org/10.1146/annurev-ento-072121-075258
Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ (2015) Seven shortfalls that beset large-scale knowledge of biodiversity. Ann Rev Ecol Evol Syst 46:523–549. https://doi.org/10.1146/annurev-ecolsys-112414-054400
Gaskett AC (2011) Orchid pollination by sexual deception: pollinator perspectives. Biol Rev 86:33–75. https://doi.org/10.1111/j.1469-185X.2010.00134.x
Ghilardi-Lopes NP, Zattara E (2022) Ciencia ciudadana y polinizadores nativos de Sudamérica. Editora Cubo, Saõ Carlos
Giannini TC, Pinto CE, Acosta AL, Taniguchi M, Saraiva AM, Alves-dos-Santos I (2013) Interactions at large spatial scale: the case of Centris bees and floral oil producing plants in South America. Ecol Modell 258:74–81. https://doi.org/10.1016/j.ecolmodel.2013.02.032
González AV, Murúa M, Ramírez PA (2014) Temporal and spatial variation of the pollinator assemblages in Alstroemeria ligtu (Alstroemeriaceae). Rev Chi Hist Nat 87:1–5. https://doi.org/10.1186/0717-6317-87-5
González CR, Elgueta M, Ramírez F (2018) A catalog of Acroceridae (Diptera) from Chile. Zootaxa 4374:427–440. https://doi.org/10.11646/zootaxa.4374.3.6
González AV, González-Browne C, Salinas P, Murúa M (2019) Is there spatial variation in phenotypic selection on floral traits in a generalist plant–pollinator system? Evol Ecol 33:687–700. https://doi.org/10.1007/s10682-019-10002-7
Groom Q, Pernat N, Adriaens T, De Groot M, Jelaska SD, Marčiulynienė D, Martinou AF, Skuhrovec J, Tricarico E, Wit EC, Roy HE (2021) Species interactions: next-level citizen science. Ecography 44:1781–1789. https://doi.org/10.1111/ecog.05790
IPBES (2016) The assessment report of the intergovernmental science-policy platform on biodiversity and ecosystem services on pollinators, pollination and food production. In: Potts SG et al (eds) Secretariat of the intergovernmental science-policy platform on biodiversity and ecosystem services. IPBES, Bonn
Johnson SD, Morita S (2006) Lying to Pinocchio: floral deception in an orchid pollinated by long-proboscid flies. Bot J Linn Soc 152:271–278. https://doi.org/10.1111/j.1095-8339.2006.00571.x
Kleiber C, Zeileis A (2008) Applied Econometrics with R. Springer-Verlag, New York. ISBN 978-0-387-77316-2, https://CRAN.R-project.org/package=AER
Koffler S, Barbiéri C, Ghilardi-Lopes NP, Leocadio JN, Albertini B, Francoy TM, Saraiva AM (2021) A buzz for sustainability and conservation: the growing potential of citizen science studies on bees. Sustainability 13:959. https://doi.org/10.3390/su13020959
Lander T (2020) Network modelling, citizen science and targeted interventions to predict, monitor and reverse bee decline. Plants People Planet 2:111–120. https://doi.org/10.1002/ppp3.10068
Murúa M, Ramírez MJ, González A (2019) Is the same pollinator species equally effective in different populations of the generalist herb Alstroemeria ligtu var. simsii? Gayana Bot 76:109–114. https://doi.org/10.4067/S0717-66432019000100109
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326. https://doi.org/10.1111/j.1600-0706.2010.18644.x
Philippi RA (1865) Aufzählung der chilenischen Dipteren. Verh Zool-Bot Ges Wien 15:595–782. https://doi.org/10.5962/bhl.title.9295
Pinheiro M, Alves-dos-Santos I, Sazima M (2017) Flowers as sleeping places for male bees: somehow the males know which flowers their females prefer. Arthropod-Plant Interact 11:329–337. https://doi.org/10.1007/s11829-017-9532-6
R Development Core Team (2022) R: A language and environment for statistical computing. Version 1.3.1093. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 15 Dec 2021
Revelle W (2022) psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University, Evanston, Illinois. R package version 2.2.5, https://CRAN.R-project.org/package=psych. Accessed 21 Dec 2021
Rezende EL, Lavabre JE, Guimarães PR, Jordano P, Bascompte J (2007) Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 448(7156):925–928. https://doi.org/10.1038/nature05956
Rondani C (1863) Diptera exotica revisa et annotata novis nonnullis descriptis. Eredi Soliani, Modena
Sabino WO, Da Silva CI, Alves-dos-Santos I (2017) Mating system and sleeping behaviour of the male and female Centris (Paracentris) burgdorfi Friese (Apidae, Centridini). J Insect Behav 30:103–118. https://doi.org/10.1007/s10905-017-9600-x
Sapir Y, Shmida A, Ne’eman G (2005) Pollination of Oncocyclus irises (Iris: Iridaceae) by night-sheltering male bees. Plant Biol 7:417–424. https://doi.org/10.1055/s-2005-837709
Schlinger EI (1981) Acroceridae. In: McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DEM (eds), Manual of Nearctic Diptera. Vol I. Research Branch, Agriculture Canada, Ottawa, pp 575–584
Seymour RS, Bartholomew GA, Barnhart MC (1983) Respiration and heat production by the inflorescence of Philodendron selloum Koch. Planta 157:336–343. https://doi.org/10.1007/BF00397405
Seymour RS, White CR, Gibernau M (2003) Heat reward for insect pollinators. Nature 426:243–244. https://doi.org/10.1038/426243a
Taylor PJ, Vise C, Krishnamoorthy MA, Kingston T, Venter S (2020) Citizen science confirms the rarity of fruit bat pollination of baobab (Adansonia digitata) flowers in Southern Africa. Diversity 12(3):106. https://doi.org/10.3390/d12030106
Watts Stella, Sapir Yuval, Segal Bosmat, Dafni Amots (2013) The endangered Iris atropurpurea (Iridaceae) in Israel: honey-bees, night-sheltering male bees and female solitary bees as pollinators. Ann Bot 111(3):395–407. https://doi.org/10.1093/aob/mcs292
Wardhaugh CW (2015) How many species of arthropods visit flowers? Arthropod Plant Interac 9:547–565. https://doi.org/10.1007/s11829-015-9398-4
Wei T, Simko V (2021) R package ‘corrplot’: Visualization of a Correlation Matrix. (Version 0.92), https://github.com/taiyun/corrplot. Accessed 21 Dec 2021
Woodcock TS, Larson BM, Kevan PG, Inouye DW, Lunau K (2014) Flies and flowers II: floral attractants and rewards. J Pollinat Ecol 12:63–94. https://doi.org/10.26786/1920-7603(2014)5
Acknowledgements
We thank all the citizen scientists of the Moscas Florícolas de Chile group who have contributed with their records in the years that the project has worked. We thank Gabriela Carrasco, Luis Eduardo, Felipe Molina, Mario Antonio, Sebastián Cordero, Flor de la Montaña, Tomás Poch, Andrés Ramírez, Claudio Salas, Matías Tobar, and Pedro Vargas for providing photographic material.
Funding
RMBS grateful to Agencia Nacional de Investigación y Desarrollo (ANID) and Fondo Nacional de Ciencia y Tecnología Grant No. FONDECYT 3200817.
Author information
Authors and Affiliations
Contributions
RMBS: conceived the idea, compiled the information from citizen science group and wrote the first draft. VDS and RMBS: analyzed the dataset and performed the statistical analysis. MM and VDS: review the first draft and provide comments. RMBS, VDS and MM: write, discussed and correct the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barahona-Segovia, R.M., Durán-Sanzana, V. & Murúa, M. This flower is our bed: long-term citizen science reveals that hummingbird flies use flowers with certain shapes as sleeping places. Arthropod-Plant Interactions 17, 1–10 (2023). https://doi.org/10.1007/s11829-022-09936-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-022-09936-7