Abstract
The pink stem stalk borer, Sesamia cretica Lederer, is the main insect pest of sugarcane, Saccharum officinarum L., in Iran and worldwide. This paper assesses the effects of six sugarcane cultivars on the feeding performance and enzymatic activity of S. cretica under controlled conditions. It also investigates the biochemical traits of sugarcane cultivars (total phenolic, flavonoids, and anthocyanins contents) and explores their relationship with the nutritional physiology of S. cretica. The study findings indicated remarkable differences in the nutritional properties and digestive function of S. cretica on various sugarcane cultivars. The S. cretica larvae reared on cultivar CP48-103 were indicated to have the highest ECI, RGR, and ECD values. In addition, the larvae fed on cultivar CP73-21 showed the lowest values of RCR and RGR. The S. cretica larvae induced the highest proteolytic activity during feeding with CP48-103, CP57-614, and CP73-21 cultivars. The fifth instar larvae demonstrated the highest and lowest amylolytic activity when fed with cultivars IRC99-01 and CP57-614, respectively. Moreover, significant variations in the phytochemical metabolites were detected among the sugarcane cultivars. Significant negative or positive correlations were found between the tested parameters of S. cretica and the biochemical characteristics of sugarcane cultivars. The cluster analysis results showed that cultivar CP73-21 was relatively unsuitable for S. cretica feeding: it was a suggested candidate to grow in regions with typically high pest infestation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is an established source of sugar and a highly effective crop with excellent photosynthetic performance and good applicability to biomass manufacturing (Arcenaux 1965; Soltani Orang et al. 2014). The pink stem borer, Sesamia cretica Lederer, is well known and broadly distributed and one of the main pests of sugarcane and maize fields, especially in southern provinces in Iran, such as Fars and Khuzestan, and many parts of the world (Ezzeldin et al. 2009; Sedighi et al. 2016; Arbabtafti et al. 2021). Maize, Zea mays L., and sugarcane, Saccharum officinarum L., are the main hosts that are seriously damaged by the stem borers of the genus Sesamia (Ranjbar Aghdam and Kamali 2002). The feeding of S. cretica larvae mitigates qualitative and quantitative attributes of sugarcane and damages the stalks during the cropping season which is inappropriate for consumption (Temerak and Negm 1979; Sadeghi et al. 2019).
Chemical pesticides have long been a solution to controlling the S. cretica population. The routine application of pesticides is associated with multiple dangerous implications to human health, significant nontarget mortality in natural enemies, and intensification of insect resistance to chemicals (Wright and Verkert 1995; Tomberlin et al. 2002). Most recently, S. cretica pest control has changed rapidly from an insecticide approach to integrated management.
Developing resistance in host plants can be an effective ecological factor in integrated pest management strategies for dealing with S. cretica in sugarcane fields. Assessing the resistance of different cultivars to insect pests can provide relevant data about their suitability or unsuitability for the target insect pests (Tsai and Wang 2001; Hemati et al. 2012a; Shishehbor and Hemmati 2021). Appropriate incorporation of resistant cultivars into pest management techniques requires knowledge of pest species’ nutritional performances and life history characteristics (Gvozdenac et al. 2018; Abedi et al. 2019).
Therefore, nutritional properties and digestive physiology are important variables in evaluating the interactions between plant with insects and the susceptibility or resistance of various host plants to pests (Hemati et al. 2012b; Shishehbor and Hemmati 2021). Although sugarcane is a globally recognized host to S. cretica, published research lacks information on this pest’s nutritional traits and the function of its digestive enzymes on different cultivars. Accordingly, this research evaluated the reaction of S. cretica to feeding on six sugarcane cultivars. Moreover, different defense characteristics of host plants affect the suitability of herbivorous insects. Plant secondary metabolites are key compounds that act as nutritional inhibitors and antidigestive, inhibitory, and repellent compounds, reducing pest growth and survival rate (Price et al. 1980; War et al. 2011).
Sugarcane stems are potential source of secondary metabolites, such as anthocyanins, flavonoids, and phenolic contents (Arcenaux 1965). Phenolic compounds are the most significant secondary plant metabolites produced through the shikimic acid pathway from primary metabolites (War et al. 2012). Anthocyanins are polyphenolic compounds (flavonoids) responsible for the blue, red, and purple colors of most fruits and flowers (Turfan et al. 2011). Hence, this research also evaluated the major phytochemical metabolites such as flavonoids, total phenolic, and anthocyanins content in various sugarcane cultivars and examined the possible relationship between these compounds and the physiological responses of the S. cretica. No research data were available regarding the correlation between tested parameters of S. cretica and the biochemical compounds of sugarcane. The findings of this study will be useful for developing new approaches to pink stem borer management, including the use of resistant sugarcane cultivars to reduce insect pests’ damage in the farm system.
Materials and methods
Sources of sugarcane cultivars
The stalks of six sugarcane (S. officinarum) cultivars (IRC99-01, SP70-1143, CP48-103, CP57-614, CP69-1062, and CP73-21) were obtained from the Khuzestan Institute of Sugarcane Research and By-products Development, Ahvaz, Iran. These cultivars are widely cultivated commercially in southern Iran in Khuzestan. All cultivars grow in the same geographical area using the same agricultural techniques.
Rearing of S. cretica
Originally, the larvae of S. cretica were collected from sugarcane fields of Ahvaz, Iran. They were reared on each sugarcane cultivar under controlled conditions: 27 ± 2 °C, 65 ± 5% RH, and a photoperiod of 16:8 (L:D) h according to the method of Sedighi (2016). The larvae were reared on cut stems of sugarcane cultivars in plastic containers (1.5 Liter, 22 cm diameter, and 8 cm depth) for two generations, and the third-generation larvae were used in the experiments.
Nutritional responses and body weight of S. cretica
Feeding performances (larval weight, food consumed, feces produced, and weight gain) of the third instar larvae until pre-pupation of S. cretica on each sugarcane cultivar were evaluated based on the Waldbauer (1968) (40 replicates for each cultivar). The weights of the third instar larvae were measured before and after feeding on stems of various sugarcane cultivars until pre-pupation. The weight of each sugarcane cultivar was measured and then the cultivar was transferred to plastic plates to feed the larvae. These larvae were fed with the stem of each cultivar and the final weight was recorded.
Larval weight, the remaining food, and feces at the end of each experiment were recorded until the feeding stopped and reached the pre-pupal stage. The weight of the eaten stem was determined by subtracting the stem residue at the end of each experiment from the total weight of the given stem. To obtain the dry weight percentage of the food and larvae, 20 specimens of food and larvae for each cultivar were weighed, oven-dried at 60 °C for 48 h, and then weighed again. The nutritional performances of S. cretica larvae are evaluated by Waldbauer (1968) formulae:
Consumption index (CI) = [(E/A)]; Approximate digestibility (AD) = [(E–F)/E]; Efficiency of conversion of ingested food (ECI) = [(P/E) × 100]; Efficiency of conversion of digestion food (ECD) = [(P/E–F) × 100]; Relative consumption rates (RCR) = [(E/W0 × T)]; and Relative growth rates (RGR) = [P/ W0 × T].
where A = average of larval dry weight over time (mg), E = dry weight of the food consumed (mg), F = dry weight of feces produced (mg), P = dry weight gain of larvae (mg), T = the feeding duration (day), and W0 = primary weight of larvae (mg).
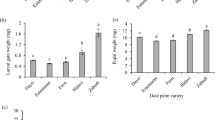
Furthermore, the fifth instar larvae and pupal weight of S. cretica were measured 24 h after their emergence and pupation on each sugarcane cultivar.
Digestive enzymatic activity
Under a stereomicroscope, the fifth instar larvae of S. cretica feeding on each sugarcane cultivar were dissected in pre-cooled distilled water. The midguts of 50 larvae of S. cretica were homogenized on ice and prepared described by Hosseininaveh (2007). Homogenates were centrifuged at 15,000 g at 4 °C for 10 min. Then, supernatant was collected and frozen (-20 °C) for enzymatic assays. All assays were done in three replications with a blank without enzymatic extract.
Amylase activity of S. cretica
The amylase activity of S. cretica larvae fed with different sugarcane cultivars was determined by the dinitrosalicylic acid (DNSA) method using 1% soluble starch and 50 mM acetate buffer at the optimal pH (Bernfeld 1955). Twenty microliters of the enzyme extract were incubated with 40 mL of soluble starch at 37 °C for 30 min. The enzymatic reaction was stopped by adding 100 ml of DNSA reagent, and the adsorption was read at 540 nm. A standard curve of absorbance against the amount of maltose released was created to facilitate the calculation of the amount of maltose released during the α-amylase assays. A series of dilutions of maltose in the universal buffer were prepared to obtain the following concentrations: 2, 1, 0.5, 0.25, and 0.125 mg mL−1.
Protease activity of S. cretica
The proteolytic activity was examined using azocasein (1.5%) as a substrate by Elpidina (2001). The reaction mixture contained 50 ml substrate solution in 50 mM acetate buffer and 15 ml enzyme extract. The mixture was incubated at 37 °C for 50 min for hydrolysis of azocasein. After incubation for 50 min, 100 mL of 30% trichloroacetic acid (TCA) was added to the reaction mixture, kept at 4 °C, and centrifuged at 15,000 g for 10 min. One hundred microliters of the supernatant were mixed with 100 mL of 2 M NaOH, and the adsorption rate was determined at 440 nm. The blank solution contained all of the listed reagents except for the enzyme solution. Appropriate blanks, to which TCA had been added before the substrate, were prepared for each treatment. A tyrosine standard curve was made with 1–15 μg mL−1 tyrosine solution. One unit of protease activity was defined as the increase in optical density per milligram of tissue protein per minute due to proteolysis of azocasein. Moreover, protein concentrations were calculated using the Bradford (1976) protein assay, and known amounts of bovine serum albumin (BSA) (2, 1.5, 1, 0.5, 0.25, 0.125, and 0.063 mg mL−1) were utilized to create a standard curve.
Biochemical properties of sugarcane cultivars
Biochemical characteristics of various sugarcane cultivars were investigated to detect their relationship with feeding indices and enzymatic activity of S. cretica. Sliced sugarcane stems were used to measure all phytochemicals of the tested sugarcane cultivars. The experiments were carried out in three replications.
Total phenol, anthocyanins, and flavonoids contents determination
The method of Slinkard and Singleton (1977) was used to assay the total phenol content in the stems of various sugarcane cultivars. Briefly, the powdered cut stems extracts were homogenized in methanol. After centrifugation, the supernatants were transferred to 1.5 mL Folin–Ciocalteu reagent 10% and sodium carbonate solution 7% were added to the mixture and the absorbance was measured at 765 nm. Gallic acid was utilized as standard compound for the quantification of total phenol content.
Also, total anthocyanins and flavonoid contents in various sugarcane cultivars were measured by the method given by Kim et al. (2003). Briefly, 2 g of powdered cut stems of sugarcane cultivars was homogenized in acidified ethanol (1 acid acetic:100 ethanol), and the absorbance was measured at 520 nm for total anthocyanins and 415 nm for total flavonoids. Also, the standard curve for total anthocyanins and flavonoids was made utilizing cyanidin and quercetin standard solutions, respectively.
Data analysis
Before statistical analysis, all data were validated using the Shapiro–Wilk test in the SPSS v. 22.0 environment. Moreover, the effect of various sugarcane cultivars on the feeding performance and enzymatic activity of S. cretica and secondary metabolites of sugarcane were analyzed by one-way ANOVA. Statistical differences of means were compared with the Tukey test at a 5% significance level. Correlation between some main physiology properties of S. cretica, including relative growth rate, relative consumption rate, efficiency of conversion of ingested food, pupal weight, amylolytic, and proteolytic activities parameters, with biochemical characteristics of various sugarcane cultivars was investigated through Pearson’s correlation test. A dendrogram of sugarcane cultivars was created based on all the parameters of S. cretica in the tested sugarcane cultivars by Ward’s method (SPSS Inc. 2007).
Results
Nutritional responses
Table 1 shows the values for the feeding performance of the third instar larvae until pre-pupation of S. cretica. Nutritional indices were significantly different between larvae that fed on various cultivars of sugarcane. Higher consumption index was observed during feeding of larvae with cultivars CP57-614 and SP70-1143 and a lower value was seen during feeding on cultivar CP48-103 (F = 4.32; df = 5, 234; P = 0.002). Larvae fed with cultivars CP57-614 and IRC99-01 showed the lowest approximate digestibility (AD) value compared to other cultivars (F = 6.92; df = 5, 234; P < 0.001). The highest value of the efficiency of conversion of ingested food (ECI) of the larvae was on cultivar CP48-103 (F = 7.45; df = 5, 234; P < 0.0001). Also, larvae-fed cultivar CP48-103 showed the highest values of efficiency of conversion of digested food (ECD) (F = 4.72; df = 5, 234; P = 0.001). Larvae reared on cultivars CP57-614, SP70-1143, and IRC99-01 showed the highest value of relative consumption rate, while the lowest value was in cultivar CP73-21 (F = 6.92; df = 5, 234; P < 0.001). The highest value of relative growth rate was in cultivar CP48-103, and the lowest value was in cultivars SP70-1143 and IRC99-01 (F = 2.86; df = 5, 234; P = 0.02).
Table 2 indicates the food consumption and body mass of S. cretica larvae on various sugarcane cultivars. No significant differences were observed in food consumed and weight of fifth instar larvae among the sugarcane cultivars (F = 0.46; df = 5, 234; P = 0.81). The highest weight gain of larvae was observed when larvae were fed on cultivars CP48-103 and CP69-1062 (F = 18.26; df = 5, 234; P < 0.001). Also, the heaviest pupal mass was measured on cultivar CP69-1062 and the lightest on cultivars CP57-614, CP73-21, and SP70-1143 (F = 25.17; df = 5, 234; P < 0.001).
Enzymatic activity of larvae
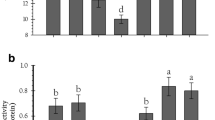
Table 3 shows the amylolytic and total proteolytic activity of the fifth instar S. cretica larvae fed with the tested sugarcane cultivars. Digestive enzymes of S. cretica reared on sugarcane cultivars showed significant differences. The highest and lowest levels of amylase activity were recorded on cultivars IRC99-01 and CP57-614 (F = 2839.54; df = 5, 12; P < 0.001). Furthermore, the larvae reared on cultivars CP48-103, CP57-614, and CP73-21 showed higher protease activity, while lower one detected in the larvae was reared on cultivars CP69-1062, SP70-1143, and IRC99-01 (F = 27.24; df = 5, 12; P < 0.001).
Biochemical traits of various sugarcane cultivars
Table 4 lists the plant secondary metabolites attributes of sugarcane cultivars. There were significant differences in the biochemical content of various sugarcane cultivars. The highest total phenolic content was measured in CP57-614 (F = 197.71; df = 5, 12; P < 0.001). The lowest amount of flavonoids was detected in CP69-1062 and SP70-1143, while the highest amount was observed in CP73-21 and IRC99-01 (F = 1981.69; df = 5, 12; P < 0.001). Moreover, the highest amount of total anthocyanin was obtained for the cultivar SP70-1143, while the lowest amount was measured for CP57-614 (F = 6644.31; df = 5, 12; P < 0.001).
Correlation analysis
Table 5 shows the analysis of correlation coefficients of nutritional performances and enzymatic activity of S. cretica with biochemical characteristics of the tested cultivars. Significant correlations were detected between S. cretica and the sugarcane cultivar’s phenolic, flavonoids, and anthocyanins contents. The proteolytic activity of S. cretica showed significant positive correlations with phenolic content (r = 0.724) and flavonoids (r = 0.672). A significant negative correlation was observed between the total phenolic content of different sugarcane cultivars and S. cretica pupal mass (r = − 0.544) and amylolytic activity (r = − 0.836). Moreover, the pupal weight of S. cretica showed positive correlations (r = 0.491) with anthocyanins content. In contrast, the proteolytic activity of larvae negatively correlated (r = − 0.682) with this biochemical parameter of sugarcane. The phenolic content of cultivars highly correlated with the relative consumption rate of larvae (r = 0.292).
The relative consumption rate, pupal weight, and amylolytic activity of S. cretica (Table 5) did not significantly correlate with the flavonoids content of the sugarcane cultivars. Furthermore, there was no significant correlation among RGR and ECI of S. cretica with the tested all biochemical characteristics content of sugarcane cultivars.
Cluster analysis
Figure 1 presents the dendrogram based on the tested parameters of S. cretica in different sugarcane cultivars. Various sugarcane cultivars were grouped in two clusters A and B. Sub-cluster A1 includes cultivars CP57-614 and SP70-1143, and sub-cluster A2 includes cultivar CP73-21. Cluster B consists of cultivars CP69-1062, IRC99-01, and CP48-103.
Discussion
For ideal growth, survival, and reproduction, insects must obtain enough necessary nutrients from host plants (Hemmati et al. 2021). Our results showed that S. cretica larvae reared on various sugarcane cultivars were able to develop and complete their life cycle on all the tested sugarcane cultivars. However, the amount of nutrition varied among the cultivars tested. The nutritional performance of insects depends on the quality and quantity of diet consumed by the host plants (Karasov et al. 2011). Nutritional indicators of an insect indicate a potential for nutrition and increase in body mass in insect pests (Nathan et al. 2005; Lee 2007).
The present research findings show that although S. cretica can complete development in all tested sugarcane cultivars. It shows that these cultivars are a good source of nutrition. Reciprocally, the feeding rate was the lowest in cultivars CP57-614, SP70-1143, and CP73-21, probably due to low nutritional level, biochemical properties, and high concentration of protein inhibitors. The results showed that the high quality of food, especially biochemical attributes of sugarcane, affects the digestive enzymes activity and nutritional indicators of S. cretica. Furthermore, the study shows an interaction between the biochemical characteristics of sugarcane and the nutritional physiology of this pest. So far, no effective step and comprehensive studies have been performed to evaluate the nutritional physiology of S. cretica on sugarcane cultivars.
Quantitative research has studied the effect of phytochemical metabolites on the efficiency and performance of herbivores. Phenolic compounds are one of the most important secondary metabolites that prevent feeding by herbivores (Haukioja et al. 2002). Sharma et al. (2009) investigated the high levels of polyphenols and tannins as plant resistance factors against Helicoverpa armigera (Hubner). Mardani-Talaee et al. (2016) reported that total phenolic contents in host plants could reduce reproductive performance and decrease insect pests’ growth rate. Moreover, Zahedi et al. (2019) showed that interactions between cucumber cultivar and Aphis gossypii Glover were affected by biochemical attributes of tested cultivars. Abedi et al. (2019) evaluated that secondary compounds negatively affect pests’ nutritional performances and growth. According to Naseri and Majd-Marani (2020), the activity of digestive enzymes and the feeding efficiency of Tribolium castaneum (Herbst) when fed with different rice cultivars were affected. These findings are consistent with the findings of the current study.
Bodyweight has been reported to be related to the food consumed and is one of the key biological indicators of the pest population (Liu et al. 2004). Also, nutritional consequences of inappropriate cultivars are reflected in pupal weight. The lowest pupal weight of S. cretica on cultivars CP57-614, CP73-21, and SP70-1143 showed that the larvae fed on these cultivars were less suitable than other sugarcane cultivars. Here, it has been seen that changes in nutrient quality or secondary composition between sugarcane cultivars can affect larval growth and can be reflected in the weight of S. cretica pupal. Bodyweight loss in cultivars CP57-614, CP73-21, and SP70-1143 might be attributed to the higher contents of biochemical characteristics in these cultivars.
According to cluster analysis in this study, larvae reared on the cultivars CP69-1062, IRC99-01, and CP48-103 presented higher nutritional physiology, indicating that these cultivars are suitable for growth and nutrition of S. cretica. In addition, the results of cluster analysis showed that CP69-1062, IRC99-01, and CP48-103 are relatively susceptible cultivars, and cultivars CP57-614, CP73-21, and SP70-1143, especially CP73-21, are relatively unsuitable cultivars for S. cretica larvae.
In this study, the feeding properties of S. cretica fed on sugarcane were negatively correlated with the levels of the phytochemical metabolites. It was shown that secondary compounds play a negative role in the growth of S. cretica. Moreover, the results showed that cultivar CP73-21 as an unsuitable host for S. cretica could be considered in the IPM programs of S. cretica in sugarcane fields. Besides, the lowest feeding indices, such as RCR and RGR by S. cretica in cultivar CP73-21, are most likely related to the poor nutritional quality of this cultivar. Larvae reared on cultivar CP73-21 had the lowest values of RCR and RGR, indicating that larvae feeding on this cultivar were less effective in converting ingested and digested food to biomass. Also, a significant correlation between relative consumption rate and biochemical attributes indicated less ability to convert ingested feed into biomass, leading to reduced food intake and larval weight and can be due to higher levels of secondary compounds in cultivars CP57-614, CP73-21, and SP70-1143.
In parallel, S. cretica enzymatic levels were adjusted based on the number of biochemical traits, such as the total phenol, flavonoids, and anthocyanins contents. The S. cretica larvae fed on cultivar IRC99-01 had the highest amylolytic activity. The lowest amylolytic activity of S. cretica, reported in cultivar CP57-614, can be related to the presence of amylase inhibitors. The total phenol content of sugarcane cultivars was negatively correlated to the amylolytic activity of larvae, suggesting that there was low feeding activity due to a higher concentration of phenol content in the tested cultivars. The enzymatic activity of S. cretica was affected by the food consumed, which has been reported in many studies on pests (Hemati et al. 2012a; Jalaeian et al. 2021). Moreover, the lowest proteolytic activity on cultivars IRC99-01, SP70-1143, and CP69-1062 can be attributed to some enzyme inhibitors that can inhibit digestive enzymes. Here, it was observed that the proteolytic activity of S. cretica positively correlated with the total phenol and flavonoids contents and negatively correlated with anthocyanins of sugarcane. Accordingly, different levels of the enzymatic activity in S. cretica larvae can be related to the biochemical differences of sugarcane cultivars. It has been reported that the physical and chemical characteristics of host plants can adversely affect the feeding and digestion processes of insects (Scriber and Slansky 1981).
Conclusion
Our results expand the knowledge on developing a new management procedure for S. cretica, including resistant cultivars in farm systems. The results indicated that cultivars CP69-1062, IRC99-01, and CP48-103 were the most suitable and CP73-21 was the least unsuitable for S. cretica feeding, with a potential application against this pest. It is recommended to investigate the effect of sugarcane cultivars on life history variables of S. cretica for the future. Furthermore, to achieve more practical knowledge for controlling this pest, the response of S. cretica toward other growth inhibitors should be studied to select suitable inhibitors to improve plant resistance against S. cretica.
References
Abedi Z, Golizadeh A, Soufbaf M, Hassanpour M, Jafari-Nodoushan A, Akhavan HR (2019) Relationship between performance of carob moth, Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) and phytochemical metabolites in various pomegranate cultivars. Front Physiol 10:1425
Arbabtafti R, Fathipour Y, Ranjbar Aghdam H (2021) Temperature-dependent demography of two geographically isolated populations of Sesamia cretica (Lepidoptera: Noctuidae). Environ Entomol 50(4):909–918
Arcenaux G (1965) Cultivated sugarcane of the world and their botanical derivation. Proc Int Soc Sugar Cane Technol 12:844–854
Bernfeld P (1955) Amylases, a and b. Meth Enzymol 1:149–158
Elpidina EN, Vinokurov KS, Gromenko VA, Rudenshaya YA, Dunaevsky YE, Zhuzhikov DP (2001) Compartmentalization of proteinases and amylases in Nauphoeta cinerea midgut. Arch Insect Biochem Physiol 48:206–216
Ezzeldin HA, Sallam AAA, Helal TY, Fouad HA (2009) Effect of some materials on Sesamia cretica infesting some maize and sorghum varieties. Arch Phytopath Plant Protec 42(3):277–290
Gvozdenac SM, Prvulovi DM, Radovanovi MN, Ovuka S, Mikli VJ, Canski JMA et al (2018) Life history of Plodia interpunctella Hübner on sunflower seeds: effects of seed qualitative traits and the initial seed damage. J Stored Prod Res 79:89–97
Haukioja E, Ossipov V, Lempa K (2002) The interactive effects of leaf maturation and phenolics on consumption and growth of a geometrid moth. Entomol Exp Appl 104:125–136
Hemati SA, Naseri B, Ganbalani GN, Dastjerdi HR, Golizadeh A (2012a) Digestive proteolytic and amylolytic activities and feeding responses of Helicoverpa armigera (Noctuidae: Lepidoptera) on different host plants. J Econ Entomol 105(4):14391446
Hemati SA, Naseri B, Ganbalan GN, Dastjerdi HR, Golizadeh A (2012b) Effect of different host plants on nutritional indices of the pod borer helicoverpa armigera. J Insect Sci 12(55):1–15
Hemmati SA, Takalloo Z, Taghdir M, Mehrabadi M, Balalaei S, Moharramipour S, Sajedi RH (2021) The trypsin inhibitor pro-peptide induces toxic effects in Indianmeal moth Plodia Interpunctella. Pest Biochem Physiol 171:104730
Hosseininaveh V, Bandani AR, Azmayeshfard P, Hosseinkhani S, Kazzazi M (2007) Digestive proteolytic and amylolytic activities in Trogoderma granarium Everts (Dermestidae: Coleoptera). J Stored Prod Res 43:515–522
Inc SPSS (2007) SPSS base 16.0 user’s guide. SPSS Incorporation Chicago, IL
Jalaeian M, Mohammadzadeh M, Mohammadzadeh M, Borzoui E (2021) Rice cultivars affect fitness-related characteristics and digestive physiology of the rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J Stored Prod Res 93:101821
Karasov WH, Martinez del Rio C, Caviedes-Vidal E (2011) Ecological physiology of diet and digestive systems. Annu Rev Physiol 73:69–93
Kim DO, Chun OK, Kim KJ, Moon HY, Lee CY (2003) Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 51:6509–6515
Lee KP (2007) The interactive effects of protein quality and macronutrient imbalance on nutrient balancing in an insect herbivore. J Exp Biol 210:3236–3244
Liu ZD, Li DM, Gong PY, Wu KJ (2004) Life table studies of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), on different host plants. Environ Entomol 33:1570–1576
Mardani-Talaee M, Nouri-Ganblani G, Razmjou J, Hassanpour M, Naseri B, Asgharzadeh A (2016) Effects of chemical, organic and bio-fertilizers on some secondary metabolites in the leaves of bell pepper (Capsicum annuum) and their impact on life table parameters of Myzus persicae (Hemiptera: Aphididae). J Econ Entomol 109:1231–1240
Naseri B, Majd-Marani Sh (2020) Assessment of eight rice cultivars flour for feeding resistance to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 88:101650
Nathan SS, Chung PG, Murugan K (2005) Effect of biopesticides applied separately or together on nutritional indices of the rice leaf folder Cnaphalocrocis medinalis. Phytoparasitica 33:187–195
Price PW, Bouton CE, Gross P, Mcpheron BA, Thompson JN, Weise AE (1980) Interaction among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Ranjbar Aghdam H, Kamali K (2002) In vivo rearing of Sesamia cretica and Sesamia nonagrioides botanephaga. J Entomol Soc Iran 22:63–78
Sadeghi R, Eshrati MR, Mortazavian SMM, Jamshidnia A (2019) The effects of the essential oils isolated from four ecotypes of cumin (Cuminum cyminum L.) on the blood cells of the pink stem borer, Sesamia cretica Ledere (Lepidoptera: Noctuidae). J Kansas Entomol Soc 92(1):390–399
Scriber JM, Slansky F (1981) The nutritional ecology of immature insects. Ann Rev Entomol 26:183–211
Sedighi L, Ranjbar Aghdam H, Imani S, Shojai M (2016) Comparative demography of Sesamia cretica Lederer (Lepidoptera: Noctuidae) on its two the most important natural hosts, maize and sugarcane. J Agr Sci Tech 18:1807–1818
Sharma HC, Sujana G, Rao DM (2009) Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea. Arthropod Plant Interact 3:151–161
Shishehbor P, Hemmati SA (2021) Investigating of secondary metabolites in bean cultivars and their impact on nutritional performance of Spodoptera littoralis (Lep.: Noctuidae). Bull Entomol Res 112:1–11. https://doi.org/10.1017/S0007485321000948
Slinkard K, Singleton VL (1977) Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic 28:49–55
Soltani Orang F, Ranjbar Aghdam H, Abbasipour H, Askarianzadeh A (2014) Effect of temperature on developmental rate of Sesamia cretica (Lepidoptera: Noctuidae) immature stages. Insect Sci 14:197
Temerak SA, Negm AA (1979) Impact and differential effect of certain biomortality factors on the eggs and newly-hatched larvae of the pink borer, Sesamia cretica Lederer on two sugarcane varieties. Z Angew Entomol 88:313–318
Tomberlin JK, Sheppard DC, Joyace JA (2002) Susceptibility of black soldier fly (Diptera: Stratiomyidae) larvae and adults to four insecticides. J Econ Entomol 95:598–602
Tsai JH, Wang JJ (2001) Effects of host plant on biology and life table parameters of Aphis spiraecola (Hom: Aphididae). Environ Entomol 30:44–50
Turfan O, Turkyılmaz M, Yemis O, Ozkan M (2011) Anthocyanin and colour changes during processing of pomegranate (Punica granatum L., cv. Hicaznar) juice from sacs and whole fruit. Food Chem 129:1644–1651
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv Insect Physiol 5:229–288
War AR, Paulraj MG, War MY, Ignacimuthu S (2011) Differential defensive response of groundnut to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J Plant Interact 6:1–11
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
Wright DJ, Verkert RHJ (1995) Integration of chemical and biological control systems for arthropods; evaluation in a multitrophic context. Pestic Sci 44:207–218
Zahedi A, Razmjou J, Rafiee-Dastjerdi H, Leppla NC, Golizadeh A, Hassanpour M, Ebadollahi A (2019) Tritrophic interactions of cucumber cultivar, Aphis gossypii (Hemiptera: Aphididae), and its predator Hippodamia variegata (Coleoptera: Coccinellidae). J Econ Entomol 112:1774–1779
Acknowledgements
This research was funded by Shahid Chamran University of Ahvaz, which is greatly appreciated.
Funding
This research was funded by Shahid Chamran University of Ahvaz, (Grant No. SCU.AP99.323).
Author information
Authors and Affiliations
Contributions
Conceptualization, AB, BH, and SAH; methodology, BH and SAH; software, AB and SAH; validation, AB, BH, and SAH; formal analysis, AB and SAH; investigation, AB, BH, HRA, and SAH; resources, AB, BH, HRA, and SAH; data curation, AB, BH, and SAH; writing—original draft preparation, AB; writing—review and editing, BH and SAH; visualization, BH and SAH; supervision, BH, HRA, and SAH; project administration, BH; and funding acquisition, BH. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any experiment on human or animal performed by any of the authors.
Additional information
Handling Editor: Vartika Mathur.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Babamir-Satehi, A., Habibpour, B., Aghdam, H.R. et al. Interaction between feeding efficiency and digestive physiology of the pink stem borer, Sesamia cretica Lederer (Lepidoptera: Noctuidae), and biochemical compounds of different sugarcane cultivars. Arthropod-Plant Interactions 16, 309–316 (2022). https://doi.org/10.1007/s11829-022-09898-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-022-09898-w