Abstract
To prevent damage by pollen beetles (Brassicogethes aeneus (Fabricius), syn. Meligethes aeneus (Fabricius)), frequent insecticide applications are often necessary. It would be an advantage if treatments for pollen beetle control would not only avoid yield losses by bud damage by overwintered pollen beetles, but also minimize pest reproduction, and also have side effects on the population development of other pests present in the crop. The effects of the neonicotinoid Biscaya (a.i. thiacloprid) and the pyrethroids Mavrik (tau-fluvalinate) and Karate Zeon (lambda-cyhalothrin) applied at different growth stages of winter oilseed rape on the abundance of overwintered pollen beetles were determined in field trials in Germany (2013–2015). In addition, effects on the two larval instars and new generation of adult pollen beetle were studied. Biscaya and Mavrik significantly reduced the number of overwintered pollen beetles up to seven days after application, whereas Karate Zeon had no effect. Application of Biscaya at the beginning of flowering resulted in a high mortality of L1-larvae in all years. The number of premature L1-larvae dropping down from the plants during the first week after application increased up to 425% compared to the control. The number of L2-larvae dropping down to the ground for pupation was significantly reduced by insecticide applications at different growth stages except for Karate Zeon. In Karate Zeon-treated plots, the number of L2-larvae dropping to the ground increased up to 42% compared to the control. In accordance with the reduced number of L2-larvae in Biscaya- and Mavrik-treated plots, fewer new-generation pollen beetles emerged in the field trials near Braunschweig, efficacy varying between 57 and 76% in Biscaya-treated plots and 32 and 57% in Mavrik-treated plots in 2014 and 2015, respectively. The results indicate that Mavrik, and especially Biscaya, are effective in controlling pollen beetles, reducing infestation pressure and thereby insecticide treatment frequency in following years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pollen beetle, Brassicogethes aeneus (Fabricius), syn. Meligethes aeneus (Fabricius) (Coleoptera: Nitidulidae) is a major pest on winter and spring oilseed rape across Europe (Williams 2010). The overwintered beetles leave their hibernation sites in March/April and often feed on pollen of different spring flowers before they colonize oilseed rape crops where they feed on the buds to get access to pollen (Müller 1941b; Free and Williams 1978; Ouvrard et al. 2016). High numbers of pollen beetles during the green bud stage can result in bud abortion and substantial loss of yield (Nilsson 1987). After ovary maturation, females lay their eggs into the buds (Fritzsche 1957; Nilsson 1988a; Ekbom and Borg 1996; Cook et al. 2004; Hervé et al. 2015). The two larval instars feed on pollen (Osborne 1964; Nilsson 1988b; Cook et al. 2004). The first immature larval instars (L1) develop within the buds, whereas the second larval instars (L2) are very active and consume pollen from several flowers during their development (Williams and Free 1978). The majority of mature L2-larvae drop down to the soil for pupation during the petal fall. After emergence, new-generation pollen beetles feed on pollen of different plant families before they seek their hibernation sites at the turn of July to August (Müller 1941a).
To minimize damage by various insect pests, crops of oilseed rape in Europe are often sprayed frequently with insecticides (Richardson 2008a). During 2007–2013, the mean number of annual insecticide treatments in winter oilseed rape in Germany was 2.7 (Freier et al. 2015). The highest intensity was targeted on cabbage seedpod weevil (Ceutorhynchus obstrictus (Marsham)) and pollen beetle (Freier et al. 2015). For more than 20 years, the main insecticide class extensively used against pests on oilseed rape have been the pyrethroids (Nauen 2005; Heimbach et al. 2006; Müller et al. 2008; Thieme et al. 2010). Frequent applications of pyrethroids and the overlapping exposure of different oilseed rape pests have resulted in high selection pressure and the development of resistance in some of these species including pollen beetle, cabbage seedpod weevil and cabbage stem flea beetle (Psylliodes chrysocephala (L.)) (Heimbach and Müller 2013). Pyrethroid resistance is also known for the rape winter stem weevil (C. picitarsis Gyllenhal) in France (Robert et al. 2015).
In 1999, decreasing pyrethroid sensitivity in pollen beetles was first detected in France (Thieme et al. 2010; Slater et al. 2011). Since then, pyrethroid resistance has been found all over Europe (Hansen 2003; Nauen 2005; Wegorek 2005; Heimbach et al. 2006; Richardson 2008b; Tiilikainen and Hokkanen 2008; Slater et al. 2011; Zimmer and Nauen 2011a). To control pyrethroid-resistant pollen beetles, other insecticides with different modes of action are necessary, which do not only prevent bud damage and yield losses by overwintered pollen beetles but ideally also diminish the reproduction rate of the targeted pest, thereby reducing the probability of a high infestation pressure and insecticide treatment frequency in the following years. Effects of insecticide application on population development of pollen beetles were reported by Kdimati (1990). In plots treated with the pyrethroid Decis (a.i. deltamethrin), bud infestation with eggs and numbers of dropping larvae were reduced resulting in lower numbers of emerging new-generation pollen beetles.
Besides other insecticides, the systemic neonicotinoid Biscaya (a.i. thiacloprid 72 g ha−1) and the non-systemic pyrethroids Mavrik (type I pyrethroid, tau-fluvalinate 48 g ha−1) and Karate Zeon (type II pyrethroid, lambda-cyhalothrin 7.5 g ha−1) are registered for control of pollen beetles. These insecticides are classified as contact and stomach poisons. Thiacloprid acts as an agonist on the insect nicotinic acetylcholine receptor (Elbert et al. 2008). Tau-fluvalinate and lambda-cyhalothrin are sodium channel modulators and interfere with nerve conduction (Roberts et al. 1999). Type I and II pyrethroids are separated by the absence (type I) or presence (type II) of an α-cyano group in their structure (Dong 2007; He et al. 2008).
In contrast to C. obstrictus which showed similar sensitivity to different active substances of pyrethroids (Heimbach and Müller 2013), differences in the susceptibility of pollen beetles are known. Type I pyrethroids are known to have a higher efficacy against resistant pollen beetles than type II pyrethroids under field conditions (Schröder et al. 2009; Smatas et al. 2012) and in laboratory tests (Wegorek et al. 2009); oilseed rape inflorescences and leaves dipped in test solutions of different insecticides resulted in type I pyrethroids (in the study tau-fluvalinate and bifenthrin) causing higher mortality of pollen beetles than type II pyrethroids (beta-cyfluthrin, zeta-cypermethrin, and esfenvalerate) (Wegorek et al. 2009). However, in a standard bioassay (IRAC 2009) applied for resistance monitoring in Germany, pollen beetles have shown a decreasing sensitivity to type I pyrethroids as well (Heimbach and Müller 2013). This is in accordance with Makunas et al. (2011); Lithuanian pollen beetle populations were tested in bioassays over three consecutive years and lower effects of type II pyrethroids than tau-fluvalinate (type I) were observed. A decreasing susceptibility to type I pyrethroids over the experimental years were also assessed.
The objective of the present study was to determine the effects of the insecticides Biscaya, Mavrik and Karate Zeon applied between the bud and full flowering stage of winter oilseed rape on the abundance of overwintered pollen beetles, the two larval instars and the new generation of pollen beetles.
Materials and methods
The effects of insecticides on the abundance and population growth of pollen beetles were studied from 2013 to 2015 in field trials at the trial sites of the Julius Kühn-Institut in the region of Braunschweig, Germany (52°32′80.29″N, 10°63′16.74″E (Wendhausen 2013), 52°21′72.27″N, 10°62′72.30″E (Sickte 2014), 52°21′00.81″N, 10°67′36.03″E (Lucklum 2015)). The winter oilseed rape cultivars “Visby” (2013 and 2014) and “Avatar” (2015) were used in the experiments. The BBCH code of Lancashire et al. (1991) was used for characterizing the growth stages of winter oilseed rape. The field trials were established in a randomized block design. The plots had a size of 30 m × 24 m (Lucklum), 40 m × 24 m (Wendhausen) and 45 m × 24 m (Sickte). The controls were arranged as included controls according to EPPO PP 1/152(4) (EPPO 2012). There were no additional isolation zones within the trial site. All samplings were done in the centre of each plot leaving sufficient distance to adjacent plots. All treatments were replicated four times. Insecticide application was carried out between the bud stage and the flowering stage, using recommended product application rates in Germany in 300 l water ha−1. In 2013, the neonicotinoid Biscaya (300 ml ha−1, a.i. 72 g thiacloprid ha−1) and the pyrethroid Karate Zeon (75 ml ha−1, 7.5 g lambda-cyhalothrin ha−1) were used. For the application of Biscaya at different growth stages, separate plots were used, whereas the three applications of Karate Zeon were carried out in a sequence to the same four plots. In 2014 and 2015, Karate Zeon was replaced by the pyrethroid Mavrik (200 ml ha−1, 48 g tau-fluvalinate ha−1). For the application of Mavrik as well as of Biscaya at different growth stages in 2014 and 2015, separate plots were used.
In addition, similar field trials at various locations in Lower Saxony, Mecklenburg-Western Pomerania and Bavaria were carried out in 2013–2015 (Table 1). The field trials were established with different cultivars and plot sizes of 36–480 m2 each in a randomized block design with four replicates. As in the field trials carried out in the region of Braunschweig, the insecticides Biscaya, Mavrik and Karate Zeon were applied with recommended product dose rates once between the green (BBCH 53) and the yellow bud stages (BBCH 59).
In the field trials near Braunschweig, the abundance of overwintered pollen beetles was assessed according to the EPPO Standard PP 1/178 (3) (EPPO 2005) just before and 1, 3–4, 6–8 and 13–15 days after application. The number of pollen beetles was recorded between 9.00 and 11.00 a.m. by beating 50 randomly selected main stems per plot over a plastic tray (31.5 cm × 25.5 cm). Subsequently, the mean number of beetles per main stem was calculated.
To collect L1- and L2-larvae dropping from inflorescences to the ground in field trials near Braunschweig, either as a direct lethal effect of insecticides or to pupate in the soil (L2), ten plastic bowls (17 cm × 12.1 cm) filled with a 10% sodium benzoate water solution for conservation were placed on the ground in the centre of each plot (approximately 1 m distance to the tramline). The plastic bowls were positioned before the first insecticide application at BBCH 53 in 2013 and at BBCH 55 in 2014 and 2015, so not to miss the beginning of the larval dropping. The bowls were checked every few days and, after the first larvae were caught at BBCH 62, were then emptied weekly until BBCH 76–78. The larvae were stored in 70% ethanol, later separated by their development stage (L1 or L2) according to Osborne (1964) and counted under the binocular microscope (eight- to tenfold magnification). The number of larvae captured in 10 bowls of 205.7 cm2 each was adapted to 1 m2. Additionally, the plant density was assessed early in spring (3 April 2013, BBCH 16–18; 10 March 2014, BBCH 30–31; 11 March 2015 BBCH 16–18) by counting the plants growing on 1 × 1 m at ten randomly selected areas per plot.
At all other trial locations, four plastic bowls (18.3 cm × 13.6 cm) per plot were placed in the field. In 2013, the plastic bowls were established at BBCH 61 (Puch), BBCH 63 (Stöckendrebber) and BBCH 70 (Cramonshagen). In 2014 and 2015, at all locations, they were set up at the bud stage and emptied weekly. The trapped larvae were stored and separated into development stages as described above. The number of larvae captured in 4 bowls of 248.9 cm2 was adapted to 1 m2. Only the results of L2-larvae will be presented.
New-generation pollen beetles were collected in field trials near Braunschweig using three soil-photoeclectors (each 0.25 m2, ecoTech GmbH) per plot, established near the tramline (approximately 1 m distance). To avoid any damage to the development of oilseed rape plants within and outside the enclosed area, the circular bases of the photoeclectors were dug into the soil before stem elongation of plants in early spring. At BBCH 76–78, the photoeclectors were enclosed by a fabric tent before the emergence of new-generation pollen beetles started. A perforated plastic bag was fixed to the opening on top of the photoeclectors to catch the beetles alive. The plastic bags were emptied twice a week between the start (BBCH 80) and the end of beetle emergence (BBCH 84).
Statistical analyses
Statistical analyses were carried out using the software R, version 3.1.2 (R Core Team 2014; packages: lme4 (Bates et al. 2015), multcomp (Hothorn et al. 2008), effects (Fox 2003), MASS (Venables and Ripley 2002), glmmADMB (Fournier et al. 2012), coin (Hothorn et al. 2006)). Data of each year were analysed separately, because of the high annual variability in infestation levels, weather conditions and field trial locations. To compare the abundance of overwintered pollen beetles per main stem in different treatments, generalized linear mixed models (GLMMs, Poisson for count data) were used. The treatment and the date were included into the model as the main effects and their interaction was tested. The replicates were integrated as a random effect. The optimal model was selected using Akaike Information Criteria (AIC) described in Zuur et al. (2009). The model was checked for dispersion. The treatments were compared pairwise for each day of assessment with the package lsmeans (Lenth 2015) for post hoc testing. P values were adjusted with the Hochberg method (Blakesley et al. 2009).
Differences between numbers of larvae in different treatments were analysed using generalized linear models (GLMs). Because the Poisson model revealed overdispersion, a negative binomial model was used. The treatment and the date were included into the model as the main effects and their interaction was tested. To account for differing numbers of plastic bowls (a few plastic bowls were tipped over or destroyed by animals), an offset was included into the model. Pairwise comparison of the treatments for each sampling period was conducted as described for overwintered pollen beetles. To compare the accumulated number of L2-larvae dropping down for pupation in the different treatments over the total sampling period, an analysis of variance (ANOVA) was performed. Assumptions on variance homogeneity and normality of residuals were visually inspected. The differences between the means were evaluated by Tukey’s HSD test. To analyse differences between the abundance of new-generation pollen beetles emerging in the different treatments, GLMMs (Poisson) were used. The total numbers of new-generation beetles collected over all sampling periods were analysed as described for L2-lavae. The emergence rate of new-generation beetles was calculated as the percentage of new-generation beetles in relation to L2-larvae dropping to the soil for pupation. The efficacy of insecticide treatments was calculated according to the formula of Abbott (1925).
Results
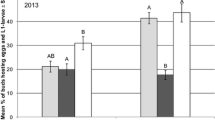
In 2013, on average 2.0 pollen beetles per main stem were recorded at BBCH 53 in the plots before treatment. One day after treatment, the number of pollen beetles per main stem was significantly reduced by 47% in plots treated with Biscaya compared to the control (GLMM, p = 0.0032) and to plots treated with Karate Zeon (GLMM, p = 0.0308) (Table 2). Three days after treatment, the pollen beetle density increased because in the meantime maximum daily temperatures > 20 °C resulted in new immigration. The lowest number of pollen beetles occurred again in plots treated with Biscaya (efficacy 33%). Pollen beetle numbers did not differ between the treatments 7 days after treatment (GLMM, p > 0.05), while 13 days after treatment the numbers significantly increased in Biscaya-treated plots in comparison to the control (GLMM, p = 0.0014).
Similar effects of Biscaya on pollen beetle densities were found following the application at BBCH 60 in 2013: 1 and 4 days after application, the pollen beetle density was lowest in Biscaya-treated plots, whereas Karate Zeon had no significant effect on beetle numbers. Seven and 14 days after treatment, there was no significant difference in the number of pollen beetles per main stem between the treatments (GLMM, p > 0.05). In the period following the insecticide application at BBCH 65, the infestation rate of pollen beetles was very low (on average 0.3 pollen beetles per main stem) with the highest number of beetles observed in Biscaya-treated plots.
In the field trial of 2014, the pollen beetle density was clearly lower than in 2013; at BBCH 55, an average of 1.5 pollen beetles per main stem were recorded in plots before treatments. After insecticide application at BBCH 55, the beetle density decreased continuously in all plots (Table 2). One and 3 days after the application of Biscaya and Mavrik, the number of pollen beetles was significantly lower than in the control (GLMM, Biscaya: p < 0.001/p = 0.0171; Mavrik: p < 0.001/p = 0.0076). One day after treatment, the efficacy of Biscaya and Mavrik was 68 and 82%, respectively. Three days after treatment, pollen beetle density values in Biscaya- and Mavrik-treated plots were both reduced by ca. 50%. At 7 and 15 days after treatment, no significant difference in pollen beetle density occurred between treatments (GLMM, p > 0.05). Because of a very low infestation rate (on average 0.08 pollen beetles per main stem) in the period following insecticide application at BBCH 62, these data are too low for meaningful analysis.
In 2015, on average 2.1 pollen beetles per main stem were recorded at BBCH 55 in the plots before treatment. As in 2014, pollen beetle density generally decreased in all plots following the application. Again the application of Biscaya and Mavrik showed similar effects on pollen beetle density (Table 2). One day after treatment, pollen beetle density decreased by 70% in Biscaya and by 96% in Mavrik-treated plots. The number of pollen beetles was significantly lower in insecticide-treated plots up to 7 days after treatment, with Mavrik having stronger effects. At 14 days after treatment, the pollen beetle density significantly increased in Biscaya-treated plots in comparison to the control (GLMM, p = 0.0002) and Mavrik-treated plots (GLMM, p = 0.0110) similar to 2013. Following the insecticide application at BBCH 62, pollen beetle density was low but the same trend was observed. The number of pollen beetles was significantly lower in Biscaya- and Mavrik-treated plots up to 3 days after treatment. At 8 days after treatment, no significant difference between treatments was detected.
In all experimental years, there was no difference in plant density between plots, so an influence on the number of dropping larvae by different plant densities can be excluded (data not presented). In all 3 years of field trials in the region of Braunschweig, the premature dropping of L1-larvae started at BBCH 62–65. In 2013, in the first sampling period from 2 to 15 May (BBCH 60–65), the number of L1-larvae (706 individuals m−2) in the plots treated with Biscaya at BBCH 60 was significantly higher than in all other treatments (Table 3). Compared to untreated plots, the number of dropping L1-larvae was increased up to 388%. Similar strong effects were recorded following the application of Biscaya at BBCH 62 in 2014 and 2015. In the Biscaya-treated plots, the number of L1-larvae increased up to 425 and 200%, respectively, compared to untreated plots. In both years, the lowest number of dropping L1-larvae was found in plots treated with Mavrik at BBCH 55.
In the second sampling period of larvae in 2013 (15–23 May, BBCH 65–69), the highest number of L1-larvae dropped down in the plots treated with Biscaya at BBCH 65, with 1122 L1-larvae m−2, and an increase of 283% compared to the control. In 2014, in the second sampling period, only low numbers of L1-larvae dropped down from the plants and there was no significant difference between treatments (GLM, p > 0.05). In 2015, the number of L1-larvae caught in plastic bowls in plots treated with Biscaya at BBCH 62 increased up to 112% compared to the control. The lowest number of L1-larvae was found in the plots treated with Biscaya and Mavrik at BBCH 55, with numbers of L1-larvae reduced by 63% compared to the control.
In all experimental years, the number of L1-larvae caught during the third and fourth weekly sampling periods decreased continuously. In 2013, there was no significant difference between treatments in the third sampling period (BBCH 69–71) (GLM, p > 0.05). In the fourth sampling period in 2013 (BBCH 71–74), significantly more L1-larvae dropped down in the control compared to Biscaya treatments at BBCH 53 (GLM, p = 0.001) and BBCH 60 (GLM, p = 0.0162), respectively. In plots treated with Biscaya at BBCH 53 and BBCH 60, the number of L1-larvae was reduced by 82 and by 70%, respectively. In 2014 and 2015, only few larvae were found in the plastic bowls in the third (BBCH 67–69) and fourth sampling periods (2014: BBCH 69–72; 2015: 69–71) and there was no significant difference between treatments (GLM, p > 0.05).
The peak of L2-larvae, dropping down to the ground for pupation, was observed at BBCH 65–69 at petal fall in all experimental years. The mean number of L2-larvae accumulated over the total period of larval drop showed lower numbers in plots treated with Biscaya (Table 3). In 2013, the highest efficacy (58%) was achieved by the application of Biscaya at BBCH 60, while the efficacy of Biscaya at BBCH 53 and BBCH 65 was 25 and 38%, respectively. Repeated application of Karate Zeon at the three different growth stages resulted in 10% higher numbers of L2-larvae dropping to the ground than in the control. Higher numbers of L2-larvae dropping down in Karate Zeon-treated plots were also observed in other field trials distributed across Germany and in different years with larval numbers dropping down in Karate Zeon-treated plots increasing up to 42% compared to the control (Table 4).
As in 2013, insecticide applications reduced the number of dropping L2-larvae in 2014 and 2015. Application of Biscaya at BBCH 55 and BBCH 62 in 2014 resulted in a significant reduction compared to the control (Tukey’s HSD, Biscaya BBCH 55: p = 0.0332; Biscaya BBCH 62: p = 0.0004) with efficacies of 34 and 59%, whereas the application of Mavrik at BBCH 55 and BBCH 62 showed efficacies of 30 and 17%, respectively (Tukey’s HSD, p > 0.05). In 2015, the number of L2-larvae was significantly reduced by the applications of Biscaya at BBCH 55 and 62 and of Mavrik at BBCH 55 compared to the control (Tukey’s HSD, Biscaya BBCH 55: p = 0.0003; Biscaya BBCH 62: p = 0.0008; Mavrik BBCH 55: p = 0.0025). In plots treated with Biscaya or Mavrik at BBCH 55, the number of L2-larvae was reduced by 64 and 53%, respectively. The treatment with Biscaya at BBCH 62 resulted in an efficacy of 58%, whereas the application of Mavrik at this growth stage reduced the number of L2-larvae only by 13%. Similar effects of Biscaya were observed in the additional field trials distributed across Germany in three consecutive years. In most trials, the numbers of L2-larvae were significantly lower compared to the control (Table 4). Mavrik also reduced the number of L2-larvae, but in most trials it was less effective than Biscaya. In contrast, higher numbers of L2-larvae were detected after Karate Zeon applications compared to the control.
The emergence of new-generation pollen beetles started in all experimental years at BBCH 80. In 2013, the efficiency of the catching method was compromised by deep cracks in the soil caused by extended drought periods and not all emerging new-generation beetles were caught. In 2014 and 2015, the number of emerging new-generation pollen beetles was significantly reduced in all Biscaya-treated plots which showed a higher efficacy at both application dates in each year (Table 3). In 2014, the application of Biscaya at BBCH 55 and 62 resulted in a reduction of new-generation beetles by 57 and by 59%, whereas the treatment with Mavrik at BBCH 55 and BBCH 62 reduced the number of new-generation beetles by 47 and 42%, respectively. In 2015, the lowest number of new-generation pollen beetles was recorded in plots treated with Biscaya at BBCH 55 (efficacy 76%), followed by the Biscaya treatment at BBCH 62 (72%) and the application of Mavrik at BBCH 55 (57%). The application of Mavrik at BBCH 62 resulted in a reduction of beetles by 32% compared to the control. The average emergence rate in all treatments was 22.9% in 2014 and 40.4% in 2015.
Discussion
In the presented field trials, the density of overwintered pollen beetles on oilseed rape crops was significantly reduced up to 7 days after the application of Biscaya and Mavrik, whereas Karate Zeon had no effect. Type I pyrethroids (tau-fluvalinate in Mavrik) are known to have a higher efficacy against pyrethroid-resistant pollen beetles than type II pyrethroids (lambda-cyhalothrin in Karate Zeon) in the field (Schröder et al. 2009; Smatas et al. 2012). Significant effects of Mavrik for at least 7 days after application are described by Vaitelyte et al. (2011). So far, no resistance to thiacloprid has been detected in European B. aeneus populations (Nauen et al. 2012; Zimmer et al. 2014). Effects seen in the field trials confirm this. A significant reduction of overwintered pollen beetles by application of Biscaya was also reported by Smatas et al. (2012). Jansen and Gomez (2014) observed significant effects on pollen beetle reduction by Biscaya for at least 8 days after application.
In addition to direct lethal effects, the application of Biscaya and Mavrik at the bud stage may have sublethal effects on pollen beetles, i.e. affecting the physiology or behaviour of individuals that survive the exposure to insecticides (Desneux et al. 2007). Sublethal effects may influence the coordination of body movements of the beetles or result in increased activity (Desneux et al. 2007). These effects may be the reason for the phenomenon observed by Gödeke (personal communication); in winter oilseed rape field trials, more adult pollen beetles dropped down from plants treated with Biscaya up to 7 days after application compared to the untreated plots, whereas the application of Mavrik did not result in higher numbers of dropping beetles.
In 2013 and 2015, pollen beetle density increased in plots treated with Biscaya at the bud stage compared to the control 14 days after treatment. This may be explained by a higher attractiveness of the inflorescences after degradation of insecticide residuals. The lower number of beetles for several days after treatment resulted in less bud damage as a result of feeding and oviposition making the plants more attractive than control plants to pollen beetles (Brandes et al. unpublished). The recolonization of Biscaya-treated plots after the decline of insecticidal activity was also reported by Tölle (2014).
It is uncertain whether the larvae hatching from eggs, which have been laid by the increased number of beetles in Biscaya-treated plots 14 days after treatment, were able to complete their development before the end of flowering. Without sufficient food, the larvae can be forced to premature pupation, resulting in a high mortality (Nielsen and Axelsen 1988). According to Nilsson (1994), the larvae should be ready to pupate at petal fall; otherwise, they have to feed on stems or pods to finish their development. This increases the risk of predation and parasitization.
The low pollen beetle density in plots treated with Biscaya and Mavrik at the bud stage contributed to a reduced infestation of buds with eggs (Brandes et al. 2018) which finally resulted in a lower number of larvae and new-generation pollen beetles. Further, the application of Biscaya in the flowering stage (BBCH 60–65) resulted in enhanced premature dropping of L1-larvae. This may have been caused by lethal effects of Biscaya or by sublethal effects impairing larval coordination; this requires further work. Uncoordinated or stumbling movements of third instar larvae of the cabbage looper (Trichoplusia ni) after topical application of pyrethroids were reported by Toth and Sparks (1990). In a larval dip laboratory assay, the L2-larvae of pollen beetle were highly sensitive to thiacloprid while they showed insensitivity to lambda-cyhalothrin (Zimmer et al. 2014). It is likely that L1-larvae are also highly sensitive to thiacloprid resulting in an enhanced dropping of larvae after application. Enhanced larval dropping caused by physical effects of spraying activity can be excluded because no such effects were seen in Mavrik- or Karate Zeon-treated plots. The L1-larvae are not ready for pupation. Once they have dropped to the ground, they are likely to fall prey to predators such as ground beetles (Carabidae), rove beetles (Staphylinidae) and spiders (Araneae) (Büchs and Alford 2003; Piper and Williams 2004; Zaller et al. 2009; Öberg et al. 2011) or they may die from desiccation or lack of food.
The assessment of the number of mature L2-larvae dropping down for pupation is very important for the determination of insecticide effects on population growth of pollen beetle. Higher numbers of L2-larvae dropping down in Karate Zeon-treated plots in the field trial at Wendhausen in 2013 (number of L2-larvae increased up to 10% compared to the control) were also observed in other field trials distributed across Germany and in different years. In Karate Zeon-treated plots, the number of larvae dropping down increased up to 42% compared to the control. The reason for this might be the elimination of antagonists such as spiders by Karate Zeon in the crop canopy resulting in an undisturbed development of pollen beetle larvae. Negative effects of lambda-cyhalothrin on the abundance of spiders have been reported by Wehling and Heimbach (1991), Devotto et al. (2007) and Liu et al. (2013). Similarly, staphylinid larvae which are known to reduce the density of pollen beetle larvae in flowers may be affected by lambda-cyhalothrin (Felsmann 2008).
Insecticide hormoligosis might be another reason for an enhanced number of L2-larvae after Karate Zeon application. Luckey (1968) described the phenomenon that sublethal effects of various stress agents, such as insecticides, can be stimulatory to insects and an increase of reproduction can be expected at low concentrations of insecticides. The highly elevated concentration of monooxygenase enzymes, which are mainly responsible for the fast degradation of pyrethroids in resistant pollen beetles (Zimmer and Nauen 2011b), may be responsible for stimulation of the females resulting in increased oviposition and consequently high numbers of larvae.
As a consequence of the reduction of overwintered pollen beetles, of effects on egg laying (Brandes et al. 2018) and of additional direct effects on L1-larvae in Biscaya-treated plots, the number of second instars was (in most field trials significantly) reduced compared to the control. A significant reduction of L2-larvae after two applications of Biscaya was also reported from field trials in 3 consecutive years by Tölle (2014). In addition, Jansen and Gomez (2014) found a reduction of L2-larvae by 84% in plots treated with Biscaya at the end of the bud stage. In the present field trials, the application of Mavrik also reduced the numbers of L2-larvae, but in most cases its action was less effective than Biscaya. In contrast to Biscaya, Mavrik did not reduce infestation of buds with eggs (Brandes et al. 2018), so it can be concluded that the effects of Mavrik on the number of L2-larvae dropping down for pupation were mainly caused by the reduction of overwintered pollen beetles after application at the bud stage.
The pollen beetle larvae dropping down to the soil can be consumed by ground beetles, rove beetles (Büchs 2003; Zaller et al. 2009; Skellern and Cook 2018) or spiders (Öberg et al. 2011), while the pupae are likely to be preyed upon in the ground by ground beetles and rove beetles (Büchs and Nuss 2000; Büchs 2003). All these natural enemies are known to be affected by insecticides (Pfiffner and Luka 2003; Felsmann 2008). In the present study, the insecticide applications were carried out in a very tight crop canopy. Spraying of oilseed rape during the flowering stage using conventional spraying techniques results in an interception value of 80, which means that only low amounts of the insecticides penetrate to soil level (EFSA 2014). Therefore, in the present field trials the predators were exposed only to low doses of insecticides, with limited effects on predation of larvae and pupae of pollen beetle on and in the soil, but this could not be tested in detail. Further important biocontrol agents are parasitoids. They can also be affected by insecticide applications, but the insecticides used in these field trials did not affect parasitization of pollen beetle larvae by the parasitoids Tersilochus heterocerus and Phradis spp. (Brandes et al. 2017).
In all field trials, the number of L2-larvae dropping down for pupation in different treatments was reflected in the number of emerging new-generation pollen beetles. The treatments with the lowest number of L2-larvae also had the fewest emerging beetles. The application of Biscaya and Mavrik resulted in significantly lower numbers of new-generation beetles than the control, with Mavrik being less effective than Biscaya, which corresponds to the number of L2-larvae dropping down for pupation. A significant reduction of the numbers of emerging new-generation pollen beetles after the application of Biscaya was also described by Tölle (2014).
The effects of Biscaya and partly also Mavrik on pollen beetle populations found in the presented field trials are not reflected in the common efficacy evaluation of insecticides according to EPPO Standard 1/178 (3) (EPPO 2005), where only the effects on overwintered pollen beetles are recorded. The application of Biscaya did not only show effects on overwintered beetles, but also on infestation of buds with eggs and larvae (Brandes et al. 2018). In addition, the application of Biscaya in flowering oilseed rape is highly effective on L1-larvae. Despite the effects on overwintered adult pollen beetles, the effects of Mavrik on infestation of buds with eggs and larvae were smaller compared to Biscaya (Brandes et al. 2018) and the effects on L1-larvae were absent. In conclusion, Biscaya is effective in controlling adult pollen beetles during the bud stage and an effect on reproduction may reduce infestation pressure and insecticide treatment frequency in the following years. Applications at the flowering stage against pollen beetle are not common and not purpose of integrated pest management. Once the plants begin to flower, pollen beetles prefer pollen in open flowers to pollen in buds without causing damage (Fritzsche 1957; Cook et al. 2007). But if other insect pests such as C. obstrictus or Dasineura brassicae require control by insecticides during the flowering stage because of exceeding the thresholds, Biscaya can reduce the reproduction of simultaneously occurring pollen beetles as a side effect.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Blakesley RE, Mazumdar S, Dew MA, Houck PR, Tang G, Reynolds CF III, Butters MA (2009) Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology 23:255–264
Brandes M, Heimbach U, Ulber B (2017) Effects of insecticide application on parasitism rates of pollen beetle larvae (Brassicogethes aeneus (Fabricius)) by tersilochine parasitoids. Arthropod Plant Interact. https://doi.org/10.1007/s11829-017-9580-y
Brandes M, Heimbach U, Ulber B (2018) Impact of insecticides on oilseed rape bud infestation with eggs and larvae of pollen beetle (Brassicogethes aeneus (Fabricius)). Arthropod Plant Interact. https://doi.org/10.1007/s11829-018-9616-y
Büchs W (2003) Predators as biocontrol agents of oilseed rape pests. In: Alford DV (ed) Biocontrol of oilseed rape pests. Blackwell Science, Oxford, pp. 279–298
Büchs W, Alford DV (2003) Predators of oilseed rape pests. In: Alford DV (ed) Biocontrol of oilseed rape pests. Blackwell Science, Oxford, pp. 181–200
Büchs W, Nuss H (2000) First steps to assess the importance of epigaeic active polyphagous predators on oilseed rape insect pests with soil pupating larvae. IOBC-WPRS Bull 23(6):151–163
Cook SM, Murray DA, Williams IH (2004) Do pollen beetles need pollen? The effect of pollen on oviposition, survival, and development of a flower-feeding herbivore. Ecol Entomol 29:164–173
Cook SM, Khan ZR, Pickett JA (2007) The use of push-pull strategies in integrated pest management. Annu Rev Entomol 52:375–400
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Devotto L, Carrillo R, Cisternas E, Gerding M (2007) Effects of lambda-cyhalothrin and Beauveria bassiana spores on abundance of Chilean soil surface predators, especially spiders and carabid beetles. Pedobiologia 51:65–73
Dong K (2007) Insect sodium channels and insecticide resistance. Invertebr Neurosci 7:17–30
EFSA (2014) European Food Safety Authority; EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA J 12(5):3662
Ekbom B, Borg A (1996) Pollen beetle (Meligethes aeneus) oviposition and feeding preference on different host plant species. Entomol Exp Appl 78:291–299
Elbert A, Haas M, Springer B, Thielert W, Nauen R (2008) Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci 64:1099–1105
EPPO (2005) European and Mediterranean plant protection organization; PP 1/178 (3), efficacy evaluation of insecticides. Meligethes aeneus on rape. EPPO Bull 35:183–185
EPPO (2012) European and Mediterranean Plant Protection Organization; PP 1/152 (4), efficacy evaluation of plant protection products. Design and analysis of efficacy evaluation trials. EPPO Bull 42(3):367–381
Felsmann DS (2008) The spatio-temporal dynamics of epigaeic predators and insect pests in different oilseed rape management systems. PhD thesis, University of Braunschweig
Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder M, Nielsen A, Sibert J (2012) AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 27:233–249
Fox J (2003) Effect displays in R for generalised linear models. J Stat Softw 8(15):1–27
Free JB, Williams IH (1978) The responses of the pollen beetle, Meligethes aeneus, and the seed weevils, Ceutorhynchus assimilis Payk. on oil-seed rape, Brassica napus and other plants. J Appl Ecol 15:761–774
Freier B, Sellmann J, Strassemeyer J, Schwarz J, Klocke B, Kehlenbeck H, Zornbach W (2015) Netz Vergleichsbetriebe Pflanzenschutz. Jahresbericht 2013—Analyse der Ergebnisse der Jahre 2007 bis 2013. Berichte aus dem Julius Kühn-Institut 178
Fritzsche R (1957) Zur Biologie und Ökologie der Rapsschädlinge aus der Gattung Meligethes. Zeitschrift für angewandte Entomologie 40:222–280
Hansen LM (2003) Insecticide-resistant pollen beetles (Meligethes aeneus F) found in Danish oilseed rape (Brassica napus L.) fields. Pest Manag Sci 59:1057–1059
He LM, Troiano J, Wang A, Goh K (2008) Environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology vol. 195, Springer, Heidelberg, pp. 71–91
Heimbach U, Müller A (2013) Incidence of pyrethroid-resistant oilseed rape pests in Germany. Pest Manag Sci 69:209–216
Heimbach U, Müller A, Thieme T (2006) First steps to analyse pyrethroid resistance of different oilseed rape pests in Germany. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 58:1–5
Hervé MR, Garcia N, Trabalon M, Le Ralec A, Delourme R, Cortesero AM (2015) Oviposition behavior of the Pollen Beetle (Meligethes aeneus): a functional study. J Insect Behav 28:107–119
Hothorn T, Hornik K, van de Wiel MA, Zeileis A (2006) A Lego system for conditional inference. Am Stat 60(3):257–263
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
IRAC (2009) Insecticide resistance action committee; IRAC susceptibility test methods series, method no: 011. http://www.irac-online.org/content/uploads/Method_011_v3_june09.pdf
Jansen JP, Gomez GS (2014) A large field trial to assess the short-term and long-term effects of 5 insecticides used to control the pollen beetle on parasitic hymenoptera in oilseed rape. IOBC-WPRS Bull 103:9–16
Kdimati H (1990) Untersuchungen zur Befallsprognose des Rapsglanzkäfers (Meligethes aeneus F.) an Winterraps. PhD thesis, University of Rostock
Lancashire PD, Bleiholder H, van den Boom T, Langelüddeke P, Strauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appli Biol 119:561–601
Lenth R (2015) lsmeans: least-squares means. R package version 2.20–23 http://CRAN.R-project.org/package=lsmeans
Liu TX, Irungu RW, Dean DA, Harris MK (2013) Impacts of spinosad and λ-cyhalothrin on spider communities in cabbage fields in south Texas. Ecotoxicology 22:528–537
Luckey TD (1968) Insecticide Hormoligosis. J Econ Entomol 61:7–12
Makunas V, Brazauskiene I, Smatas R (2011) Resistance of Meligethes aeneus to pyrethroids in Lithuania. Zemdirbyste Agric 98:431–438
Müller HJ (1941a) Beiträge zur Biologie des Rapsglanzkäfers Meligethes aeneus F. Zeitschrift für Pflanzenkrankheiten Pflanzenschutz 9:385–435
Müller HJ (1941b) Weitere Beiträge zur Biologie des Rapsglanzkäfers. Meligethes aeneus F. (Ueber das Winterlager und die Massenbewegung im Frühjahr). Zeitschrift für Pflanzenkrankheiten Pflanzenschutz 12:529–595
Müller A, Heimbach U, Thieme T (2008) Pyrethroid sensitivity monitoring in Germany of oilseed rape pest insects other than pollen beetle. EPPO Bull 38:85–90
Nauen R (2005) Insecticide resistance in European agriculture: research instead of rumours. In: Proceedings brighton crop protection conference—crop science & technology 3, 123–130
Nauen R, Zimmer CT, Andrews M, Slater R, Bass C, Ekbom B, Gustafsson G, Hansen LM, Kristensen M, Zebitz CPW, Williamson MS (2012) Target-site resistance to pyrethroids in European populations of pollen beetle, Meligethes aeneus F. Pestic Biochem Physiol 103:173–180
Nielsen PS, Axelsen J (1988) Developmental time and mortality of the immature stages of the pollen beetle (Meligethes aeneus F.) under natural conditions. J Appl Entomol 105:198–204
Nilsson C (1987) Yield losses in summer rape caused by Pollen Beetles (Meligethes spp.). Swed J Agric Res 17:105–111
Nilsson C (1988a) The pollen beetle (Meligethes aeneus F.) in winter and spring rape at Alnarp 1976–1978. II Oviposition Växtskyddsnotiser 52(6):139–144
Nilsson C (1988b) The number of larval instars of Meligethes aeneus (F.) in southern Sweden. Växtskyddsnotiser 52(6):151–152
Nilsson C (1994) Pollen beetles (Meligethes spp) in oil seed rape crops (Brassica napus L.): biological interactions and crop losses. PhD thesis, Swedish University of Agricultural Sciences
Öberg S, Cassel-Lundhagen A, Ekbom B (2011) Pollen beetles are consumed by ground- and foliage-dwelling spiders in winter oilseed rape. Entomol Exp Appl 138:256–262
Osborne P (1964) Morphology of the immature stages of Meligethes aeneus (F.) and M. viridescens (F.) (Coleoptera, Nitidulidae). Bull Entomol Res 55:747–759
Ouvrard P, Hicks DM, Mouland M, Nicholls JA, Baldock KCR, Goddard MA, Kunin WE, Potts SG, Thieme T, Veromann E, Stone GN (2016) Molecular taxonomic analysis of the plant associations of adult pollen beetles (Nitidulidae; Meligethinae), and the population structure of Brassicogethes aeneus. Genome 59:1101–1116
Pfiffner L, Luka H (2003) Effects of low-input farming systems on carabids and epigeal spiders—a paired farm approach. Basic Appl Entomol 4:117–127
Piper R, Williams I (2004) Incidence and feeding activity of epigeic, predatory invertebrates within winter oilseed rape in the UK with comparisons between integrated and conventional crop management. IOBC-WPRS Bull 27(10):281–288
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Richardson DM (2008a) Summary of findings from a participant country pollen beetle questionnaire. EPPO Bull 38:68–72
Richardson DM (2008b) Pollen beetle in the UK; the start of a resistance problem? EPPO Bull 38:73–74
Robert C, Ruck L, Carpezat J (2015) Integrated pest management of the rape winter stem weevil (Ceutorhynchus picitarsis) in France. In: 14th International Rapeseed Congress. Saskatoon, 5–9 July 2015
Roberts TR, Hutson DH, Jewess PJ, Lee PW, Nicholls PH, Plimmer JR (1999) Pyrethroids. In: Roberts TR, Hutson DH (eds) Metabolic pathways of agrochemicals. Part two: insecticides and fungicides. The Royal Society of Chemistry, Cambridge, pp 579–726
Schröder G, Pölitz B, Wolff C, Krüger B (2009) Möglichkeiten der gezielten Bekämpfung von Pyrethroid-resistenten Rapsglanzkäferpopulationen—Ergebnisse von Ringversuchen mehrerer Bundesländer. Gesunde Pflanzen 61:19–30
Skellern MP, Cook SM (2018) Prospects for improved off-crop habitat management for pollen beetle control in oilseed rape. Arthropod Plant Interact. https://doi.org/10.1007/s11829-018-9598-9
Slater R, Ellis S, Genay JP, Heimbach U, Huart G, Sarazin M, Longhurst C, Müller A, Nauen R, Rison JL, Robin F (2011) Pyrethroid resistance monitoring in European populations of pollen beetle (Meligethes spp.): a coordinated approach through the Insecticide Resistance Action Committee (IRAC). Pest Manag Sci 67:633–638
Smatas R, Makunas V, Brazauskiene I, Petraitiene E (2012) Sensitivity of pollen beetle (Meligethes aeneus F.) to insecticides with different modes of action and their efficacy in the field conditions. Zemdirbyste-Agric 99:197–202
Thieme T, Heimbach U, Müller A (2010) Chemical control of insect pests and insecticide resistance in oilseed rape. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, Heidelberg, pp 313–335
Tiilikainen TM, Hokkanen HMT (2008) Pyrethroid resistance in Finnish pollen beetle (Meligethes aeneus) populations—is it around the corner? EPPO Bull 38:99–103
Tölle ML (2014) Factors regulating the population dynamics and damage potential of pollen beetle (Meligethes aeneus F.) on crops of oilseed rape. PhD thesis, University of Göttingen
Toth SJ, Sparks TC (1990) Effect of temperature on toxicity and knockdown activity of cis-Permethrin, esfenvalerate, and l-cyhalothrin in the cabbage looper (Lepidoptera: Noctuidae). J Econ Entomol 83(2):342–346
Vaitelyte B, Petraitiene E, Smatas R, Brazauskiene I (2011) Control of Meligethes aeneus, Ceutorhynchus assimilis and Dasineura brassicae in winter oilseed rape (Brassica napus L.). Zemdirbyste-Agric 98:175–182
Venables WN, Ripley BD (2002) Modern applied statistics with S (Venables WN, Ripley BD (eds)), Springer, New York
Wegorek P (2005) Preliminary data on resistance appearance of Pollen beetle PB (Meligethes aeneus F.) to selected pyrethroids, organophosphorous and chloronicotynyls insecticide, in 2004 year in Poland. Resistant Pest Manag Newsl 14(2):19–21
Wegorek P, Mrówczynski M, Zamojska J (2009) Resistance of pollen beetle (Meligethes aeneus F.) to selected active substances of insecticides in Poland. J Plant Prot Res 49:119–128
Wehling A, Heimbach U (1991) Untersuchungen zur Wirkung von Pflanzenschutzmitteln auf Spinnen (Araneae) am Beispiel einiger Insektizide. Nachrichtenblatt des deutschen Pflanzenschutzdienstes 43:24–30
Williams IH (2010) The major insect pests of oilseed rape in Europe and their management: an overview. In: Williams IH (ed) Biocontrol-based integrated management of oilseed rape pests. Springer, Heidelberg, pp 1–43
Williams IH, Free JB (1978) The feeding and mating behavior of pollen beetles (Meligethes aeneus Fab.) and seed weevils (Ceutorhynchus assimilis Payk.) on oil-seed rape (Brassica napus L.). J Agric Sci 91:453–459
Zaller JG, Moser D, Drapela T, Frank T (2009) Ground-dwelling predators can affect within-field pest insect emergence in winter oilseed rape fields. Biocontrol 54:247–253
Zimmer CT, Nauen R (2011a) Pyrethroid resistance and thiacloprid baseline susceptibility of European populations of Meligethes aeneus (Coleoptera: Nitidulidae) collected in winter oilseed rape. Pest Manag Sci 67:599–608
Zimmer CT, Nauen R (2011b) Cytochrome P450 mediated pyrethroid resistance in European populations of Meligethes aeneus (Coleoptera: Nitidulidae). Pestic Biochem Physiol 100:264–272
Zimmer CT, Köhler H, Nauen R (2014) Baseline susceptibility and insecticide resistance monitoring in European populations of Meligethes aeneus and Ceutorhynchus assimilis collected in winter oilseed rape. Entomol Exp Appl 150:1–10
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This study was supported by the Union zur Förderung von Oel-und Proteinpflanzen e.V. Special thanks to the staff of the plant protection services of the federal states Mecklenburg-Western Pomerania, Lower Saxony and Bavaria for conducting external field trials. Furthermore thanks to Dr. Doreen Gabriel and Dr. Anke Dietzsch for statistical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Heikki Hokkanen.
Rights and permissions
About this article
Cite this article
Brandes, M., Heimbach, U. & Ulber, B. Effects of thiacloprid, tau-fluvalinate and lambda-cyhalothrin on overwintered pollen beetles (Brassicogethes aeneus (Fabricius)) and their offspring in oilseed rape. Arthropod-Plant Interactions 12, 823–833 (2018). https://doi.org/10.1007/s11829-018-9621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-018-9621-1