Abstract
Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae) is a major soybean (Glycine max (L.) Merrill) pest and reduces grain quality and yield worldwide. In the context of integrated pest management strategies, plant resistance stands out as an extremely valuable tool for the management of pest populations. Here, we evaluated the resistance of several soybean entries to P. guildinii using tests of attractiveness and feeding preference. We also evaluated trichome number and length as well as pod hardness to evaluate the relationships between these parameters and the resistance to stink bug. D 75-10169, PI 171451, PI 229358, PI 227687, “IAC 100,” IAC 78-2318, PI 274454, PI 274453 and “IAC 19” were less attractive and less consumed by stink bugs. D 75-10169, PI 227687 and PI 274454 received low probe numbers and a short consumption duration per probe; “IAC 100” and PI 274453 received low probe numbers; PI 171451 and PI 229358 received short probe durations; and “IAC 19” received the highest number of probes. There was no correlation between trichome density and length with the attractiveness and feeding preference of the adult insects; however, pod hardness results suggested that this morphological factor may influence the number of probes performed by the insect. PI’s entries, D 75-10169, “IAC 100” and “IAC 19” expressed antixenosis resistance and should be appropriate for use in soybean breeding programs aimed at developing entries with higher resistance to pest insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean, Glycine max (L.) Merrill (Fabales: Fabaceae), is one of the world’s primary agricultural crops, with the USA being the largest producer, followed by Brazil and Argentina (Castanheira and Freire 2013). Among the main agricultural pests, bugs from the Pentatomidae family (Chocorosqui and Panizzi 2004; Leskey and Hogmire 2005) are particularly significant. One of the predominant species of this family is the neotropical redbanded stink bug, Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae), which can be found on many crops, including beans (Phaseolus vulgaris (L.)), peas (Pisum sativum (L.)), alfalfa (Medicago sativa (L.)), cotton (Gossypium hirsutum (L.)), coffee (Coffea spp. (L.)), guava (Psidium guajava (L.)) and sunflower (Helianthus annuus (L.)) (Panizzi and Slansky 1985).
In the USA, the incidence of P. guildinii has increased in recent years and this species has become the most common stink bug in areas with significant soybean production, such as Louisiana (Baur and Baldwin, 2006; Smith et al. 2009; Tindall and Fothergill, 2011). This species of Pentatomidae predominates in many locations where soybean is cultivated in Brazil (Oliveira and Panizzi 2003). In Argentina, P. guildinii and Nezara viridula (Linnaeus) (Hemiptera: Pentatomidae) are the main economically significant pests and cause great economic impacts on soybean production (Massoni and Frana 2005).
Both the nymphal stages and adults of these insects can damage soybeans by inserting their stylets into the seeds to extract nutrients, which can induce abortion or decrease seed yield and quality, as well as favor the growth of certain fungi, such as Nematospora coryli (Peglion), which can cause yeast strain disease (Panizzi et al. 2000; McPherson and McPherson, 2000). The presence of these insects has also been associated with a delay in the maturation of plants of some entries, as a result of the injection of toxins (Vicentini and Jimenez 1977) and, with the increased incidence of other pests and diseases, such as the beetle Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae) and the pathogen Fusarium spp. (Todd and Womack 1973). In particular, P. guildinii stands out as the stink bug species that causes the most significant qualitative and quantitative losses for soybean crops (Corrêa-Ferreira and Azevedo 2002) and has the greatest impact on leaf retention in plants (Sosa-Gómez and Moscardi 1995).
Methods for controlling the redbanded stink bug on soybeans are hampered by the low susceptibility of this species to the active ingredients in the most widely available insecticides, requiring a greater number of applications of broad-spectrum insecticides relative to other stink bugs (Temple et al. 2009; Temple 2011). In addition, the overuse of insecticides can negatively affect the environment, eliminate natural enemies of this pest, and select for insect populations resistant to the active ingredients in these insecticides (Byrne et al. 2003; Lin et al. 2009).
Plant resistance is considered to be an essential strategy for integrated pest management practices. Effective plant resistance is longer lasting and more efficient and less expensive than chemical control methods, and it reduces the risk of pests developing resistance to the active ingredients in pesticides. Host-plant resistance mechanisms are divided into three categories: antixenosis, antibiosis and tolerance (Smith 2005).
Antixenosis mechanisms are characterized by the presence of morphological, physical or chemical plants traits, that affect the feeding, digestion, matinge and/or oviposition behaviors of insects. With respect to these factors, the presence of chemical compounds which act as repellents or feeding deterrents, can determine the expression of antixenosis effect. Morphological characteristics, such as pubescence and tissue hardness, have also been described as important factors for the defense of plants against herbivores (Panda and Khush 1995).
With respect to soybean crops, some cultivars and introductions (PI’s) have already been identified as resistant to insects expressing the three mechanisms, which in some cases has been associated or not. However, there are few studies involving the species P. guildinii, aiming to characterize the mechanisms of plant resistance against this insect. The objective of this study was to evaluate soybean entries against the redbanded stink bug under laboratory conditions, in order to check the expression of antixenosis, through testing of attractiveness and feeding preference. Regression analyses were also performed to correlate attractiveness and feeding preference with trichome density and length, as well as with pod hardness.
Materials and methods
Soybean entries
We evaluated 17 soybean entries (Table 1). The inbred lines PI 171451, PI 229358, PI 227687, PI 274453 and PI 274454 (PI) were chosen because they showed multiple characteristics of resistance to insects (Kogan 1989; Lourenção et al. 1989). D 75-10169, IAC 78-2318 and IAC 74-2832 are derived from crosses between PI 229358 and PI 274454 (Miranda et al. 1979; Lourenção and Miranda 1987).“IAC 17,” “IAC 19” and “IAC 24” expressed antixenosis and antibiosis characteristics against N. viridula (Lourenção et al. 2000; Miranda et al. 2003; Piubelli et al. Piubelli et al. 2003a), lepidopterans and whitefly (Valle and Lourenção 2002; Vieira et al. 2011; Silva et al. 2012; Valle et al. 2012). “IAC 100” expressed resistance to several pest insects, including N. viridula (McPherson et al. 2007). L1-1-01 is derived from “IAC-100”. “Conquista” and “Coodetec 208” cultivars are widely planted throughout Brazil.
Testing was conducted using pods from plants sown in 5-L pots with sterilized soil and fertilizer (Raij et al. 1997). Each vessel held two soybean plants and was maintained in a greenhouse, free from insect infestation.
Piezodorus guildinii stock rearing
P. guildinii individuals were maintained under controlled conditions (14:10 h L:D at 26 ± 2 °C and 65 ± 10 % RH), according to the methods of Corrêa-Ferreira (1985) and Borges et al. (2006), with some modifications. Initially, egg masses, nymphs and adults were collected from cultivated soybean entries different from those used in this study.
Adults were maintained in plastic cages (8 L; 22 cm Ø and 20 cm in height), covered with organdy to allow adequate ventilation. The bottom surfaces of the cages were lined with filter paper to absorb excrement and maintain sanitary conditions. Each cage contained 25 insect couples, which were maintained on a natural diet of green snap-bean pods (P. vulgaris (L.)) (5 pods/cage) and raw peanuts (Arachis hypogaea (L.)), in portions of 50 g/cage deposited in Petri dishes. Food was replaced and the cages were cleaned every 4 days to avoid fungal contamination. Pieces of cotton moistened with distilled water were placed in Petri dishes to meet the suitable hydration needs of the bugs and to maintain the moisture levels within the cages. To serve as oviposition sites and shelter for the insects, disks of dry cotton were placed equidistantly along the bottom surface of the cage, as well as suspended along the top edge of the cage using hooks fashioned from number-2 clips. To ensure that the eggs were not consumed by the adults (Panizzi 1991), eggs were collected daily and placed in Petri dishes (8.5 cm Ø) lined with moistened filter paper and containing a bean pod to serve as a food source for the insects in the first nymphal stage. When the insects reached the second stage of development, they were transferred to rearing cages prepared as described previously.
Attractiveness and feeding preference
The tests of attractiveness and feeding preference were conducted under laboratory conditions (described above), using pods from different entries, and the results were analyzed based on phenological group (early, semi-early and late). The pods were collected from greenhouse grown plants at phenological stage R6, as described by Fehr and Caviness (1977), because it has been demonstrated that adult and nymph stink bugs raised on soybean pods from this stage of development show better performance (Oliveira and Panizzi 2003).
The tests were performed in round aluminum arenas (60 cm Ø × 10 cm height) covered with glass plates and lined at the base with filter paper. Two pods of each entry were placed into each arena in a random but equidistant formation. Next, two adult couples per entry, of up to 48 h of age, were released into the center of each arena; the insects were fasted for 24 h prior to the beginning of the tests.
Insect counts were performed for each entry at 15, 30, 45, 60, 120 and 180 min after release. Meanwhile, pod consumption by the insects was observed for 120 min by assessing the number of probes made by the insects as well as the duration of each probe. The number of probes was determined by visual inspection, as described by Rossetto et al. (1981). The duration of each probe was measured using a digital timer; the timer was started when the insect inserted its stylet into a pod and it was stopped when the stink bug withdrew its stylet from the pod.
These tests were carried out using a randomized complete block experimental design with eight repetitions for each maturation group of soybean studied. Video recordings of the experiments were also performed in case the data needed to be reassessed. At the end of each repetition, the insects were discarded.
Density and length of trichomes and pod hardness
To correlate the morphological characteristics of the pods of soybean entries with the feeding preferences of P. guildinii, we assessed the trichome number, trichome length and hardness of R6 pods.
To measure trichome number, we used a stereomicroscope, Nikon SMZ-685 (Nikon, Melville, New York, USA), with graph paper as a length reference. We examined the middle of each pod (on the second grain) and counted the total number of trichomes present in a 25 mm2 area, as described by Paron and Lara (2005). Trichome length was determined using a video-camera system, Opton TA-0124XS (Carl Zeiss, Jena, Oberkochen, Germany) installed on an optical microscope. The images were transferred to a computer and evaluated using the EDN-2 software program (Fig. 1). The equipment was calibrated using a transmitted blade (Carl Zeiss).
An analysis of pod hardness was performed using a CT3 Texture Analyzer (Brookfield; Middleboro, Massachusetts, USA), calibrated for a penetration depth of 5 mm at a speed of 2.0 mm s−1 with a TA 9/1000 point. The measurement results are expressed in grams-force per centimeter (gf/cm) and represent the maximum force required for the point to enter the pulp of the pod, simulating the process by which insects insert their mouthparts into a pod. The evaluations were standardized using pods from plants at phenological stage R6, and resistance was always tested using the second grain of the pod.
To quantify the trichome number and length, 8 and 20 pods were tested from each entry, respectively. To quantify pod hardness, 10 pods were evaluated for each entry. These trials were conducted using a completely randomized design, with each pod representing a repetition.
Statistical analysis
The data obtained from tests were submitted to analysis of variance, with normality determined using the Shapiro–Wilk test and homoscedasticity determined using Levene’s test (Winer et al. 1991).When significant effects were found for the treatments, Fisher’s LSD tests were used (P ≤ 0.05) to compare the means performed using the statistical software program PROC MIXED-SAS 9.2 (SAS Software 2001). Additionally, for the final attractiveness test, we calculated an attractiveness index (AI), which was defined as follows: AI = (2 × G)/(G + P), where G was equal to the number of insects attracted to the treatment and P was equal to the number of insects attracted to the standard cultivar (commercial material was more susceptible within each phenological group). The index varied from 2 (attractive) to 0 (repellent), with a value of 1 indicating neutrality (Lin et al. 1990).
Correlation analyses were performed for the following parameters: trichome number with attractiveness, probe number and time per probe; trichome length with attractiveness, probe number and time per probe; and pod hardness and probe number.
Results
Atractiveness
No differences in attractiveness were observed between the soybean entries (early, semi-early or late) for the different assessment periods (15, 30, 45, 60, 120 and 180 min). However, considering the averages from all of the evaluations (Table 2), some entries were less attractive than others to the redbanded stink bug.
Among the early group, D 75-10169, PI 171451 and PI 229358 attracted fewer adults of P. guildinii, differing from “Coodetec 208” (F = 2.50; df = 5, 42; P = 0.0453). Based on the attractiveness index (Table 2), D 75-10169, PI 229358 and PI 171451 were classified as repellent, relative to the commercial standard “Coodetec 208”. “IAC 17” and “IAC 23” were classified as neutral, with attractiveness rates similar to that of the standard.
For the semi-early group, PI 227687, “IAC 100” and IAC 78-2318, were less attractive to insects than the standard (F = 6.32; df = 5, 42; P = 0.0002), with the former two entries being classified as repellent, relative to the commercial standard “IAC 24”. “IAC 18” was found to be most attractive to the insects.
Among the late maturity group, PI 274454, PI 274453 and “IAC 19” were found to be the least attractive to P. guildinii (F = 4.05; df = 4, 35; P = 0.0084). These entries were classified as repellent, whereas L1-1-01 was classified as neutral, concerning to the standard “Conquista,” which was the most attractive to the insects (Table 3).
Probe number and time per probe
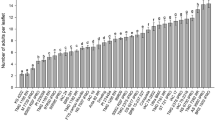
In the early group, “IAC 17” and PI 171451 had the highest number of probes, differing (F = 3.74; df = 5, 42; P = 0.0095) from D 75-10169 and “Coodetec 208” (Fig. 2). However, for the time per probe, “Coodetec 208” differed from the other entries, with a moderately high value. D 75-10169, PI 229358 and PI 171451 exhibited the lowest mean values for this parameter (F = 47.06; df = 5, 42; P < 0.001) (Fig. 3).
For the semi-early cycle, “IAC 18” and “IAC 24” had the highest average number of probes (F = 5.80; df = 5, 42; P = 0.0007), and these values were significantly different from those of PI 227687 and “IAC 100” (Fig. 2). For the time per probe, PI 227687 differed from the other entries, with the exception of IAC 74-2832, which showed the lowest average value (F = 2.90; df = 5, 42; P = 0.0347) (Fig. 3).
Among the entries of late maturity, “IAC 19”, “Conquista” and L1-1-01 were the most probed (F = 6.84; df = 4, 35; P = 0.0007), significantly different from PI 274454 and PI 274453 (Fig. 2). Time per probe was low for PI 274454, which differed from the other entries, with the exception of “IAC 19” (F = 3.45; df = 4, 35; P = 0.0239) (Fig. 3).
Density and length of trichomes and pod hardness
“IAC 17” and “Coodetec 208” stood out as having the highest average trichome densities (F = 6.93; df = 5, 54; P < 5.98) in the early group, differing from PI 171451, PI 229358 and “IAC 23”. There was no difference in trichome length in this group (F = 1.81; df = 5, 114; P = 0.1156). “Coodetec 208” presented the highest average pod hardness, differing from all the other entries (F = 30.07; df = 5, 42; P < 5.98).
In relation to the entries of the semi-early group, “IAC 100” and IAC 78-2318 had the highest numbers of trichomes (F = 37.88; df = 5, 54; P < 5.98), significantly different from the other entries. No difference was observed between the entries in terms of trichome length (F = 0.60; df = 5, 114; P = 0.6990). With respect to pod hardness, PI 227687 held the highest average value (F = 23.53, df = 5, 42; P < 5.98), whereas “IAC 18” and “IAC 24” were less hard.
Among the late maturity group, L1-1-01 and PI 274453 showed higher trichome densities (F = 11.21; df = 4, 35; P < 5.98) relative to PI 227454, “Conquista” and “IAC 19”. No difference was observed in trichome length among the entries (F = 2.41; df = 4, 95; P = 0.0546). “Conquista” presented the lowest pod hardness relative to the other entries (F = 11.21; df = 4, 35; P < 0.0001).
Correlation analysis
Based on the calculated coefficients (r), the correlations were not highly significant among the evaluated parameters (Table 4). However, negative correlations were found for all groups with respect to probe number and pod hardness and were stronger for the early and semi-early groups.
Discussion
Attractiveness
The causes of antixenosis for resistant plants subject to insect infestation can be derived from morphological factors, such as trichomes and tissue hardness, nutritional deficiency, chemical compounds, or a combination of any of these factors (Panda and Khush 1995). With respect to the chemical compounds produced by soybeans, some have been identified as repellents and can prevent or reduce contact between insects and plant surfaces (Smith 2005). In particular, saponins and protease inhibitors are some of the substances present in soybeans that can inhibit insect attack (Sirisingh and Kogan 1982).
The lowest preference for pods of entries D 75-10169, PI 229358 and PI 171451 (early); PI 227687 and “IAC 100” (semi-early); and PI 274454, PI 274453 and “IAC 19” (late), suggests the volatilization of compounds that repel or inhibit feeding by P. guildinii.
It has previously been shown that the lines PI 229358, PI 227687 and PI 274454 expressed antixnosis type resistance against Anticarsia gemmatalis (Hübner) (Lepidoptera: Noctuidae), which preferentially rejected these lineages in choice tests (Hoffmann-Campo et al. 1994). PI 227687 also provoked repellency to Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) caterpillars and adults of Epilachna varivestis (Mulsant) (Coleoptera: Coccinellidae), being verified the presence of volatile derivatives of their leaves (Liu et al. Liu and Norris 1989).
The entry D 75-10169 was also less attractive to adults of B. tabaci biotype B (Valle et al. 2012) and its expression of resistance may be related to the way in which this line was developed, which included some entries, such as PI 229358, with characteristics of resistance to certain insects. Similarly, “IAC 19” was developed by IAC from crosses involving D 72-9601, which showed resistance to caterpillars and also derived from PI 229358, which showed resistance to other phytophagous arthropods (Valle and Lourenção, 2002). Considering the results obtained from analyzing the offspring, these entries may contain a large part of genes which confer the characteristic of antixenosis of PI 229358.
Similarly, the resistance displayed by “IAC 100” may be related to its parental lines, PI 229358 and PI 274454, in addition to its derivation from crosses between “IAC 12” and IAC 78-2318, which may also have been a source of multiple resistance to insects (Lourenção et al. 1987; Hoffmann-Campo et al. 1994; Rossetto et al. 1995). This cultivar likely inherited some of the genes conferring the antixenosis type resistance in these materials. Recent studies have demonstrated that many entries with “IAC 100” in their genealogy, express resistance to N. viridula (McPherson et al. 2007) and to various lepidopteran soybean defoliators (McPherson and Buss 2007). This demonstrates that this entry can be a good parent that is able to generate novel entries with a promising combination of characteristics of tolerance to insects and productivity (Pinheiro et al. 2005; Maia et al. 2009).
Probe number and time per probe
Based on the attractiveness results, we expected that certain entries would be less attractive to the insects than others, possibly due to differences in the stimuli that initiate feeding, resulting in shorter probe durations and variation in the number of probes.
Within their respective groups, D 75-10169 (early), PI 227687 (semi-early) and PI 274454 (late), which displayed repellent effects against P. guildinii, showed low probe numbers and short probe durations. These findings suggest that in addition to the release of volatile repellents, these entries may also present fewer stimuli for feeding initiation (relating to the low number of probes) and may contain compounds that are unpalatable to P. guildinii adults, or suppressants (which inhibit the initiation of feeding) and deterrents (which inhibit the maintenance of feeding) (Smith 2005).
Studies involving PI 227687, N. viridula and Chrysodeixis includens (Walker) (Lepidoptera: Noctuidae), demonstrated that the expression of resistance in this lineage was related to chemical factors, that cause feeding deterrence and affect the development of these insects (Smith 1985; Piubelli et al. 2003b). Recently, studies involving A. gemmatalis identified the detrimental effect of a flavonoid compound, called rutin (quercetin 3-O-rutinoside), which is present in the leaves of PI 227687 and had detrimental effects against caterpillars, causing deterrence and adverse metabolic effects (Piubelli et al. 2005; Hoffmann-Campo et al. 2006).
Rutin is also found in “IAC 100” and PI 274454 (Piubelli et al. 2005). Furthermore, PI 274454 also contains high concentrations of genistin (phytoestrogen from 7-O-glucoside), which is another flavonoid that has been linked to the resistance against pest insects by soybean plants (Piubelli et al. 2005; Hoffmann-Campo et al. 2006).
The entries “IAC 100” and PI 274453 showed significant values of time per probe, however, both had low probe numbers and expressed repellency toward P. guildinii adults within their respective groups. In a similar study, “IAC 100” also received less number of probes of N. viridula in their pods (Campos et al. 2010). These findings indicate that these entries may release repellent and/or supressant volatiles to insects.
In contrast, “IAC 18,” “IAC 24,” L1-1-01 and “Conquista” received many probes, with high time per probe, suggesting that they are susceptible to P. guildinii. “Coodetec 208” showed long probe times with relatively few probes, suggesting that this entry contains compounds that stimulate feeding, also characterizing as susceptible to the redbanded stink bug.
It should be emphasized that high probe numbers can also indicate the presence of antixenotic effects, because deterrent compounds could disrupt feeding by the stink bugs, prompting the need for more probes to begin feeding anew. In this respect, PI 171451 and PI 229358, which were relatively unattractive to the stink bugs and showed low time per probe and intermediate probe numbers, might also be considered resistant to P. guildinii adults. “IAC 19,” was also characterized as resistant, showing an intermediate time per probe, a high average number of probes and repellency to the insect.
Density and length of trichomes and pod hardness
In addition to the biochemical factors cited above, attractiveness parameters (e.g., probe number and time per probe) can also be influenced by morphological factors, such as pilosity and tissue hardness, which can interfere with the insect’s mobility and act as structural barrier for feeding, respectively (Webster 1975). These factors, in association with the chemical compounds discussed above, are characteristic of antixenosis type resistance for feeding (Smith 2005).
Mature soybean pods have more trichomes than immature pods, and this can hinder insect mobility while simultaneously encouraging stink bugs to move. Therefore, insects on mature pods spend more time seeking suitable locations for probes, which reduces the amount of time available for feeding and consequently, reduces probe number and time per probe (Molina and Trumper 2012).
Considering the above facts, there was the assumption that the entries that were less attractive to P. guildinii and that showed fewer probes and shorter time per probe might have greater numbers of trichomes. However, among the early group, the PI’s, less attractive to insects also showed low trichome densities. In addition, D 75-10169, which was also resistant (Table 2), showed an intermediate trichome density. Indeed, we found a positive correlation between the presence of these structures and attractiveness, suggesting that as trichome number is decreased, infestation by stink bug adults also decreases.
In contrast, “IAC 100” (semi-early), which was not very attractive, showed intermediate consumption with a high number of trichomes, suggesting a possible negative relationship between trichome density and dietary preference. Similarly, “IAC 18” (semi-early) and “Conquista” (late), which showed high attractiveness and a high time per probe of P. guildinii, had low trichome densities.
Other inconsistencies were verified with PI 227453 (many trichomes) and PI 227454 (few trichomes), which were not attractive to P. guildinii and received relatively few probes by this insect.
The fact that we found no strong or significant correlations (Table 4) between attractiveness and feeding preference parameters with trichome density and length in the studied strains suggests the presence of other factors that are more important in determining feeding preferences in adult redbanded stink bugs.
However, it should be emphasized that preference tests on the pods were only performed with adult insects in this study. Therefore, one cannot rule out the influence of the above factors on stink bug populations during the initial stages of development. Based on the literature (Lourenção et al. 1997; Oliveira and Panizzi 2003), the presence of dense trichomes can negatively affect the behavior of stink bug nymphs, affecting feeding and leading to death by starvation.
In addition to trichomes, other morphological characteristics can affect feeding in stink bugs, including the size of the air space between the pod wall and the seeds (Panizzi and Silva 2009), as well as the nature of the seed coat, which can contain high levels of lignins to protect the seeds from herbivore attack (Boesewinkel and Bouman 1995). For example, research conducted on Aphis glycines Matsumura (Hemiptera: Aphididae) demonstrated that high lignin concentrations in soybean leaves are a defense mechanism used by these plants (Hu et al. 1993).
In this context, we evaluated pod hardness and it was noted that although there was not a highly significant correlation, this factor was associated with the feeding variation among the entries, given that in the three groups of these soybean entries, the correlation between the probe number and pod hardness was negative.
Studies conducted with P. guildinii and N. viridula revealed that probe number in both species was decreased when insects fed on soybeans from more mature phenological stages (Molina and Trumper 2012). Mature soybean pods have thicker cell walls with a greater proportion of lignin, creating a stronger physical barrier to prevent damage from herbivores (Capeleti et al. 2005; Saes Zobiole et al. 2010; Molina and Trumper 2012).
“Coodetec 208,” PI 227687, PI 274453 and PI 274454, which showed high hardness levels in their respective groups, were also the entries with the lowest numbers of probes, suggesting a decrease in the probe number as a function of the increase in hardness of the integument of the pod. PI 171451, PI 229358, “IAC 18,” “IAC 24” and “Conquista,” which were among the most highly probed entries, showed relatively low hardness values.
In contrast, “IAC 17,” “IAC 19” and L1-1-01 showed relatively high probe numbers but also showed high or intermediate hardness relative to their groups. Conversely, D 75-10169 and “IAC 100,” received few probes but showed intermediate hardness. This suggests that the primary determinant of resistance or susceptibility in these soybean entries may be linked not to the morphological factor of pod hardness but to one of the other factors discussed above.
Therefore, the feeding preferences of P. guildinii are likely associated with a complex combination of morphological factors, such as pod hardness, as well as with biochemical factors, together or independently, these factors affect insect behavior through repellence and feeding inhibition. In addition, other mechanisms used by soybean plants for protection against stink bug attack have also been reported, including a short period of pod-filling, a higher seed yield per plant, the capacity to reject damaged immature pods and replace them with new ones, the senescence of normal leaves when the plant reaches ripeness, and resistance to the yeast N. coryli (Rossetto et al. 1995).
In this study, we observed significant levels of antixenosis type resistance in the PI’s entries, D 75-10169, “IAC 100” and “IAC 19”. These results may be useful for future soybean breeding programs aimed at developing entries that are resistant to this species of stink bug. In addition, “IAC 100” and “IAC 19,” are commercially available germplasms that can be sown in regions with a history of P. guildinii infestation, or in regions where the influence of this stink bug is increasing. Therefore, the use of these cultivars, in association with other pest control techniques, may allow efficient management of the redbanded stink bug and reduce the population levels of this insect.
References
Baur ME, Baldwin J (2006) Red banded stink bug trouble in Louisiana. Lousiana Agric 49:9–10
Boesewinkel FD, Bouman F (1995) The seed: structure and function. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp 1–24
Borges M, Birkett M, Aldrich JR, Oliver JE, Chiba M, Murata Y, Laumann RA, Barrigossi JA, Pickett JA, Moraes MCB (2006) Sex attractant pheromone from the rice stalk stink bug, Tibraca limbativentris Stal. J Chem Ecol 32:2749–2761
Byrne FJ, Castle S, Prabhaker N, Toscano NC (2003) Biochemical study of resistence to imidacloprid in B biotype Bemisia tabaci from Guetemala. Pest Manag Sci 59:347–352
Campos M, Knutson A, Heitholt J, Campos C (2010) Resistance to seed feeding by southern green stink bug, Nezara viridula (Linnaeus), in soybean, Glycine max (L.) Merrill. Southwest Entomol 35:233–239
Capeleti I, Ferrarese MLL, Krzyzanowski FC, Ferrarese Filho O (2005) A new procedure for quantification of lignin in soybean (Glycine max (L.) Merril) seed coat and their relationship with the resistance to mechanical damage. Seed Sci Technol 33:511–515
Castanheira EG, Freire F (2013) Greenhouse gas assessment of soybean production: implications of land use change and different cultivation systems. J Clean Prod 54:49–60
Chocorosqui VR, Panizzi AR (2004) Impact of cultivation systems on Dichelops melacanthus (Dallas) (Heteroptera: Pentatomidae) population and damage and its chemical control on wheat. Neotrop Entomol 33:487–492
Corrêa-Ferreira BS (1985) Criação massal do percevejo verde, Nezara viridula (L.). Embrapa-CNPSo, Londrina
Corrêa-Ferreira BS, Azevedo J (2002) Soybean seed damage by different species of stink bugs. Agric For Entomol 4:145–150
Fehr WR, Caviness CE (1977) Stages of soybean development. Iowa State University, Ames
Hoffmann-Campo CB, Mazzarin RM, Lustosa PR (1994) Mecanismos de resistência de genótipos de soja: teste de não -preferência para Anticarsia gemmatalis Hübner, 1818. (Lep.: Noctuidae). Pesqui Agropecu Brasileira 29:513–519
Hoffmann-Campo CB, Neto JAR, Oliveira MCN, Oliveira LJ (2006) Detrimental effect of rutin on Anticarsia gemmatalis. Pesqui Agropecu Brasileira 41:1453–1459
Hu Q, Zhao JW, Cui DW (1993) Relationship between content of secondary catabolite lignin in soybean and soybean resistance to the soybean aphid. Plant Prot 19:8–9
Kogan M (1989) Plant resistance in soybean insect control. In: Pascale AJ (ed) World research conference IV. Orientácion Gráfica Editora, Buenos Aires, pp 1519–1525
Leskey TC, Hogmire HW (2005) Monitoring stink bugs (Hemiptera: Pentatomidae) in midatlantic apple and peach orchards. J Econ Entomol 98:143–153
Lin H, Kogan M, Fischer D (1990) Induced resistance in soybean to the mexican bean beetle (Coleoptera: Coccinelidae): comparisons of inducing factors. Environ Entomol 19:1852–1857
Lin CY, Wu DC, Yu JZ, Chen BH, Wang CL, Ko WH (2009) Control of silverleaf whitefly, cotton aphid and kanzawa spider mite with oil and extracts from seeds of sugar apple. Neotrop Entomol 38:531–536
Liu SH, Norris DM (1989) Lyne P (1989) Volatiles from the foliage of soybean, Glycine max, and lima bean, Phaseolus lunatus: their behavioral effects on the insects Trichoplusia ni and Epilachna varivestis. J Agric Food Chem 37:496–501
Lourenção AL, Costa AS, Miranda MAC. Sources of resistance to insect pests and virus vectors in the soybean germplasm tested at Instituto Agronomico, SP, Brasil. In: World Soybean Research Conference, 4 (1989) Buenos Aires. Anais… Gráfica SRL, Buenos Aires, pp 1578–1581
Lourenção AL, Miranda MAC (1987) Resistência de soja a insetos: VIII. IAC 78-2318, linhagem com resistência múltipla. Bragantia 46:65–72
Lourenção AL, Miranda MAC, Nagai V (1987) Resistência de soja a insetos: VII. Avaliação de danos de percevejos em cultivares e linhagens. Bragantia 46:45–57
Lourenção AL, Miranda MAC, Pereira JCVNA, Ambrosano GMB (1997) Resistência de soja a insetos X. Comportamento de cultivares e linhagens em relação a percevejos e desfolhadores. An Soc Entomol Brasil 26:543–550
Lourenção AL, Pereira JCVNA, Miranda MAC, Ambrosano GM (2000) Avaliação de danos causados por percevejos e por lagartas em genótipos de soja de ciclos precoce e semiprecoce. Pesq Agropec Brasileira 35:879–886
Maia MCC, Vello NA, Rocha MDM, Fonseca Júnior NS, Lavorante OJ, Pinheiro JB, Dias CTS, Assis GML (2009) Seleção de linhagens experimentais de soja para características agronômicas e tolerância a insetos. Bragantia 68:85–97
Massoni F, Frana J (2005) Si no es en soja¿las chinches donde están? Información técnica de cultivos de verano, Publicación miscelánea 104, Campaña, pp 100–102
McPherson RM, Buss GR (2007) Evaluating lepidopteran defoliation resistance in soybean breeding lines containing the stink bug (Hemiptera: Pentatomidae) resistance IAC-100 cultivar in their pedigrees. J Econ Entomol 100:962–968
McPherson JE, McPherson RM (2000) Stink bugs of economic importance in America North of Mexico. CRC Press, Boca Raton
McPherson RM, Buss GR, Roberts PM (2007) Assessing stink bug resistance in soybean breeding lines containing genes from germplasm IAC-100. J Econ Entomol 100:1456–1463
Miranda MAC, Rossetto CJ, Rossetto D, Braga NR, Mascarenhas HAA, Teixeira JPF, Massariol A (1979) Resistência de soja a Nezara viridula e Piezodorus guildinii em condições de campo. Bragantia 38:181–188
Miranda MAC, Braga NR, Lourenção AL, Miranda FTS, Uneda SH, Ito MF (2003) Descrição, produtividade e estabilidade da cultivar de soja IAC-24, resistente a insetos. Bragantia 62:29–37
Molina GAR, Trumper EV (2012) Selection of soybean pods by the stink bugs, Nezara viridula and Piezodorus guildinii. J Insect Sci 12:1–16
Oliveira EDM, Panizzi AR (2003) Performance of nymphs and adults of Piezodorus guildinii (Westwood) (Heteroptera: Pentatomidae) on soybean pods at different development stages. Brazilian Arch Biol Technol 46:187–192
Panda N, Khush GS (1995) Host plant resistance to insects. CAB International, Wallingford
Panizzi AR (1991) Ecologia nutricional de insetos sugadores de sementes. In: Panizzi AR, Parra JRP (eds) Ecologia nutricional de insetos e suas implicações no manejo de pragas. Manole, Brasília, pp 253–287
Panizzi AR, Silva FAC (2009) Insetos sugadores de sementes (Heteroptera). In: Panizzi AR, Parra JRP (eds) Bioecologia e nutrição de insetos. Embrapa, Brasília, pp 465–522
Panizzi AR, Slansky F Jr (1985) Review of phytophagous pentatomids (Hemiptera: Pentatomidae) associated with soybean in the Americas. Florida Entomol 68:184–203
Panizzi AR, McPherson JE, James DG, Javahery M, McPherson RM (2000) Stink bugs (Pentatomidae). In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. CRC Press LLC, Boca Raton, pp 421–474
Paron MJFO, Lara FM (2005) Relação entre tricomas foliares de genótipos de feijoeiro comum, Phaseolus vulgaris L. e resistência a Diabrotica speciosa Germar, 1824 (Coleoptera: Chrysomelidae). Ciênc Agrotec 29:894–898
Pinheiro JB, Vello NA, Rossetti CJ, Zucchi MI (2005) Potential of soybean genotypes as insect resistance sources. Crop Breed Appl Biot 5:294–301
Piubelli GC, Hoffmann-Campo CB, Arruda IC, Lara FM (2003a) Nymphal development, lipid content, growth and weight gain of Nezara viridula (L.) (Heteroptera: Pentatomidae) fed on soybean genotypes. Neotrop Entomol 32:127–132
Piubelli GC, Hoffmann-Campo CB, Arruda IC, Franchini JC, Lara FM (2003b) Flavonoid increase in soybean as a response to Nezara viridula injury and its effecton insect-feeding preference. J Chem Ecol 29:1221–1233
Piubelli GC, Hoffmann-Campo CB, Moscardi F, Miyakubo SH, de Oliveira MC (2005) Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? J Chem Ecol 31:1509–1524
Raij B, Cantarella H, Quaggio J (1997) Recomendações de adubação e calagem para o Estado de São Paulo. Fundação IAC, Campinas
Rossetto CJ, Lourenção AL, Igue T, Miranda MAC (1981) Picadas de alimentação de Nezara viridula em cultivares e linhagens de soja de diferentes graus de suscetibilidade. Bragantia 40:109–114
Rossetto CJ, Gallo PB, Razera LF, Bortoletto N, Igue T, Medina PF, Tisseli Filho O, Aquilera V, Veiga RFA, Pinheiro JB (1995) Mechanisms of resistance to stink bug complex in the soybean cultivar IAC-100. An Soc Entomol Brasil 24:517–522
Saes Zobiole LH, de Oliveira Jr RS, Kremer RJ, Constantin J, Bonato CM, Saraiva Muniz A (2010) Water use efficiency and photosynthesis of glyphosate-resistant soybean as affected by glyphosate. Pestic Biochem Phys 97:182–193
Sas Software (2001) SAS/STAT: user’s guide, version 8.1. SAS Institute, Cary
Silva JPGF, Baldin ELL, Souza ES, Lourenção AL (2012) Assessing Bemisia tabaci (genn.) biotype B resistance in soybean genotypes: antixenosis and antibiosis. Chil J Agric Res 72:516–522
Sirisingh S, Kogan M (1982) Insects affecting soybeans in storage. In: Sinclair JB, Jackobs JA (ed) Soybean seed quality and stand establishment. Proceedings of a Conference for Scientists of Asia. Urbana-Champaign: University of Illinois, College of Agriculture, pp 77–82
Smith CM (1985) Expression mechanisms and chemistry of resistance in soybean (Glycine max L. Merr.) to soybean looper, Pseudoplusia includens (Walker). Insect Sci Appl 6:243–248
Smith CM (2005) Plant resistance to arthropods molecular and conventional approaches. Springer, Dordrecht
Smith JF, Luttrell RG, Greene JK (2009) Seasonal abundance, species composition and population dynamics of stink bug in production fields of early and late soybeans in South Arkansas. J Econ Entomol 102:229–236
Sosa-Goméz DR, Moscardi F (1995) Retenção foliar diferencial em soja provocada por percevejos (Heteroptera: Pentatomidae). An Soc Entomol Brasil 24:401–404
Temple JH (2011) Redbanded stink bug, Piezodorus guildinii (Westwood): pest status, control strategies, and management in Louisiana soybean. Dissertation, Louisiana State University
Temple JH, Leonard BR, Davis JA, Fontenot K (2009) Insecticide efficacy against red banded stink bug, Piezodorus guildinii (Westwood), a new stink bug pest of Louisiana soybean. Midsouth Entomol 2:68–69
Tindall KV, Fothergill K (2011) First records of Piezodorus guildinii in Missouri. Southwest Entomol 36:203–205
Todd JW, Womack H (1973) Secondary infestations of cigarette beetle in soybean seed damaged by southern green stink bug. Environ Entomol 2:720
Valle GE, Lourenção AL (2002) Resistência de genótipos de soja a Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotrop Entomol 31:285–295
Valle GE, Lourenção AL, Pinheiro JB (2012) Adult attractiveness and oviposition preference of Bemisia tabaci biotype B in soybean genotypes with different trichome density. J Pest Sci 85:431–442
Vicentini R, Jimenez HA (1977) El vaneo de los frutos en soja. Instituto Nacional de Tecnología Agropecuaria, Paraná
Vieira SS, Bueno AF, Boff MIC, Bueno ECOF, Hoffman-Campo CB (2011) Resistance of soybean genotypes to Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae). Neotrop Entomol 40:117–122
Webster JA (1975) Association of plant hairs and insect resistance. An annotated bibliography. USDA-ARS Misc. Publ, 1927:1–18
Winer BJ, Brown DR, Michels KM (1991) Statistical principles in experimental design, 3rd edn. MacGraw-Hill, New York
Acknowledgments
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Amparo à Pesquisa do Estado de São Paulo for funding the present study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Smith.
Rights and permissions
About this article
Cite this article
Silva, J.P.G.F., Baldin, E.L.L., Canassa, V.F. et al. Assessing antixenosis of soybean entries against Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod-Plant Interactions 8, 349–359 (2014). https://doi.org/10.1007/s11829-014-9316-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-014-9316-1