Abstract
During seed germination trials of Hypericum hookerianum, seedlings of Lake View accession from Palni hills of Southern India segregated into green- (97.44 %) and red-pigmented (2.56 %) types. Seedlings cultured in Murashige and Skoog (1962) basal medium developed into fast growing green and slow growing red plant types in 6 weeks, the latter showing increased concentrations of total phenols, anthocyanins and flavonoids and 19-fold higher concentration of hypericin. Hypocotyls/cotyledons of red seedlings cultured using 2.325 μM kinetin (KIN) produced hypericin-rich (4.38 ± 0.18 mg/g DW), stunted (0.5–1.2 cm) shoots which ceased to grow in 8 weeks. Segments (4–6 mm) of these shoots sub-cultured in the dark for 4 weeks followed by 2-week light exposure and repeated subculture enabled mass multiplication of productive (3.93 ± 0.06 mg hypericin/g DW) shoots. Green hypocotyls and cotyledons subjected to 4 + 2 weak dark–light treatment also produced 9.18 ± 2.44 and 4.25 ± 0.96 comparable hypericin-rich (3.73 ± 0.21 mg/g DW) shoots. Red and green seedling explants cultured in basal medium in the dark produced 6.82 ± 0.75 cm etiolated shoots with reduced leaves which synthesized 2.27 ± 0.15 mg hypericin/g DW on illumination. Green cotyledons cultured in the dark using 2.45 μM indole-3 butyric acid (IBA) formed calluses which on illumination formed 12.64 ± 3.8 productive (3.86 ± 0.31 mg hypericin/g DW) 0.5- to 1.5-cm-long shoot clusters. Phenotypic segregation of seedlings, the ability of both red and green seedling explants to multiply in the dark and produce hypericin on illumination, and IBA-induced indirect shoots producing significant amounts of metabolite compared to wild plants (0.35 ± 0.09 mg/g DW) and green shoot cultures (0.91 ± 0.03 mg/g DW) are new to Hypericum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Hypericum consists of many ethnomedicinal plants used by local communities all over the world. Hypericum perforatum L. (St. John’s wort) of Europe is the most popular and commercially recognized ethnomedicinal species especially useful in the treatment of neurological disorders and mild to moderate depression. Among diverse classes of biologically active secondary metabolites produced by this species, naphthodianthrones (hypericin and pseudohypericin) and prenylated phloroglucinol derivatives (hyperforin, adhyperforin) are pharmaceutically important accounting for most of its medicinal properties (Savio et al. 2011). In plants grown in natural environments, the production and quality of these compounds may be affected by a host of factors including genotype, varying environmental conditions and biotic stresses (Filippini et al. 2010). Preparations from cultivated plants based on total hypericins vary from 7.72 to 38.5 % of the labeled claims and commercial samples in the form of capsules show 13- to 17-fold difference between highest and lowest levels of hypericin (Zobayed and Saxena 2004; Shaw et al. 2005). Southwell and Bourke (2001) reported up to 50-fold variation in the content of hypericin and pseudohypericin in the leaves of St. John’s wort in summer- and winter-grown plants. In addition to variability within species and populations, plant samples also become contaminated with pollutants and pathogenic and insect infestations that can compromise product quality. The advantages of using in vitro cultures instead of cultivated plants for achieving consistent production of the hypericins and regulation of their synthesis have been discussed (Kirakosyan et al. 2004). Gadzovska et al. (2005) recommended specific use of 6-benzylaminopurine (BAP) for hypericin production in tissue cultures of H. perforatum, while Karakas et al. (2009) found concentrations of BAP impacting hypericin content in H. triquetrifolium. However, Coste et al. (2011) found enhanced production of hypericins in shoot cultures of H. maculatum raised in the presence of 0.4 mg/l of either BAP or KIN. As a light-activated naphthodianthrone, hypericin gets transformed to pseudohypericin in the presence of light and it effectively induces light-mediated apoptosis in cancer cells (Karioti and Bilia 2010). It is more stable than hyperforin and implicated in antitumoral, antiretroviral, and mild antidepressant activities of the pharmaceutical preparations. Therefore, hypericin is more commonly studied for its biological properties than pseudohypericin and hyperforin.
The widespread interest in the anti-depressant activity of H. perforatum paved the way for investigating other species of Hypericum. In fact, such other species as H. triquetrifolium, H. montanum, H. montbretti, H. sinarium and H. sampsonii have already been found to contain even higher concentrations of hypericin than H. perforatum, encouraging the establishment of in vitro cultures, cultivation, and pharmacological evaluation protocols (Liu et al. 2007; Ayan and Çirak 2008; Kusari et al. 2009). Tissue cultures of Hypericum are established from diverse explant types including seedling parts, nodes, leaf tissue, and adventitious roots. Both agar and liquid nutrient formulations have been employed for raising hypericin-rich tissue and cell cultures (Liu et al. 2007; Karakas et al. 2009; Oluk et al. 2010). Significant variations observed in production potential and bioactive ingredients of these cultures were related to genotype of the mother plant, seasonal changes, phases of plant growth, type of source material used for extraction, time of harvest, and the plant cultivation method (Zobayed and Saxena 2004). Manipulation of the culture environment had significant effects on induced synthesis of secondary metabolites and, depending on the culture system, metabolite degradation and/or biotransformation of the metabolites may also occur. A systematic study on biomass production efficiency and accumulation of bioactive molecules in different culture systems showed comparatively higher concentrations of hypericin in plants multiplied in gelled media (Zobayed et al. 2004). Karppinen et al. (2006) favored use of shoot cultures for superior extraction resulting in high yield of hypericin and the production of less variable pharmaceutical preparations (Karppinen 2010) in H. perforatum. Although most of the earlier investigators used MS nutrient formulation with great success, Danova et al. (2012) preferred Gamborg’s B5 vitamins for improved production of hypericins in shoot cultures of H. rumeliacum and H. tetraperum.
Hypericum hookerianum is a lesser known evergreen woody shrub of montane forests distributed in parts of India, Bangladesh, Myanmar, China, and Thailand. There is no published work on in vitro culture of this species for multiplication or for biosynthesis of active ingredients. In this paper, we report consistent formation of a small percentage of red seedlings from an accession of H. hookerianum and shoots rich in hypericin multiplied from both the green and red phenotypes. Further, we demonstrate light-induced substantial synthesis of hypericin in etiolated shoots differentiated upon cotyledon and cotyledon callus tissues of normal green seedlings.
Materials and methods
Seed culture
Ripe berries were collected from a small population of Hypericum hookerianum occurring in the Shola forest of the Lake view area in Kodaikanal (2,200 m) in Palni hills of the Western Ghats in Southern India. Seeds released from the fruits were cleaned by washing in running tap water and then in 1 % Labolene detergent (Thermo Fisher Scientific (India), Mumbai) for 5 min before blotting over filter paper, surface decontamination by immersion under constant stirring in 0.1 % HgCl2 solution containing a drop of Tween-20 for 4 min and rinsing thrice in sterile distilled water. Seeds in groups of 120–140 were dispersed on water gelzan (1.5 %) medium devoid of nutrients in 9-cm pre-sterilized plastic Petri dishes (Tarzons, Kolkata). The medium was autoclaved at 121 °C and 1.1 kg/cm pressure for 18 min before dispensing into the Petri dishes. Dishes were sealed with Parafilm-M and the seeds incubated for germination at 25 ± 2 °C under 12 h photoperiod (50–60 μEm−2 S−1). Observations on seed germination were made at weekly intervals and data on percentage germination and segregation of the seedling types based on pigmentation were collected after 8 weeks.
Seedling culture
Eight-week-old green and red-pigmented seedlings of Lake View accession were separated and used for further experiments. They were washed once in sterile distilled water and seedlings of 1.2–1.8 cm transplanted individually in 15 ml of Murashige and Skoog (1962) basal gelzan nutrient medium supplemented with 3 % (w/v) sucrose in 150 × 25-mm culture tubes or in groups of 6–8 spread over 50 ml medium in 250-ml flasks and cultured under 12 h photoperiod for 6 weeks in the culture room. After 6 weeks, the green and red-pigmented plants raised from the seedlings were scrutinized for morphological attributes (fresh weight of the plantlet, branches of the stem, internode length, length of the tap root) and total chlorophyll, phenol, anthocyanin, flavonoid, and hypericin contents. Unless otherwise stated, 15 randomly selected plants were used for each determination and the data presented are the mean of three separate determinations.
Due to the relatively poor growth of the red seedlings in the basal medium, shoot multiplication potential of the 8-week old red seedlings was tested by dissecting out hypocotyls (1.0–1.5 cm) and cotyledons (0.8–1.0 cm) of both red and green seedlings and culturing them in medium supplemented with 2.325 μM KIN for 6–8 weeks. These organ cultures incubated in light (12 h photoperiod) for 6 weeks, as well as under continuous dark for 4 weeks to produce etiolated shoots followed by light exposure (12 h photoperiod) for 2 weeks to induce hypericin synthesis, were monitored for bud/shoot formation, pigmentation, and shoot growth. Green and red shoots differentiated upon the hypocotyl and cotyledon cultures were extracted and estimated for hypericin following standard procedures.
Multiplication of hypericin yielding shoots
Since the buds and shoots differentiated upon the red-pigmented hypocotyls and cotyledons showed stunted growth and ceased to grow after 8 weeks of culture in the light, seedling-derived shoots (0.5–1.2 cm) were chopped into 4–6 mm segments, transplanted in nutrient medium fortified with 2.325 μM KIN and sub-cultured in the dark. After 4 weeks, the multiplied etiolated shoots were exposed to light for 2 weeks to induce hypericin synthesis. Likewise, the cotyledons and hypocotyls of green seedlings were also cultured in MS medium supplemented with 2.325 μM KIN in the dark and were exposed to light after 4 weeks to test their ability to multiply shoots and synthesize hypericin. Shoots multiplied and exposed to light at 6 (4 + 2)-week intervals were divided and repeatedly sub-cultured in light and dark cycles to amass shoots rich in active principles.
Induction of hypericin synthesis in etiolated shoot cultures
Hypocotyls and cotyledons of in vitro-raised green seedlings were dissected out and cultured in MS gelzan basal medium and medium supplemented with 2.45 μM IBA in 9.0-cm-diameter pre-sterilized plastic dishes sealed with Parafilm-M and incubated both in the light and the dark at 25 ± 2 °C. For incubation in the dark, the dishes were stacked in aluminium boxes and covered with a layer of black cloth. After 6–8 weeks of culture in the dark, the etiolated shoots measuring more than 6 cm differentiated upon the hypocotyls and cotyledons in the basal medium and callus with rudimentary buds proliferated upon both the hypocotyl and cotyledon segments in presence of 2.45 μM IBA were exposed to continuous illumination (50–60 μEm−2 S−1). Changes in the hypericin content of shoots derived from hypocotyls and cotyledons and shoots/buds and shoots differentiated upon the cotyledonary calli were determined after 2 weeks, as against the control shoots of the wild mother plant from which the seedling cultures were raised, and also the green shoots derived from the hypocotyl and cotyledon cultures in light.
Extraction and quantification of hypericin
Air-dried 200 mg plant samples respectively of young shoots of Lake View accession, red-pigmented buds and shoot cultures established from the hypocotyl segments (Fig. 1c, d), chlorophyllous shoot cultures raised in the light, light-exposed red pigment forming etiolated shoots differentiated upon green cotyledons, and red-pigmented shoot clusters differentiated from etiolated cotyledonary calli, and appropriate control tissues were extracted with 10 ml chloroform under sonication in a Sonics Vibra Cell Sonicator (USA). After removing the chloroform, each sample was re-extracted thrice with 10 ml methanol, evaporated to 3 ml and filtered through 0.2-μm Waters nylon syringe filters.
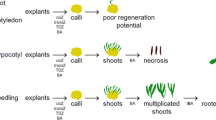
a Seedlings of Vattakkanal (left) and Lake View (right) accessions of Hypericum hookerianum showing segregation of the latter into green- and red-pigmented types in water gelzan medium. b Green and hypericin-rich red plant types raised after 6 weeks seedling subculture in MS Gelzan medium containing 3 % sucrose. c Shoots and buds differentiated upon red-pigmented hypocotyl explant after 8 weeks of culture with 2.325 μM KIN. d Hypericin-rich shoot culture established by subculturing 4–6 mm red-pigmented shoot segments in MS + 2.325 μM KIN in the dark for 4 weeks followed by 2 weeks light exposure
HPLC analysis
High performance liquid chromatography analysis of the filtered extracts was carried out using a UV–Visible detector working at 590 nm in a Shimadzu 1100 system. The hypericin elution program was done isocratically from a C18 (125 × 4 mm) reversed phase column using a mobile phase consisting of (A) acetonitrile:(B) methanol and water (70:30) in the ratio (A:B) 20:80. Aliquots (50 μl each) of green and red samples were injected into the column at 0.25 ml/min flow rate. The elution was monitored at 590 nm and the eluted hypericin was identified on the basis of the retention time by comparison with retention time of the standard (Sigma-Aldrich).The amount of hypericin in individual samples was calculated from the calibration curve prepared in the range of 0.5–100 μg/ml (r 2 > 0.99). The mean value obtained from three separate analysis was taken for calculation.
Determination of total chlorophyll, phenol, anthocyanin and flavonoid concentrations
Chlorophyll, anthocyanin, and flavonoids were extracted by homogenizing 500 mg fresh aerial parts consisting of leaves and stems of 8-week-old green- and red-pigmented plants in a glass pestle and mortar using 80 % cold acetone, 0.01 % acidified methanol, and 80 % aqueous methanol, respectively, and individual extracts filtered through a 200-μm nylon screen. The residues were re-extracted once with the solvent and the combined extracts of each sample were centrifuged at 4,000 rpm in a Kubota 6200 high speed centrifuge. Supernatants were collected and made up to 20 ml each with respective solvent before measuring absorbance at 645 and 663 nm for chlorophylls, 506, 510, and 700 nm for anthocyanins and 510 nm for flavonoids. Total chlorophyll concentration in each sample was calculated using the constants of Porra (2002). The Folin–Ciocalteu method was used to determine total phenolic content of the sample (0.1 ml) as described by Singleton and Rossi (1965). Absorbance was measured at 765 nm. The calibration curve was prepared by employing gallic acid at concentrations of 0.4–1.6 mM. Anthocyanins in acidified methanol extract estimated following the method of Wrolstad et al. (1990). Flavonoids in the methanol extracts were estimated by the aluminium chloride method described by Zhishen et al. (1999). All the values are expressed on fresh weight basis and each value represents mean ± SD of four different determinations.
Morphological characters
Morphology of the 8-week-old chlorophyllous and red-pigmented plants was compared by measuring the height of the shoot canopy and fresh weight of the plants and also by counting the number of branches of the shoots.
Results
Seeds of Lake View accession germinated at a frequency of 96.53 %. Imbibition and swelling of seeds in 3–5 days followed by the emergence of plumule and tap root in 3 weeks and complete liberation of the seedlings from the seed coats occurred in 6–7 weeks. However, 2.56 % of the germinated seedlings were distinctly red-pigmented and slow growing compared to the majority green seedlings (Fig. 1a). Freehand sections of the stems of the red-pigmented seedlings showed sparsely distributed red-pigmented cells in the sub-epidermal cortical region (not shown). Unlike the green seedlings, the red ones did not survive for more than 5 weeks after emergence in water gelzan medium devoid of nutrients. The green and red seedlings sub-cultured for 6 weeks in MS basal medium supplemented with 3 % sucrose developed into fast growing, profusely branched chlorophyllous and slow growing, weakly branched red-pigmented plants, respectively (Fig. 1b). Red pigmentation of the latter plants was confined to aerial parts and the pigment-free root system was as much or more developed than the green plants. The impressive growth and morphological features of the chlorophyllous plant type were further evident from increased height, wet weight, number of branches, and total chlorophyll while the red ones had matching phytochemical profile with a 19-fold high concentration (0.39 mg/g FW) of hypericin (Table 1). Hypocotyls and cotyledons of the phytochemically desirable red seedlings were preferentially cultured in medium supplemented with 2.325 μM KIN to produce red-pigmented buds and slow growing 0.5–1.2 cm shoots in 6 weeks (Fig. 1c) which contained up to 4.38 ± 0.18 mg/g DW hypericin. Further growth of these buds and shoots ceased and necrosis of shoot tips and blackening of the stem occurred after 8 weeks. However, cultured hypocotyls and cotyledons of green seedlings quickly responded with callus free bud formation in a week followed by development of 6.58 ± 2.39 chlorophyllous shoots of 4.67 ± 0.78 cm each in 6 weeks. In both green and red seedlings, the number of prolific/stunted shoots differentiated upon the cotyledons (4.25 ± 0.96) as the case may be, were invariably less compared to hypocotyls (9.18 ± 2.44). Shoots proliferated upon both red and green hypocotyls and cotyledons in the dark were etiolated and elongated (<4.5 cm), while in the light only shoots initiated upon the green explants grew vigorously and stunted and slow growing red ones eventually declined.
Etiolated shoots differentiated upon red hypocotyls and cotyledons in the dark and normal chlorophyllous shoots upon green hypocotyls and cotyledons in the light in nutrient medium supplemented with 2.325 μM KIN contained no and a low concentration of hypericin (0.91 ± 0.03 mg/g DW). Successful intervention by culturing the red and green hypocotyls and cotyledons in the dark for 4 weeks to produce 4.42 ± 2.10 etiolated shoots at 91–96 % (26–29 out of 30 hypocotyls) and 56–68 % (19–22 out of 32 cotyledons) efficiencies followed by 2 weeks light exposure to induce hypericin biosynthesis enabled both shoot production and biosynthesis. Further, division of the slow growing and stunted 0.5–1.2 cm red shoots differentiated upon hypericin rich hypocotyls in light into 4–6 mm segments and subcultured in the dark in the presence of 2.325 μM KIN permitted significant multiplication of shoots. Each shoot segment developed at least 3–5 buds and 5.45 ± 1.69 shoots of 2.3 ± 0.79 cm through the 4-week culture period in the dark which on the 2-week light exposure turned red leaving only the shoot tips green (Fig. 1d). In both hypocotyl and cotyledon derived shoots, starting from the etiolated shoot base, red pigmentation increased in intensity upwards towards the tip of the shoot during the 2-week period. These shoots contained significantly higher concentration (3.93 ± 0.06 mg/g DW) of hypericin than the chlorophyllous shoots raised from the green hypocotyls in light and young shoots of the wild Lake View accession (0.35 ± 0.09 mg/g DW). Successive subculture in the dark and illumination of the resultant etiolated shoots at 6-week (4 weeks dark + 2 weeks light) intervals enabled multiplication and amassing of hypericin rich red-pigmented shoot lines through the 6-month period.
Isolated green and red hyopocotyls and cotyledons of Lake View seedlings were also cultured in both light and dark in MS basal medium and medium supplemented with 2.45 μM IBA to induce possible synthesis of hypericin. While 2–4 buds formed in the light developed into 2.18 ± 0.20 cm shoots after 6 weeks of subculture, 3–5 etiolated shoots of 6.82 ± 0.75 cm, formed essentially from the midrib and veins of cotyledons and cut end portions of both hypocotyls and cotyledons in the dark during the same period, turned red on illumination within 2 weeks. The long etiolated shoots so formed were thin and fragile, consisting of long (2.42 ± 0.47 cm) internodes and reduced leaves compared to the relatively shorter and healthy chlorophyllous shoots having less than 1.0 cm internode and expanded leaves grown in the light. Interestingly, the greening of the etiolated shoots occurred slowly while red pigmentation of the majority of the shoots was dominant masking chlorophyll synthesis (Fig. 2). Unlike the shoot-forming hypocotyls and cotyledons cultured for 6 weeks in the basal medium in the dark, explants cultured in the dark in medium supplemented with 2.45 μM IBA showed marginal semi-friable callus proliferation followed by the emergence of 3–18 shoot buds and simultaneous formation of 2–4 roots. On exposure to light, the bud-forming calli turned red and reddish brown while the buds developed into shoot clusters with simultaneous greening and expansion of leaves. The major bud clusters comprising 12.64 ± 3.8 buds turned green and grew slowly into 0.5–1.5 cm shoots while 4–6 buds of the smaller clusters grew vigorously to attain 2.8–3.2 cm length in 2–4 weeks. Some of these shoot clusters turned red in 1–2 weeks growing slowly or ceasing to grow thereafter. The three samples assayed for hypericin, i.e. red-pigmented greening etiolated shoots proliferated upon the hypocotyls and cotyledons in basal medium (Fig. 2), chlorophyllous shoots raised from hypocotyls/cotyledons in MS medium supplemented with IBA under illumination, and red shoots differentiated upon cotyledonary calli, contained 2.27 ± 0.15, 1.01 ± 0.02, and 3.86 ± 0.31 mg/g DW hypericin, respectively. Attempts to culture explants of red-pigmented shoots with IBA supplementation in light were not successful.
Discussion
Hypericum is a genus of herbs, shrubs, and rarely trees mostly growing in arid and semiarid parts of the world (Camas and Caliskan 2011). Since seed dormancy is characteristic of these plants, hot water, acid, and gibberellic acid treatments are followed to increase seed germination in different species including the popular H. perforatum (Cirak 2007; Camas and Caliskan 2011). The uniformly high percentage seed germination of the H. hookerianum Lake View accession recorded in water gelzan medium without prior seed treatment, in contrast to the species of the arid and temperate zones studied by other workers, may be attributed to the entirely different montane forest ecosystem in which this species grows. The unique Shola forests of high altitudes in the Palni and Nilgiri hills of the Western Ghats are bathed by both southwest and northeast monsoon rains of Asia, maintaining a high moisture content through most of the year, and the stream banks in particular provide ideal habitats for the species to flourish with healthy seed production free from desiccation. Such seeds collected from ripe fruits long before their dispersal are free from dormancy-inducing factors and germinate under a favorable culture environment to register a high frequency of germination.
Seedlings of many Hypericum species have been used for in vitro multiplication of plants and production of secondary metabolites (Zobayed et al. 2004; Oluk et al. 2010; Bacila et al. 2010). In the present study, seeds of the Lake View accession germinating at a rate of 97.44 % segregated into chlorophyllous and red-pigmented seedling types (Fig. 1a) which is new to Hypericum as a whole. That the red pigmentation of the 2.56 % seedlings was due to the presence of the naphthodianthrone, hypericin, was discovered by chemical analysis of the plants raised from the cultured seedlings. Of the two seedling phenotypes, the red ones were deficient in growth and could be sustained only through subculture with carbon supplementation. The prolific growth of the green seedlings into extensively branched plants with large canopy and shoot characters, and an equally impressive phytochemical profile of the red lines with significantly high concentrations of phenols, anthocyanins, flavonoids, and hypericin within 6 weeks of subculture were quite contrasting (Fig. 1b; Table 1), presumably justifying the long-known inverse link between growth and product accumulation in tissue cultures. In addition to the hypericin-producing red seedlings and the red seedling-derived hypericin-rich plants (0.39 mg/g FW), the overproducing buds and shoots differentiated upon the hypocotyl (Fig. 1c), and cotyledon cultures of both red (4.38 ± 0.18 mg/g DW) and green (3.73 ± 0.21 mg/g DW) seedlings, the red shoot clusters indirectly formed on cotyledonary calluses (3.86 ± 0.31 mg/gDW), and the shoots multiplied by subculturing stem explants (3.93 ± 0.06 mg hypericin/g DW) also grew slowly. Possibly due to synthesis and accumulation of hyper-concentrations of hypericin under the culture conditions, these red seedlings and shoots of different tissue origin ceased to grow any further or in some cases declined due to shoot necrosis and blackening of the stem. These observations suggest that once produced in substantial concentrations, the photosensitive hypericin may not only inhibit further growth of the shoots but may also be toxic. Consequently, the over-producing red-pigmented shoots containing as much as 4.38 ± 0.18 mg/g DW hypericin (Fig. 1c) had to be divided and derivative 4–6 mm explants sub-cultured in the dark for multiplication. In animal cells, hypericin is an inhibitor of protein kinases causing mitotic death of cells by retardation at the G2/M phase of cell cycle and formation of polynucleate cells (Erenpreisa and Cragg 2001). Thus far, specific interference of hypericin in plant cell division and tissue growth has not been reported.
Although both hypocotyls and cotyledons of red and green seedlings cultured in nutrient medium supplemented with 2.325 μM KIN were caulogenic, the former had nearly double the shoot-forming potential than the cotyledons, thereby indicating the availability of a larger number of meristematic centers than the cotyledons. Since seedling explants cultured in the dark in MS basal medium produced 3–5 etiolated shoots especially from the veins and cut ends of the explants, and the shoots so formed synthesized up to 2.27 ± 0.15 mg/g DW hypericin only on light exposure, light may not be an indispensable factor for multiplication as it is for hypericin biosynthesis. However, for normal growth of the shoots free of etiolation and reduced leaves observed in dark-grown shoots, provision of light is essential. Since hypericin synthesis and hypericin-induced abnormalities and toxicity were light-dependent, it was only logical that the latter negative responses were reversed by division and subculture of the red-pigmented shoots in the dark to induce bud and shoot multiplication before re-exposing to light for the biosynthesis expected of a sustainable production system. It was also noted that seedling explants of both red and green seedlings cultured in the dark were efficient in producing 3–7 shoots and synthesizing a significant concentration (3.73 ± 0.21 mg/g DW) of hypericin after dark and light (4 + 2 week) cycles. These concentrations are significantly higher than the values reported by other workers in shoot cultures of Hypericum species (Karppinen et al. 2006; Karakas et al. 2009; Coste et al. 2011).The observed red pigmentation progressing upwards from the etiolated shoot base leaving the shoot tip green after 2 weeks of light exposure is not described for any Hypericum species. This presumably indicates increased availability of the precursors and cofactors required for hypericin synthesis as the shoots matured and developed competence for secondary metabolic activity during photoactivation.
The relative ease of conducting studies on seedling explant cultures to raise callus-free shoots in the dark followed by synthesis of the product (hypericin) in the light imputes specificity of the combined shoot multiplication and hypericin production process to the light–dark cycle, which may act as a stress to induce product synthesis otherwise not possible in explants cultured under 12 h photoperiod or in the dark. None of the previous studies have dealt with this process, even though differentiated shoot cultures of H. perforatum are reported to produce more hypericins compared to undifferentiated cell suspension cultures (Kirakosyan et al. 2004). In addition, the observed production of biosynthetic shoots even from green hypocotyls and cotyledons makes the system all the more interesting. Since natural segregation of 2.5 % red seedlings and red pigmentation of the seedling tissues as such may not contribute to product synthesis, it is necessary that green seedlings of all the accessions are scrutinized for biosynthesis in vitro to validate the light–dark cycle-based growth and production. Under the conditions described, experimental intervention involving 4-week culture in the dark in the presence of 2.325 μM KIN and 2-week light exposure of the seedling and shoot segment cultures is a reliable option to achieve shoot multiplication and product synthesis through at least 6 subcultures in the Lake View accession. Experiments are underway to enhance hypericin production using green seedling explant cultures of available accessions of H. hookerianum. Since hypericin content is taken as an indicator of GMP in Hypericum-derived products in the pharmaceutical industry, the demand for hypericin is such that a feasible system for sustainable production is necessary (Bais et al. 2002). The shoot culture system described may be a significant step forward to realize this objective. Data presented in Table 1 and Fig. 1b suggest that, unlike the unequally growing red and green shoots, root characters of both green and red lines were comparable, thereby suggesting confinement of the growth inhibitory influences to the hypericin-producing shoot portions of the plants. It appears that the metabolite is tightly bound and not leaking into adjacent tissues or into the medium. Since light-activated product synthesis had a negative influence on continued tissue growth, the necessity of having a dark phase for multiplication and a light phase for product synthesis was experimentally justified.
Plant tissues often produce secondary metabolites in duress and stress. Physical, chemical, environmental, biotic, and nutritional factors are known to influence hypericin synthesis in tissue cultures (Walker et al. 2002; Zobayed and Saxena 2004; Pavlik et al. 2007; Cui et al. 2010). The literature is replete with instances of different growth regulators inducing hypericin production in tissue cultures. While 6-benzylaminopurine is an obligate requirement for enhanced production of hypericin in H. perforatum (Gadzovska et al. 2005) and H. triquetrifolium (Karakas et al. 2009), Padmesh et al. (2008) and Padmesh (2012) preferred α-naphthalene acetic acid for induced synthesis of the metabolite in tissue cultures of H. hookerianum. In the present study, the light-exposed etiolated hypocotyl/cotyleonary shoots produced hypericin in basal and IBA-supplemented media while the red-pigmented hypocotyl/cotyledon explants were cultured using KIN supplementation for both shoot multiplication and hypericin production. The uniformly long (6.82 ± 0.75 cm) and etiolated shoots of hypocotyl/cotyledon raised in basal medium may be the simplest experimental system described so far to achieve photoactivated synthesis of hypericin. Production of up to 2.27 ± 0.15 and 3.86 ± 0.31 mg/g DW hypericin by the directly and indirectly formed shoots (Fig. 2) suggest that the inexpensive cotyledon culture is suitable at least for demonstration purposes. Production of innumerable seeds by each plant in nature, high percentage seed germination under controlled conditions, remarkable regeneration potential of the seedling explants, and amenability to multiply and produce make seedling culture worth investigating for scaled-up production of hypericin.
References
Ayan AK, Çirak C (2008) Hypericin and pseudohypericin contents in some Hypericum species growing in Turkey. Pharm Biol 46:288–291
Bacila I, Coste A, Halmagyi A, Deliu C (2010) Micropropagation of Hypericum maculatum Cranz an important medicinal plant Rom. Biotechnol Lett 15(1):86–91
Bais HP, Walker TS, McGrew JJ, Vivanco JM (2002) Factors affecting the growth of cell suspension cultures of Hypericum perforatum L. (St. John’s wort) and production of hypericin. In Vitro Cell Dev Biol Plant 38(1):58–65
Camas N, Caliskan O (2011) Breaking of seed dormancy in Hypericum leptophyllum Hochst., an endemic Turkish species. J Med Plant Res 5(32):6968–6971
Cirak C (2007) Seed germination protocols for ex situ conservation of some Hypericum species from Turkey. Am J Plant Physiol 2:287–294
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult 106:279–288. doi:10.1007/s11240-011-9919-5
Cui XH, Murthy HN, Wu CH, Paek KY (2010) Adventitious root suspension cultures of Hypericum perforatum: effect of nitrogen source on production of biomass and secondary metabolites. In Vitro Cell Dev Biol Plant 46:437–444
Danova K, Nikolova-Damianova B, Denev R, Dimitrov D (2012) Influence of vitamins on phenolic content, morphological development, and stress response in shoot cultures of Hypericum spp. Plant Cell Tissue Organ Cult 110:383–393. doi:10.1007/s11240-012-0159-0
Erenpreisa J, Cragg MS (2001) Mitotic death: a mechanism of survival? Cancer Cell Int 1:1–7
Filippini R, Piovan A, Borsarini A, Caniato R (2010) Study of dynamic accumulation of secondary metabolites in three subspecies of Hypericum perforatum. Fitoterapia 81:115–119
Gadzovska S, Maury S, Ounnar S, Righezza M, Kascakova S, Refregiers M, Spasenoski M, Joseph C, Hagege D (2005) Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol Biochem 43:591–601
Karakas O, Toker Z, Tilkat E, Ozen HC, Onay A (2009) Effects of different concentrations of benzylaminopurine on shoot regeneration and hypericin content in Hypericum triquetrifolium Turra. Nat Prod Res 23:1459–1465. doi:10.1080/14786410701664528
Karioti A, Bilia AR (2010) Hypericins as potential leads for new therapeutics. Int J Mol Sci 11:562–594
Karppinen K (2010) Biosynthesis of hypericins and hyperforins in Hypericum perforatum (St. John’s wort)-precursors and genes involved. Academic dissertation submitted to Faculty of Science, University of Oulu, Oulu. ISBN 978-951-42-6310-1
Karppinen K, György Z, Karppinen M, Tolonen A, Jalonen J, Neubauer P, Hohtola A, Haggman H (2006) In vitro propagation of Hypericum perforatum L. and accumulation of hypericins, pseudohypericins and phloroglucinols. Propag Ornam Plants 6(4):170–179
Kirakosyan A, Sirvent TM, Gibson DM, Kaufman PB (2004) The production of hypericins and hyperforin by in vitro cultures of St. John’s wort (Hypericum perforatum). Biotechnol Appl Biochem 39:71–81
Kusari S, Zühlke S, Košuth J, Čellárová E, Spiteller M (2009) Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J Nat Prod 72:1825–1835
Liu X-N, Zhang X-Q, Sun J-S (2007) Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum. Plant Growth Regul 53:207–214
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Oluk EK, Orhan S, Karakaş O, Çakir A, Gönüz A (2010) High efficiency indirect shoot regeneration and hypericin content in embryogenic callus of Hypericum triquetrifolium Turra. Afr J Biotechnol 9(15):2229–2233
Padmesh P (2012) Isolation and characterization of candidate gene(s) involved in the biosynthesis of hypericins-an aromatic naphthodianthrone from Hypericum spp. PhD thesis, University of Kerala, Thiruvananthapuram
Padmesh P, Seeni S, Reji JV, Nair GM (2008) A novel process for the production of hypericin from shoot and callus cultures of Hypericum hookerianum Wight & Arn. Indian Patent No. 224656
Pavlik M, Vacek J, Klejdus B, Kuban V (2007) Hypericin and hyperforin production in St. John’s wort in vitro culture: influence of saccharose, polyethylene glycol, methyl jasmonate and Agrobacterium tumefaciens. J Agric Food Chem 55:6147–6153
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Savio LEB, Astarita LV, Santarem ER (2011) Secondary metabolism in micropropagated Hypericum perforatum L. grown in non-aerated liquid medium. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-011-0058-9
Shaw AK, Avery BA, Wyandt CM (2005) Content analysis and stability evaluation of selected commercial preparations of St. John’s wort. Drug Dev Ind Pharm 3(9):907–916
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic phospho -tungstic acid reagents. Am J Enol Vitic 16(3):144–158
Southwell IA, Bourke CA (2001) Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s wort). Phytochemistry 56:437–441
Walker TS, Bais PH, Vivanco JM (2002) Jasmonic acid induced hypercin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 60:289–293
Wrolstad RE, Skrede G, Lea P, Enersen G (1990) Influence of sugar on anthocyanin pigment stability in frozen strawberries. J Food Sci 55(4):1064–1065
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zobayed SMA, Saxena PK (2004) Production of St. John’s wort plants under controlled environment for maximizing biomass and secondary metabolites. In Vitro Cell Dev Biol Plant 40:108–114
Zobayed SMA, Murch SJ, Rupasinghe HPV, Saxena PK (2004) In vitro production and chemical characterization of St. John’s wort (Hypericum perforatum L. cv New Stem). Plant Sci 166:333–340
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varghese, R.J., Sooriamuthu, S. Differences in hypericin synthesis between experimentally induced seedling shoot cultures of Hypericum hookerianum Wight & Arn.. Plant Biotechnol Rep 7, 511–518 (2013). https://doi.org/10.1007/s11816-013-0289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-013-0289-9