Abstract
The development of efficient, recyclable, broad-spectrum photocatalysts was the primary objective in the field of photocatalytic wastewater degradation. Herein, a novel highly efficient ternary magnetic semiconductor composite was synthesized by integrating SrFe12O19, MoS2 nanoflower clusters, and SnS2 nanoflowers using high-temperature calcination and a one-step solvothermal method. The synthesized heterojunction nanocomposite was characterized using numerous analytical techniques, and its photocatalytic activity was evaluated under half sunlight intensity irradiation. The integration of SnS2 with SrFe12O19 and MoS2 effectively modified the crystal structure and morphology of SnS2 nanoflowers, leading to an increase in active sites while overcoming the significant electron–hole recombination rates of the individual components. The SrFe12O19/SnS2/MoS2 composite achieved 98.69% degradation of MB dye at a suitable pH of 6 and a period of 120 min of irradiation. Additionally, it maintained an excellent magnetic phenomena which contributed to it effortless to collect and reclaim from the residual mixture. After three cycles, the MB dye degradation remained at 84.07%, demonstrating its endurance and resilience. The scavenger test identified the superoxide radical as the primary agent responsible for dye destruction. This work provides study presents a synthesis method for highly efficient photocatalysts using in natural visible-light that can be recovered by simply applying an external magnetic field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of science and technology, economy and industry, a vast array of chemical manufacturing goods have become a part of people’s daily lives [1,2,3]. While these goods deliver multiple conveniences, but are also synonymous with adverse environmental impacts and wasteful use of energy. Organic dye-containing effluent from the textile industry is released in substantial amounts into rivers and groundwater; such contaminants are hazardous and slow to break down [4]. Fujishima and Honda pioneered the use of photocatalysis to address environmental pollution in 1972 by employing TiO2 to catalytically decompose water into H2 and O2 under UV-light irradiation [5]. This delivers an alternative viewpoint on the treatment of sewage. Photocatalysis is a promising emerging technology that may advance the fields of new energy and environmental protection by using solar energy to degrade pollutants in water [6]. Accordingly, the performance of photocatalysts is a critical component of this technology. Thus, the development of effective, environmentally sustainable, visible-light-responsive photocatalysts has emerged as a fresh field of interest for investigation [7].
Currently, researchers are focusing on combined semiconductors and tiny particles in which quantization properties have been approximated, along with semiconductors on passive supports [8]. Photocatalytic semiconductor materials with controlled morphologies have produced numerous favorable results in environmental remediation processes [9, 10]. Metal sulfides are of particular interest owing to their narrow band gap (approximately 1.8 eV), high solar energy utilization, and excellent carrier mobility, which allow them to meet the demanding thermodynamic requirements of reactions including the decomposition of aquatic hydrogen [11, 12], CO2 reduction [13, 14], and degradation of organic pollutants [15]. SnS2, a representative sulfide, is a visible-light photocatalyst with significant development potential owing to its relatively small band gap (2.1 eV), laminar framework, vast relative area of surface, multiple sites of action, superior chemical rigidity, non-toxic nature, and a relatively inexpensive price [16,17,18]. For instance, Liu et al. prepared SnS2 nanosheets with major (0 0 1) facets by liquid-phase stripping. When compared with bulk SnS2, SnS2 nanosheets exhibit superior photocatalytic capacity due to their significantly effective charge separation [18]. Wang et al. synthesized SnS2 via the melting process, which features a flower-like hierarchical structure formed by nanoflake assembly. The SnS2 nanoflowers synthesized by low-temperature melting approach illustrated superior photocatalytic abilities than the SnS2 nanoplates manufactured by hydrothermal method [19]. Tragically, the photocatalytic capabilities of SnS2 composites is limited by the rapid compounding of photogenerated electrons and holes both on the exterior and inside the material’s bulk [20, 21]. Therefore, it is required to develop effective methods to further ameliorate the photocatalysis activity.
In recent years, constructing heterojunction-modified semiconductor photocatalytic materials has been one of the most effective strategies to ameliorate the photocatalytic activity of a single semiconductor, because of their enhanced light absorption ability as well as accelerated charge carrier transfer and separation [22]. Up-to-date, several attempts have been made to alter the electron band structure, laminar framework, and charge separation capacity of metal sulfides to boost the activity of photocatalytic decomposition. The photogenerated charge separation in SnS2 layered materials can be improved by forming heterojunctions with other semiconductor photocatalysts (such as g-C3N4 [17], ZnS [23], Bi2S3 [24], SnO2 [25], and MgFe2O4 [16]). As a two-dimensional transition-metal disulfide and narrow-band-gap semiconductor, MoS2 has garnered significant attention owing to its high carrier mobility, exceptional photostability, expansive specific surface area, distinctive photoelectrochemical properties, and adjustable band gap energy (1.2–1.9 eV) [26]. MoS2 has been widely utilized in a wide range of applications, including photocatalysis [4, 27,28,29], sensors [30,31,32], dye-sensitized batteries [33,34,35], and lithium-ion batteries [36, 37]. Moreover, the band structure of MoS2 can well match that of SnS2, and therefore, a SnS2/MoS2 heterojunction was synthesize to promote the photocatalytic activity of SnS2. While coupling SnS2 to MoS2 alone has made strides in addressing some of the issues related to their photoresponsivity, these materials still struggle to achieve efficient recycling or rapid charge carrier separation. The separation of these photocatalysts from a mixture following a photocatalyst reaction remains a challenge, limiting their applications [38]. Ferrite can quickly and efficiently recycle photocatalysts; thus, hard magnetic ferrites have attracted considerable attention for magneto-optical applications owing to their high magnetic energy products, high saturation magnetization strength and coercivity, and strong thermal stability [39, 40]. SrFe12O19 exhibits characteristics, such as corrosion resistance, high mechanical stability, and cost-effectiveness [41]. It is encouraging to observe that adding a third semiconductor SrFe12O19 enables rapid separation from the mixture and significantly boosts the photocatalytic efficiency by improving redox capacity and electron–hole pair mobility. The literature indicates that there is an appropriate match between the energy band structure of SrFe12O19 and MoS2 [42]. Hence, it is anticipated that adding SrFe12O19 into SnS2/MoS2 will substantially decrease electron–hole complexation as well as improve photocatalytic capacity. These photocatalysts can be easily separated and recovered using a magnetic field [43].
In this study, a novel, highly efficient ternary SrFe12O19/SnS2/MoS2 magnetic semiconductor composite photocatalyst with double-heterojunction was synthesized by growing SrFe12O19 particles and MoS2 nanoflower clusters on SnS2 nanoflower carriers using high-temperature calcination and a one-step solvothermal method. Furthermore, the influence of the SrFe12O19 content and dye pH on the photocatalytic performance and the mechanism by which the ternary heterojunction with a unique flower-like morphology formed on the surface were evaluated.
Materials and Methods
Strontium chloride hexahydrate (SrCl2·6H2O), ferric chloride hexahydrate (FeCl3·6H2O), sodium molybdate dihydrate (Na2MoO4·2H2O), tin tetrachloride pentahydrate (SnCl4·5H2O), thiourea (CH4N2S), sodium hydroxide (NaOH), anhydrous ethanol (C2H5OH), isopropanol (C3H8O), and concentrated nitric acid (HNO3) were purchased from Chengdu Kolon Chemical Co., China. P-benzoquinone (C6H4O2) and disodium ethylenediaminetetraacetic acid (C10H14N2Na2O8·2H2O) were purchased from Shanghai McLean Biochemical Technology Co., China. All reagents were analytically pure and did not need any extra purification. Ultrapure water was used in all procedures.
The crystal phase compositions of the samples were determined by X-ray diffraction (SmartLab-9, Rigaku, Japan) using a Cu Kα (λ = 0.154 nm) radiation source over a 2θ scanning range of 10°–80° at a scanning speed of 0.05°s−1. The morphology and energy-dispersive spectra (EDS) of the samples were tested and characterized by a field-emission scanning electron microscope (FE-SEM, JSM-7800F, Nippon Electronics JEOL, Japan). The microstructure and lattice fringe of the samples were examined by high-resolution transmission electron microscopy (HRTEM, JE-2100F, Nippon Electronics JEOL, Japan). X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, USA) was used to analyze the elemental valences and chemical composition of the composite photocatalyst. The specific surface areas of the samples obtained through N2 adsorption apparatus (ASAP 2460, Micromeritics, USA). Ultraviolet–visible (UV–Vis) diffuse reflectance spectra (DRS) absorption spectra was performed using a spectrophotometer (Beijing Puxi General Instrument Co., Ltd., China) with a BaSO4 reference material. The photoluminescence (PL) spectrum spanning 350–800 nm was obtained with a fluorescence spectrometer (FLS1000, Edinburgh Instruments Ltd., UK). The magnetic properties of the samples were characterized using an MPMS3 magnetic test system (Quantum Company, USA). Electrochemical impedance spectroscopy (EIS) was performed using a PGSTAT302N comprehensive electrochemical workstation (Metrohm Corp., Switzerland).
Synthesis of SrFe12O19 Hexagonal Single Crystal Particles
SrFe12O19 was prepared by an established wet chemical method [42].
Synthesis of Magnetic Composite SrFe12O19/SnS2/MoS2
One-step solvothermal synthesis was utilized to generate magnetic materials [23]. Na2MoO4·2H2O (1 mmol), SnCl4·5H2O (1 mmol), and thiourea (4 mmol) were dissolved in ultrapure water (10 mL) by ultrasonication for 30 min, following which isopropanol (40 mL) was added and the combination was mechanically swirled approximately 20 min to ensure an even distribution. SrFe12O19 with molar ratios to SnS2 with 1:16, 1:8, 1:4, and 1:2 was dissolved in the mixture and dispersed throughout 1 h of mechanical and ultrasound agitation. After that, the mixture was inserted to a high-pressure reaction kettle and allowed to sit for 22 h at 200 °C. Following the reaction, the solution was chilled to 20 °C and separated. After being washed with water and ethanol four times, the samples had been filtered and dehydrated for 12 h at 80 °C. The composites synthesized with SrFe12O19, SnS2, and MoS2 in mass ratios of 1:16:16, 1:8:8, 1:4:4, and 1:2:2 were labeled SSM-I, SSM-II, SSM-III, and SSM-IV, respectively. Similar steps were utilized to synthesize pristine SnS2 and MoS2, omitting the preliminary addition of SrFe12O19. A schematic of the synthesis of the magnetic composites is shown in Fig. 1.
Photocatalytic Performance
The photocatalytic properties of the materials were evaluated by examining their ability to decompose methylene blue (MB) solutions in the absence of visible light. A catalyst sample (30 mg) was dispersed in an MB solution (80 mL, 20 mg/L) and swirled in the dark approximately 30 min to accomplish an equilibrium of adsorption and desorption [44,45,46]. The reaction was then performed at 26 °C under simulate half sunlight intensity, which was close to the actual lighting in nature. After 20 min, an aliquot (5 mL) of the solution was centrifuged to determine the absorbance at 664 nm. To minimize the impact of volume reduction on subsequent catalysis, stirring was maintained during the photocatalytic degradation experiment to keep the catalyst uniformly dispersed. This ensured that the reduction of the catalyst was proportional to the solution volume when samples were collected. Equation (1) has been utilized to compute the MB degradation ratio [42]:
where η is the degradation ratio, A0 is the absorbance of the MB solution after achieving adsorption–desorption equilibrium, and At is the absorbance of the MB solution after illumination for time t. Further, the photocatalytic stability and recyclability of the SrFe12O19/SnS2/MoS2 composite photocatalysts were evaluated using cyclic tests under the same conditions. The degradation efficiency of the SrFe12O19/SnS2/MoS2 composite was examined over three consecutive cycles in the photodegradation of a 20 mg/L solution of MB at pH 6, with a catalyst loading of 375 mg/L. Following each photodegradation experiment, the photocatalysts were retrieved using an external magnet, cleansed with ethanol and distilled water, and subsequently dried in an oven at 60 °C.
Active Radicals Capturing Research
The major active radicals trapping text was performed in the similar manner to the photocatalytic decomposition experiment, with the only difference being the inclusion of various scavengers [47]. A solution of BQ (1 mmol/L), a solution of EDTA-2Na (1 mmol), and a solution of IPA (1 mmol) were employed as scavengers for superoxide anion (⋅O2−), hydrogen peroxide (h+), and hydroxyl radical (⋅OH), respectively.
Results and Discussion
Photocatalyst Characterization
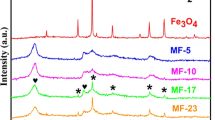
The X-ray diffraction (XRD) patterns of SrFe12O19, SnS2, MoS2, and SSM composites with various compositions are presented in Fig. 2. Over the 2θ range of 10°–80°, sharp and intense peaks corresponding to pure SrFe12O19 (JCPDS:84–1531) are observed at 30.39°, 32.40°, 34.23°, 37.19°, 40.47°, 42.55°, 55.20°, 56.89°, and 63.15°. These peaks are attributed to the (1 1 0), (1 0 7), (1 1 4), (2 0 3), (2 0 5), (2 0 6), (2 1 7), (2 0 11), and (2 2 0) crystal planes [40, 42], in the order. The highest-intensity spikes at 14.98°, 28.19°, 32.12° and 49.96° correspond to the (0 0 1), (1 0 0), (1 0 1) and (1 1 0) crystal planes of pure SnS2 (JCPDS:23–0677), respectively [18]. The characteristic spike at 34.19° is associated with the (0 1 2) crystal plane of MoS2 (JCPDS:17–0744), which exists in an amorphous state with low crystallinity [22]. The diffraction peak of the (0 0 1) crystal plane of single-phase SnS2 is enhanced by the solvothermal synthesis as a characteristic of its layered material properties [48]. This layered structure is also confirmed by the SEM morphology. The XRD spectrum of the SrFe12O19/SnS2/MoS2 composite photocatalyst is similar to that of pure SrFe12O19, indicating that SrFe12O19 is the dominant crystalline phase. The peaks at 28.19° and 52.45° still show the characteristic peaks of SnS2. However, in the presence of a magnetic field environment with SrFe12O19 ferrite particles, the co-synthesis of SrFe12O19 and MoS2 via one-step solvothermal synthesis alter the chemical reaction environment, crystallization, and growth dynamics of SnS2, creating stress or defects that reduce or shift the intensity of the (0 0 1) diffraction peak [48,49,50]. Moreover, in the composite of SnS2 with SrFe12O19 and MoS2, the introduction of components such as Mo, Sr, Fe and O, also results in some doping or vacancies, significantly affecting the intensity of specific diffraction peaks, further reducing or shifting the (0 0 1) peak intensity [49, 50].
The morphology of the photocatalysts and their constituents was characterized using FE-SEM. The 3D flower-like SnS2 microspheres consist of randomly arranged 2D SnS2 nanosheets (Fig. 3a). Hexagonal SrFe12O19 (Fig. 3c) and cluster-like nanoflower MoS2 (Fig. 3b), with an average diameter of approximately 200 nm, are properly distributed on the 3D SnS2 microspheres. FE-SEM images of the composite catalysts are shown in Fig. 3d-3g. Groups of SnS2 blooms are wrapped around each other, while the SrFe12O19 nanosheets and MoS2 blooms uniformly penetrate the SnS2 nanoflakes. This mixed structure increases the number of exposed interface regions between SrFe12O19, MoS2, and SnS2. Notably, the agglomeration of SrFe12O19 nanoparticles in the composite increases with the SrFe12O19 content (Fig. 3f, g), hindering their insertion into the SnS2 nanosheets. The changes in the layered appearance of SSM in the SEM morphology and the most prominent (1 0 0) plane observed in the HRTEM confirm the structural changes of SnS2 before and after compositing. These changes facilitate the exposure of the (1 0 0) plane of SnS2, increasing the active sites and accelerating the kinetics of electron and ion diffusion, thereby enhancing photocatalytic activity [51]. The strategy of altering and exposing diffraction peaks through compositing makes SnS2 composites a promising candidate for efficient photocatalytic materials.
Figure 4 shows the HRTEM images of the internal structure of the SSM-II composite. The pristine SnS2 and MoS2 nanosheets are thin and the SnS2 coating on the hexagonal SrFe12O19 microcrystals indicates association between SrFe12O19 and SnS2 (Fig. 4a, b). The junction area between the three materials (Fig. 4c, d) exhibits individual crystalline regions of SrFe12O19, SnS2, and MoS2, signifying the synthesis of SSM mixtures. Streaks consisting of SrFe12O19, SnS2, and MoS2 lattices are also shown. The lattice with an interlayer gap of 0.315 nm is linked with the SnS2 (1 0 0) plane [16], while the lattices with interlayer gaps of 0.338 and 0.235 nm are linked with the SrFe12O19 (1 0 5) and MoS2 (1 0 4) crystal planes, respectively [22, 43]. EDS maps of the photocatalysts, presented in (Fig. 5), confirm the presence and uniform distribution of O, S, Fe, Sr, Mo, and Sn in the SSM-II. The synthesized SSM composite not only exhibits the crystal structures of SrFe12O19, SnS2, and MoS2 but also benefits from the induced crystal structure defects and the exposure of crystal planes during the compositing process, which collectively enhance its photocatalytic activity.
Physicochemical Properties
The XPS analysis of the magnetic composite photocatalyst SSM-II revealed the presence of Mo, Sn, O, S, Fe, and Sr (Fig. 6a). The binding energy of O 1 s (530.99 eV, Fig. 6b) corresponds to the lattice oxygen in crystalline SrFe12O19. Sr 3d5/2 and Sr 3d3/2 are accountable for the peaks situated at 133.38 and 139.3 eV, respectively. According to a previous investigation, Sr 3d3/2 explains the existence of Sr2+ and Sr 3d5/2 is generated from the chemical link Sr–O (Fig. 6c) [52]. The existence of ions containing Fe3+ in strontium ferrite provides the reason for the binding energies of the Fe 2p3/2 peaks (711.68 and 716.24 eV, Fig. 6d) [42]. The Sn 3d curves are extremely smooth and symmetrical (Fig. 6e). The Sn 3d5/2 and Sn 3d3/2 areas in SnS2 are represented by the peaks approximately 486.60 eV and 494.94 eV, respectively. The peaks at 228.8 and 231.9 eV in the Mo 3d spectrum correspond to Mo 3d5/2 and Mo 3d3/2, respectively, demonstrating a 4 + Mo oxidative form. The generation of Mo6+ by the moderate oxidization of MoS2 is to blame for the satellite peak (235.7 eV) [53]. A 2 S peak (226.24 eV) is also attributed to MoS2 (Fig. 6f). The XPS profile of S 2p (Fig. 6g) shows peaks at 161.53 and 162.83 eV that correspond to S 2p3/2 and S 2p1/2, respectively, reflecting the spin orbit separation of S. An additional peak at 169.13 eV is attributed to polysulfide S2−. SnS2 and MoS2 were effectively incorporated into SrFe12O19, as indicated by the existence of S 2p and S2− [14].
Nitrogen Adsorption
Other crucial factors affecting the performance of the photocatalytic material include the specific surface area and pore size of the catalyst. The N2 adsorption–desorption isotherms of the MoS2, SnS2, SrFe12O19, and SSM-II samples are shown in Fig. 7. The N2 adsorption–desorption results show a type IV isotherms with type H3 hysteresis loops, indicating that the material possesses a mesoporous characteristics [25]. The composite sample SSM-II exhibits the similar curve as does pure SnS2, indicating that the composite retains the mesoporous characteristics of pure SnS2 [54]. Compared to SSM-II and SnS2, SrFe12O19 and MoS2 exhibits a lower adsorption rate and does not show clear characteristics of type III or type IV adsorption isotherms, which is consistent with the specific surface area test results. The low adsorption rate of strontium ferrite is unfavorable for photocatalytic degradation, but its adsorption rate can be improved through composite formation. Table 1 lists the specific surface areas (SBET) and pore volumes of all materials. SnS2 possesses the largest specific surface area owing to its spherical nanoflower structure. The addition of SrFe12O19 gradually destroyed the original flower-like spherical structure of SnS2, reducing the specific surface area of the composite. However, the specific surface areas of the composites are significantly higher than those of MoS2 and SrFe12O19, subsequently reducing the probability of electron–hole recombination and improving the degradation efficiency.
Electrochemical Impedance and Optical Property
Performance in photocatalysis depends on by the capacity of the composites to absorb light. Figure 8a shows the UV–vis absorption spectra of SrFe12O19, MoS2, SnS2, and SSM materials with various compositions. All the photocatalytic materials exhibit strong absorption over the full range of wavelengths. The SSM composites exhibit a higher UV–vis intensity than do pure SrFe12O19, SnS2, and MoS2, with SSM-II having the strongest absorbance. The forbidden bandwidth is estimated by Eq. (2) [55]:
where h, v, a, and A are Planck’s constant, optical frequency, the absorption coefficient, and a constant, respectively [56]. The index n depends on the electronic transition of the semiconductor, which is directly forbidden. The bandgaps of SnS2, SrFe12O19, SSM-I, SSM-II, SSM-III, and SSM-IV were found to be 2.29, 1.61, 0.72, 1.11, 0.97, and 0.87 eV, respectively (Fig. 8b, c). Nonetheless, the photocatalytic capacity was boosted by the recombination of SSM-II, which considerably decreased the bandgap and thus substantially prevented the crossover of carriers generated by photons. Information about the division of electron–hole pairs generated by photons can be acquired via PL measurements, which can serve as an indicator of photocatalytic performance. Compared to pristine SrFe12O19, the photoluminescence intensity of the SSM composites is significantly lower, with SSM-II possessing the lowest photoluminescence intensity among the composite samples (Fig. 8d), which effectively promotes the efficient transfer of electrons at the interface and suppresses the formation of electron–hole pairs, thus extending the lifetime of the photogenerated carriers.
UV–Vis DRS profiles of as-prepared MoS2, SnS2, SrFe12O19, SSM-I, SSM-II, SSM-III, and SSM-IV (a); corresponding Tauc plots (ɑhν)2 as a function of hν of SnS2, SrFe12O19, SSM-I, SSM-II, SSM-III, and SSM-IV (b); magnified area showing the Eg values of SnS2 and SrFe12O19 (c); PL spectra of pure SnS2, SrFe12O19, and SSM composites (d); EIS images of as-prepared SnS2, MoS2, SrFe12O19, SSM-I, SSM-II, SSM-III, and SSM-IV (e)
The charge-transfer behavior is investigated using EIS, and the corresponding Nyquist plots are displayed in Fig. 8e. The high-frequency range of the Nyquist graphs for SrFe12O19, SnS2, MoS2, and SSM depicts comparable semicircles, the diameters of which increase in the following order: SSM (SSM-I, SSM-II, SSM-III, and SSM-IV) < SnS2 < MoS2 < SrFe12O19. The greatest conductivity and minimum charge-transfer resistance are indicated by SSM-II, which enhances the charge-transfer efficiency between the interfaces. The diameters of SSM-I, SSM-III, and SSM-IV are nearly identical, indicating that the photocatalytic performance of the materials is influenced by their SrFe12O19 content. Among them, SSM-II is more advantageous for the generation and movement of electrons and holes than the other samples [57].
Photocatalytic Activity
The disintegration of MB during exposure to visible light has been utilized to evaluate the photocatalytic capacity of the photocatalysts. The absorbing spectrum of the SSM-II catalytic material for the MB dye under visible light illumination are displayed in Fig. 9a. The absorption spike characteristic of MB is observed at 664 nm, and MB is uniformly absorbed over the full range of wavelengths. After 30 min of treatment in the dark to establish adsorption–desorption equilibrium, the degradation efficiencies of the pure SrFe12O19, SnS2, and MoS2 materials reach 2.50%, 30.00%, and 41.26%, respectively, after 120 min of light exposure. The SSM composite photocatalyst demonstrates increased photocatalytic activity, with SSM-II exhibiting the maximum photocatalytic activity (98.69%) after 120 min of light exposure. Additionally, the first-order kinetic diagram of the decomposition of MB by the synthesized photocatalysts were adapted (Fig. 9c), and the decomposition rate constant (k) values were calculated (Table 2) utilizing to the Langmuir–Hinshelwood kinetic model (3) [42]:
The rate constants of SSM-II are approximately 123, 14.47, and 20.5 times higher than those of pure SrFe12O19 (0.0002 min−1), SnS2 (0.0017 min−1), and MoS2 (0.0012 min−1), respectively; other SSM composites also show higher rate constants than monophasic materials, which can be improved by incorporating SrFe12O19. However, the photocatalytic activity of the SSM material decreases as the SrFe12O19 content increases. Excess SrFe12O19 prevents the exposure of SnS2 active sites, resulting in a smaller effective interfacial contact area. Owing to constant agitation of the aqueous solution and ultraviolet reflection from various particle regions, a limited quantity of photons penetrated the interior heterojunction surface via gaps among the material petals. Thereby producing photogenerated carriers at the heterojunction and extending their lifespan. Moreover, the SrFe12O19 nanosheets uniformly penetrated the SnS2 nanoflowers, and the SnS2 nanosheets interacted with microscopic particles of MoS2 to catalyze transport of electrons and increase the specific surface area of the catalyst. To sum up, the incorporation of SrFe12O19 provides molecules of dye extra spots for adsorption and enables the SSM composite photocatalyst to rapidly degrade the target pollutants.
Impact of Initial pH
The pH of the solution is a critical and influential operational factor for the degradation of dyes, as it impacts the speed at which dye molecules are adsorbed and desorbed on the exterior of the catalyst, alters the potential of the valence band, and affects the physicochemical characteristics [57, 58]. The impact of pH on the extent of dye degradation by the SSM-II composite is demonstrated in Fig. 10. The pH was adjusted by the addition of dilute aqueous solutions of NaOH (1 M) and HCl (1 M). A weakly acidic environment is the most suitable for the degradation of MB by the SSM composites. The surface charge of the catalyst also depends on the pH of the solution. The quantity of the ionic dye adsorbed by the material is significantly different at pH 6 owing to the electrostatic repulsion between the positive charge on the catalyst surface in the ionic dye and acidic solution. Under alkaline conditions, the produced OH− ions combine with H+ ions on the photocatalyst surface, thereby reducing the adsorption efficiency [59]. The lessened oxidative potential of •OH at higher pH levels (2.8 V at pH 0 as opposed to 1.9 V at pH 7) could be further linked to the decrease in the rate of MB deteriorate at higher pH levels.
Analysis of Active Species Capture Experiments
To identify the SSM-II composite catalyst’s photocatalytic response process, research employing free radical capture have been utilized to examine the active species in the photocatalytic process. During 120 min of degradation with the addition of various scavengers, the photocatalytic decomposition rate for 80 mL of the MB (20 mg/L) is demonstrated in Fig. 11. BQ was used as a capturing agent for •O2−, whereas IPA and EDTA-2Na were employed as scavengers of •OH and h+, respectively. The decomposition ratio reaches 98.69% during 120 min of light in the absence of a trapping agent. The addition of EDTA-2Na and IPA reduces the degradation rates to 92.56% and 92.00%, respectively, includes little impact on the efficiency of photocatalysis, demonstrating that the majority of effective catalytic species are neither h+ and •OH. The photocatalytic degradation rate in the presence of BQ is only 85.13%, suggesting that •O2− is the primary active species in the overall photocatalytic process.
Photocatalyst Reusability
Figure 12 demonstrates the reusability and stability of the SSM-II photocatalyst. SSM-II maintains a degradation efficiency of 84.07% over three cycles (Fig. 12a b). The crystal structure of the SSM-II material was evaluated using XRD after three cycles of MB degradation under half sunlight intensity (Fig. 12c). The crystal structure is identical both prior to and following photodegradation, based to the patterns of XRD.
Magnetic Recovery Capability
Figure 13 shows the hysteresis return curves of pure SrFe12O19 and SSM-II. The hysteresis curve of SSM-II exhibits the typical characteristics of hard magnetic materials [60]. The narrow hysteresis return lines indicate that SSM-II has a lower hysteresis loss under alternating magnetic fields. The saturation magnetization strength (Ms) of SSM-II (13.78 emu g−1) is lower than that of SrFe12O19; however, both materials can be separated from aqueous solutions equally quickly. The resistance of magnetic materials to demagnetization increases with the coercive force, Hci. The coercive forces of monophasic SrFe12O19 and SSM-II are 3910.25 and 4161.5 Oe, respectively; thus, SSM-II is slightly more resistant to demagnetization. Photographs of SSM-II and SrFe12O19 exposed to a magnetic field (Fig. 13, inset) illustrate their facile recovery from the dye solution for subsequent recycling.
Photocatalytic Mechanism
We indicate a tenable process of degradation of the double heterojunction generated by SSM mixed materials, which accounts for our observations. The enhanced photocatalytic activity of the SSM composites relative to those of pure MoS2 and SnS2 was attributed to the formation of type II heterojunctions between the SnS2, MoS2, and SrFe12O19 semiconductors. Equations (4) and (5) were utilized to calculate the potentials of the valence band (EVB) and conduction band (ECB), which are shown in Fig. 14.
Here, Ee is the potential energy (roughly 4.5 eV for a typical hydrogen electrode), X is the absolute value of electronegativity, and Eg is the bandgap [61, 62].
Excitation of the MoS2 and SnS2 nanoflowers and hexagonal SrFe12O19 plates under simulated sunlight (k > 420 nm) caused the photoelectrons to migrate from the VB to the CB to initially generate pairings of photoelectron-hole, followed by the formation of electrons and holes on the CB and VB, respectively. Figure 14 shows a possible photocatalytic mechanism. Because the CB of SrFe12O19 (− 0.79 eV) is more negative than that of SnS2 (− 0.59 eV) and the VB of SnS2 (1.7 eV) is more positive than that of SrFe12O19 (0.8 eV), the electrons generated via photosynthetic in the CB of the hexagonal SrFe12O19 plate transition to the CB of the SnS2 nanoflowers, and the photogenerated holes transition from the MoS2 nanoflowers to the hexagonal SrFe12O19 plate. Carriers flow across the heterojunction contact, which facilitates photogenerated electron–hole pair separation and hinders recombination. Similarly, MoS2 and SrFe12O19 also form a type II heterojunction with an internal electric field. Moreover, the magnetic field produced by the magnetic material matrix, in conjunction with the internal electric field, significantly improves the photocatalytic activity. SrFe12O19 creates a stable magnetic field in the ternary composite catalytic material, and some photogenerated electrons move in the direction of the magnetic field. The spiraling motion of the magnetic field and photogenerated carriers accelerate electron migration.
Conclusions
A magnetically recyclable double-heterojunction nanophotocatalyst, SrFe12O19/SnS2/MoS2, was prepared via one-step solvothermal synthesis. The prepared SrFe12O19/SnS2/MoS2 hybrid photographic catalyst demonstrated both electromagnetic recovery and efficient responsiveness in natural visible-light. The uniformly interspersed SrFe12O19 particles and MoS2 florets on the SnS2 nanoflowers effectively modified the crystal structure and morphology of SnS2, provided abundant catalytically active sites, and decreased the recombination rate that the electron–hole pairs generated by photons reacted. At pH 6, the SrFe12O19/SnS2/MoS2 compound obtained an excellent MB decomposition rate of 98.69% under half sunlight intensity. The photocatalytic process ratio of SrFe12O19/SnS2/MoS2 composites was approximately 123, 14.47, and 20.5 times those of pure SrFe12O19, SnS2, and MoS2, respectively. The ratio of photocatalytic decomposition of SrFe12O19/SnS2/MoS2 remained as high as 84.07% after three cycles, demonstrating its stability. The magnetic response of SrFe12O19/SnS2/MoS2 facilitates the recycling of contaminated water. Further, owing to its flower-like morphology, it serves as an efficient, environmentally benign photocatalyst for wastewater treatment in a weak sun lighting.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, its supplementary information files.
References
T. LuukkonenS, O. Pehkonen, Crit. Rev. Environ. Sci. Technol. 47, 1 (2016)
K. Yin, Y. Xu, X. Li, X. Jin, J. Clean. Prod. 197, 815 (2018)
C. Su, Y. Song, M. Umar, Ocean Coast. Manag.Manag. 204, 105550 (2021)
J. Luo, R. Li, Y. Chen, X. Zhou, X. Ning, L. Zhan, L. Ma, X. Xu, L. Xu, L. Zhang, Sep. Purif. Technol.Purif. Technol. 210, 417 (2019)
A. Fujishima, K. Honda, Nature 238, 37 (1972)
R. Lavanya, T. Ramakrishnappa, K. M. Girish, K. Suresh Kumar, M. Radhakrishna Reddy, H. R. Prakash, Bulletin of Materials Science 47, 34 (2024).
M.P. Mazhari, H. Khojasteh, N. Sharifi, P. Aspoukeh, S.M. Mousavi, J. Sol-Gel Sci. Technol. 110, 156 (2024)
N. Akram, J. Guo, W. Ma, Y. Guo, A. Hassan, J. Wang, Sci. Rep. 10, 1939 (2020)
Z. Li, X. Meng, Z. Zhang, J. Photochem. Photobiol., C 35, 39 (2018)
Z. Cui, H. Wu, K. Bai, X. Chen, E. Li, Y. Shen, M. Wang, Physica E E 144, 115361 (2022)
X. Zhou, J. Zou, S. Zhang, M. Pan, W. Gong, Chin. J. Catal.Catal. 38, 287 (2017)
R. Zhang, L. Bi, D. Wang, Y. Lin, X. Zou, T. Xie, Z. Li, J. Colloid Interface Sci. 578, 431 (2020)
X. Yang, W. Xin, X. Yin, X. Shao, Chem. Phys. Lett. 651, 127 (2016)
L. Ding, Z. Zhang, Z. Wen, F. You, N. Hao, J. Wei, J. Qian, K. Wang, Sens. Actuators, B Chem. 367, 132033 (2022)
B. Xue, H. Jiang, T. Sun, F. Mao, J. Wu, Mater. Lett. 228, 475 (2018)
X. Yuan, H. Wang, Y. Wu, X. Chen, G. Zeng, L. Leng, C. Zhang, Catal. Commun.. Commun. 61, 62 (2015)
K. Mohammadi, M. Sadeghi, R. Azimirad, J. Mater. Sci. 28, 10042 (2017)
Y. Liu, X. Mi, J. Wang, M. Li, D. Fan, H. Lu, X. Chen, Inorganic Chemistry Frontiers 6, 948 (2019)
J. Wang, Y. Chen, Colloid and Interface Science Communications 45, 100522 (2021)
L. Dashairya, M. Sharma, S. Basu, P. Saha, J. Alloy. Compd. 774, 625 (2019)
A. Zhang, Z. Nan, J. Therm. Anal. Calorim.Calorim. 139, 217 (2019)
J. Kang, C. Jin, Z. Li, M. Wang, Z. Chen, Y. Wang, J. Alloy. Compd. 825, 153975 (2020)
X. Chen, C. Zhao, H. Wu, Y. Shi, C. Chen, X. Zhou, Materials 15, 3786 (2022)
D. Park, M. Kim, J. Kim, J. Alloy. Compd. 925, 166623 (2022)
Z. Mei, G. Wang, S. Yan, J. Wang, Acta Physico Chimica Sinica 37, 2009097 (2020)
Y. Zeng, N. Guo, Y. Song, Y. Zhao, H. Li, X. Xu, J. Qiu, H. Yu, J. Colloid Interface Sci. 514, 664 (2018)
X. Man, L. Yu, J. Sun, S. Li, Funct. Mater. Lett.. Mater. Lett. 09, 1650065 (2016)
M. Wu, L. Li, N. Liu, D. Wang, Y. Xue, L. Tang, Process. Saf. Environ. Prot.Saf. Environ. Prot. 118, 40 (2018)
K. Zhu, L. Luo, T. Peng, Journal of Wuhan University of Technology-Mater. Sci. Ed. 34, 883 (2019)
Y. Wang, Y. Ni, Anal. Chem. 86, 7463 (2014)
M. Ghanei-Motlagh, M.A. Taher, Biosens. Bioelectron.. Bioelectron. 109, 279 (2018)
X. Lin, F. Wang, X. Shan, Y. Miao, X. Chen, M. Yan, L. Zhang, K. Liu, J. Luo, K. Zhang, Appl. Surf. Sci. 546, 149074 (2021)
L. Song, T. Wang, W. Jing, X. Xie, P. Du, J. Xiong, Mater. Res. Bull. 118, 110522 (2019)
E.S. Sowbakkiyavathi, V. Murugadoss, R. Sittaramane, S. Angaiah, J. Solid State Electrochem.Electrochem. 24, 2289 (2020)
J. Ye, C. Cheng, J. Lin, C. Huang, T. Yeh, C. Hsieh, Surf. Coat. Technol. 394, 125855 (2020)
C. Sun, K. Zhao, Y. He, J. Zheng, ACS Appl. Mater. Interfaces 11, 20762 (2019)
J. Wan, Y. Hao, Y. Shi, Y. Song, H. Yan, J. Zheng, R. Wen, L. Wan, Nat. Commun.Commun. 10, 3265 (2019)
H. Kaur, A. Rai, S.S. Bhatia, Mod. Phys. Lett. A 34, 1950313 (2019)
N.A. Algarou, Y. Slimani, M.A. Almessiere, F.S. Alahmari, M.G. Vakhitov, D.S. Klygach, S.V. Trukhanov, A.V. Trukhanov, A. Baykal, J. Market. Res. 9, 5858 (2020)
P. Maltoni, T. Sarkar, G. Barucca, G. Varvaro, F. Locardi, D. Peddis, R. Mathieu, The Journal of Physical Chemistry C 125, 5927 (2021)
Q. Wu, Z. Yu, Y. Wu, Z. Gao, H. Xie, J. Magn. Magn. Mater.Magn. Magn. Mater. 465, 1 (2018)
S. Chen, Y. Di, H. Li, M. Wang, B. Jia, R. Xu, X. Liu, Appl. Surf. Sci. 559, 149855 (2021)
T. Xie, L. Xu, C. Liu, Y. Wang, Appl. Surf. Sci. 273, 684 (2013)
Z. Yang, Y.W. Chen, Y.F. Jin, Z. Jin, H.S. Xie, X.S. Cong, D.G. Teng, ACS Omega 9, 11356 (2024)
H. Peng, X. Yang, P. Zhang, Y. Zhang, C. Liu, D. Liu, J. Gui, RSC Adv. 7, 50216 (2017)
Y. Liu, P. Feng, Z. Wang, X. Jiao, F. Akhtar, Sci. Rep. 7, 1845 (2017)
Y. Li, S. Tan, Y. Meng, Y. Xia, L. Gao, W. Chen, J. Mater. Sci. Mater. Electron. 33, 13887 (2022)
N.S. Das, A. Roy, A. Chowdhury, Lett Appl NanoBioScience 12, 47 (2022)
N. Anitha, M. Anitha, J. Raj Mohamed, S. Valanarasu, L. Amalraj, Journal of Materials Science: Materials in Electronics 29, 11529 (2018).
J. Zhou, Z. Zhang, X. Kong, F. He, R. Zhao, R. Wu, T. Wei, L. Wang, J. Feng, Appl. Surf. Sci. 510, 145442 (2020)
J. Wu, R. Zhao, H. Xiang, C. Yang, W. Zhong, C. Zhang, Q. Zhang, X. Li, N. Yang, Appl. Catal. BCatal. B 292, 120200 (2021)
D.D. Mishra, G. Tan, J. Phys. Chem. Solids 123, 157 (2018)
Y. Zeng, N. Guo, H. Li, Q. Wang, X. Xu, Y. Yu, X. Han, H. Yu, Sci. Total. Environ. 659, 20 (2019)
X. Hu, G. Wang, J. Wang, Z. Hu, Y. Su, Appl. Surf. Sci. 511, 145499 (2020)
Y. Liu, X. Xu, J. Zhang, H. Zhang, W. Tian, X. Li, M.O. Tade, H. Sun, S. Wang, Appl. Catal. BCatal. B 239, 334 (2018)
X. Yuan, X. Xue, H. Ma, S. Guo, L. Cheng, Nanotechnology 28, 375705 (2017)
S. Xing, Z. Zhou, Z. Ma, Y. Wu, Appl. Catal. BCatal. B 107, 386 (2011)
M. Abbas, B.P. Rao, V. Reddy, C. Kim, Ceram. Int. 40, 11177 (2014)
S. Miao, Z. Zha, Y. Li, X. Geng, J. Yang, S. Cui, J. Yang, J. Photochem. Photobiol., A 380, 111862 (2019)
A.H. Mady, M.L. Baynosa, D. Tuma, J.J. Shim, Appl. Catal. BCatal. B 203, 416 (2017)
M. Sattari, M. Farhadian, A. Reza Solaimany Nazar, M. Moghadam, Journal of Photochemistry and Photobiology A: Chemistry 431, 114065 (2022).
B. Akhsassi, Y. Ettahiri, B. Bakiz, A. Taoufyq, S. Villain, C. Favotto, F. Guinneton, J.R. Gavarri, A. Benlhachemi, Colloids Surf. A 673, 131762 (2023)
Acknowledgements
Special thanks to Editage (www.editage.cn) for their English language editing assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, B., Cai, Q., Di, Y. et al. Magnetic Recyclable Double-Heterojunction SrFe12O19/SnS2/MoS2 Nanophotocatalyst: Synthesis and Visible-Light Catalytic Degradation Performance Study. Korean J. Chem. Eng. 41, 2581–2595 (2024). https://doi.org/10.1007/s11814-024-00204-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00204-3