Abstract
In polymer electrolyte membrane fuel cell (PEMFC), the cost and durability are main challenges to commercialization. To achieve the long-term durability target, the durability of PEMFC materials should be further improved. In this paper, we investigated the chemical and electrochemical degradation of the expanded polytetrafluoroethylene (e-PTFE) support and examined how it influences the mechanical properties of the e-PTFE support. We conducted the Fenton test and open circuit voltage (OCV) holding and analyzed the chemical and physical structural changes of the e-PTFE support by utilizing Fourier transform infrared spectroscopy (FT-IR), scanning electron microscope (SEM), and tensile test. After the chemical and electrochemical degradations, the molecular bonds of e-PTFE were broken, and the microstructures of e-PTFE were deformed. As the result the mechanical properties of e-PTFE showed significant decrease. Consequently, our results show that the e-PTFE support is deteriorated chemically during PEMFC operation, and this chemical degradation lead reduction of mechanical strength.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increase in CO2 emissions resulting from the use of fossil fuels, there has been a growing demand for renewable energy. Hydrogen energy, among various renewable energy sources, has garnered significant attention, leading to the commercialization and market expansion of fuel cells. Fuel cells come in various types, such as solid oxide fuel cell (SOFC), direct methanol fuel cell (DMFC), polymer electrolyte membrane fuel cell (PEMFC), and phosphoric acid fuel cell (PAFC), depending on the type of electrolyte used [1,2,3,4]. Among these, PEMFC offers advantages, including fast start-up, high current density, and low operating temperature, making it suitable for a wide range of applications, from power generation to transportation. However, the commercialization of PEMFC still faces challenges in term of costs and durability [5, 6].
To address cost issues, efforts have been made to reduce the amount of Pt catalyst loading and the cost of the stack components [7,8,9]. In addition, various methods have been employed to improve the durability of the membrane electrode assembly (MEA) to address durability concerns. In the electrode catalyst, Pt-based catalysts such as Pt–Co have been adopted, which not only enhance the activity of the catalyst but also improve its durability [10,11,12,13]. In the electrolyte membrane, radical scavengers are added to mitigate the chemical degradation of the membrane [14], and porous support such as expanded polytetrafluoroethylene (e-PTFE) is impregnated in the center of the membrane to mitigate the mechanical degradation [15,16,17]. Despite these efforts, the durability of PEMFC does not meet the long-term targets. The US Department of Energy (DOE) aims for 8000 h of PEMFC durability for light-duty vehicle (LDV), and 25,000 h (ultimately 30,000 h) for medium-duty vehicle (MDV) and heavy-duty vehicle (HDV) [18]. To achieve the long-term target, the durability of PEMFC materials should be further improved.
In PEMFC, a reinforced membrane has been used since the early 2000s [19], and e-PTFE is commonly used as a support for reinforced membrane. e-PTFE has excellent properties, such as low dielectric constant, chemical stability, high crystallinity, and high porosity [20]. Therefore, it is suitable for use as a membrane support in PEMFC. Numerous studies have demonstrated that the use of e-PTFE support improves the durability of the membrane and prolongs its lifetime [21,22,23,24,25,26]. In PEMFC, the expansion and contraction of the membrane during the humidification and drying cycle are the primary cause of membrane mechanical degradation. The reinforced membrane exhibits superior planar dimensional stability during the process of humidification and drying processes, as compared to the unreinforced membrane [17, 27]. Therefore, the stability of the plane dimension of the reinforced membrane improves durability by reducing mechanical stress.
In practical conditions of PEMFC, both the ionomer and support within a reinforced membrane undergo chemical and mechanical degradation simultaneously. Although e-PTFE is known for being chemically stable, it can be degraded chemically during extended operation of PEMFC. The chemical degradation of the support can reduce its mechanical strength. Therefore, the chemical durability of e-PTFE is crucial to achieve the PEMFC long-term target. However, there has been a lack of research on the chemical durability of e-PTFE as a support material in PEMFC.
In this study, we investigated the chemical and electrochemical degradation of the e-PTFE support and examined how it influences the mechanical properties of the e-PTFE support. We conducted Fenton tests to chemically degrade e-PTFE support and confirmed the chemical and physical structural changes. In addition, to observe effects of electrochemical degradation of e-PTFE under PEMFC operation, we fabricated the reinforced membrane using e-PTFE and conducted open circuit voltage (OCV) holding experiments. We analyzed the chemical and physical structural changes of the e-PTFE support by utilizing Fourier transform infrared spectroscopy (FT-IR), scanning electron microscope (SEM), and tensile test.
Material and Methods
Fenton Test
The e-PTFE support (Donaldson, USA) used in the experiment was 10 μm thick, and the Fenton reaction was conducted while controlling the temperature and stirring speed in an open glass reactor. The Fenton solution was prepared by adding ferrous sulfate (FeSO4 7H2O, ≥ 99%, Sigma Aldrich) to a 20% hydrogen peroxide solution (30% H2O2 in H2O, Sigma Aldrich) at a concentration of 80 ppm. To ensure uniform contact between the support and the Fenton solution, the support was affixed to a square frame, and the solution was stirred. The Fenton test was carried out for a 3 h.
Fabrication of MEA
The reinforced membrane is fabricated to a thickness of 25 μm using perfluoro sulfonic acid (PFSA) ionomer powder (E-21669D, 3 M Co., USA) with e-PTFE support. A Pt/C catalyst was purchased from CNL Energy Co., and the Pt/C catalyst with a loading 0.4 mg/cm2 is used on both the anode and cathode. The MEA is made by sandwiching the catalyst layer on both sides of the membrane and hot pressing them together.
Single Cell Operation
PEMFC cell tests were performed in a 25 cm2 single cell. This cell was assembled with MEA, gas diffusion layer, Teflon gaskets, bipolar plates and end plates. The cell temperature, relative humidity (RH) and gas flow rate were controlled using a station (CNL Energy Co., Korea). I-V curves were measured at 70 °C, 100% RH, and anode/cathode stoichiometry of 1.5/2.0. Linear sweep voltage (LSV) measurements were conducted using a potentiostat (Solartron Metrology Ltd, SI 1287) at scan rate of 1 mV/s within a voltage range of 0 to 0.4 V. During LSV measurement, the cell was supplied with H2 (40 mL/min) at the anode and N2 (200 mL/min) at the cathode.
Accelerated Stress Test
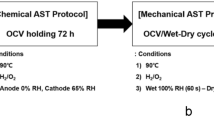
OCV holding was performed as the electrochemical durability accelerated stress test (AST) of the membrane. Following the DOE MEA chemical stability protocol [5]. The AST protocol was performed in anode-H2 and cathode-O2 at the OCV, 90 °C and 30% RH, and the anode and cathode gas flow rates were injected at a stoichiometric ratio of 10/10 with an equivalent flow rate of 0.2 A/cm2 and a back pressure of 150 kPa was applied. The evaluation time followed the DOE polymer membrane chemical durability target standard (OCV < 20% decrease, hydrogen crossover current density (HCCD) > 15 mA/cm2, short resistance < 1.0 kΩ*cm2).
Analysis
To compare the chemical structure of the e-PTFE support, FT-IR analysis was performed using an attenuated total reflection (ATR) accessory attached to FT-IR spectrometer (Nicolet iS5, Thermo Fisher Scientific, USA). The surface change of the support due to degradation was analyzed using a SEM (JSE-7100 F, JEOL, Japan), and the beam potential was 5–15 kV. The mechanical strength of the support was analyzed by tensile strength (TAXTplus, Stable Micro Systems, UK). The e-PTFE specimens, approximately 40 × 10 × 0.01 mm3 in size, were cut for tensile test. The tensile test was conducted at an elongation rate of 120 mm/min in at room temperature and atmospheric pressure.
Results and Discussion
Chemical Degradation of e-PTFE by Fenton Test
Figure 1 shows the fluoride emission rate (FER) in e-PTFE during Fenton test depending on the temperature of the Fenton reaction. The FER increased as the reaction temperature increased. It is due to increase in reaction rate that occurs with the temperature rise. The FER of e-PTFE is relatively low because e-PTFE support has chemical stability. However, this result shows that even though e-PTFE is chemically stable, it can still be degraded by radicals.
FT-IR spectroscopy was used to analyze the molecular bond change in the e-PTFE support after the Fenton test (Fig. 2). Clear changes were observed at 1148 and 1210 cm−1, corresponding to symmetric and asymmetric vibrations of CF2 groups in the PTFE [28]. With an increase in the Fenton reaction temperature, the absorbance of CF2 groups decreased, which correlates with the FER results. As the reaction temperature increases, the degradation rate of radicals/H2O2 increases, leading to a greater breakage of CF2 bond of the e-PTFE support. This shows that the chemical degradation caused by radicals can break molecular bond of the e-PTFE.
Change of Physical Structure of e-PTFE After Fenton Test
Figure 3 shows the change in the surface of the e-PTFE support before and after the Fenton test. After the Fenton test, significant structural deformations occurred in e-PTFE support. Tangled fibrils adhered to each other at junctions, nodes and fibrils were thickened, the micropores between the fibrils enlarged, and the number of micropores decreased. As the temperature of the Fenton test increased, these deformations became more pronounced. As the nodes and fibrils become thicker, the number of adjacent nodes and fibrils simultaneously decreases. Therefore, the increase in thickness appears to be a result of the adjacent fibrils and nodes sticking together due to degradation. Consequently, this result shows that the chemical degradation of radicals transforms the dense microstructure of the e-PTFE support.
Change of Mechanical Properties of e-PTFE After Fenton Test
Figure 4 shows Stress–Strain (S–S) curve of the support after the Fenton test at 80 °C, and Table 1 presents its tensile mechanical properties. The Young's modulus decreased from 40.8 MPa to 39.5 MPa before and after the Fenton test. Young’s modulus indicates the stiffness of an elastic material, which depends on the force of interatomic bonds [29]. During the Fenton test, the radicals attack the CF2 bonds of the e-PTFE support. The weakened interatomic bonds of the support led to a reduction in Young’s modulus. When the stiffness of a support decreases, it becomes more susceptible to deformation. This implies that the dimensional stability of e-PTFE becomes lower after the Fenton test. Dimensional stability is crucial in a reinforced membrane, as increased dimensional changes can make it easier for cracks to appear in the membrane. Additionally, the yield stress decreased from 1.7 MPa to 1.5 MPa, and the yield strain decreased from 3.2 to 3.0% after the Fenton test. The ultimate stress decreased from 31.6 MPa to 26.4 MPa. Consequently, the elasticity and the toughness of the e-PTFE support decreased after the Fenton test. We suggest that the overall changes in the physical properties of e-PTFE after chemical degradation are attributed to the altered structure of the support following degradation. The collapse of the densely intertwined micro-fibril structures due to degradation has resulted in a reduction in physical strength. These results show that e-PTFE, which is chemically stable, can undergo changes in its physical structure through chemical degradation by radicals.
Electrochemical Degradation of Reinforced Membrane by OCV Holding
A MEA was fabricated using an e-PTFE support to investigate the electrochemical degradation of the e-PTFE support during actual fuel cell operation. Subsequently, OCV holding, which is an AST protocol to evaluation the electrochemical durability of MEA, was conducted in a unit cell (Fig. 5). The OCV holding was carried out under conditions of low humidity, high temperature, and oxygen atmosphere at the cathode to accelerate membrane degradation [5, 30, 31]. Figure 6 shows the changes in the I–V curve during the OCV holding. The performance of cell decreased with OCV holding time. The current density at 0.6 V decreased to 26% and OCV decreased to 5% at OCV holding 144 h, as compared to the initial state. These performance decreases indicate that the electrochemical degradation of MEA. Notably, the sharp drop in OCV after OCV holding 144 h implies that hydrogen permeability of membrane increases due to electrochemical degradation.
Figure 7 shows the changes in LSV during OCV holding. Until OCV holding 96 h, HCCD hardly changed, and it even decreased. This is attributed to the occurrence of S–O–S cross-linking within the membrane during degradation [32]. In early stages of electrochemical degradation, sulfone groups in membrane form the S–O–S cross-linking. This bridge creates a dense membrane structure, which reduces hydrogen crossover. After OCV holding 96 h, there was a slight increase in HCCD, attributed to the breaking of S–O–S bridges as a result of degradation. After OCV holding 144 h, HCCD doubled compared to its initial value. This increase of HCCD is attributed to the reduction in membrane thickness of the MEA caused by electrochemical degradation.
To investigate the degradation of the support within the MEA after OCV holding, e-PTFE support was extracted using propanol. The MEA was immersed in 2-propanol solution (99.5%, SAMCHUN) for 30 min to eliminate ionomer and catalyst. Subsequently, the e-PTFE support was extracted and air-dried for approximately 6 h at room temperature and atmospheric pressure. During the e-PTFE support extraction process, e-PTFE shrinks as it is immersed in the solution and dries. This shrinkage occurs because propanol causes a shrinkage in the morphology of the e-PTFE [33]. As the result, the extracted e-PTFE support undergoes inevitable morphological changes, leading to variations in FT-IR, SEM, and S–S curve analyses compared to the pristine state (Fig. 1S, 2S, 3S). The changes of e-PTFE after extraction are described in detail in supplementary material. Therefore, to clearly understand the changes in the e-PTFE support after MEA degradation, the e-PTFE supports were extracted from both non-degraded MEA and degraded MEA, respectively, followed by an analysis of e-PTFE degradation.
Figure 8 shows the changes of the FT-IR spectrum of the e-PTFE support in degraded MEA during OCV holding. No peaks associated with the ionomer were observed in the extracted e-PTFE support [28]. Therefore, the e-PTFE supports were successfully extracted from MEA. After OCV holding, the absorbance of CF2 groups in the e-PTFE support decreased similarly to that observed after Fenton reaction. However, the reduction of CF2 peak in e-PTFE was greater after OCV holding than after the Fenton test. This result shows that long-term degradation during PEMFC operation can sufficiently deteriorate even the e-PTFE support present inside the membrane. Furthermore, when the Pt catalyst is dissolved and deposited in membrane by electrode degradation during PEMFC operation, the chemical degradation inside membrane can be accelerated, because radicals can be generated at the Pt catalyst inside the membrane [34,35,36]. In such cases, the support in the membrane may undergo more severe chemical degradation. Therefore, the chemical durability of the membrane support is significantly important during long-term operation.
Change of Mechanical Structure After OCV Holding
Figure 9 shows the structure change of e-PTFE after OCV holding. After OCV holding, the e-PTFE support in the membrane was deformed similarly observed to the results after Fenton test. Adjacent nodes and fibrils were stuck to each other, thickening material. The number of micropores decreased, and the pore size was larger. These results show that the electrochemical degradation during PEMFC operation deteriorates the e-PTFE support chemically and deforms the microstructure.
Change of Mechanical Properties and Structure of e-PTFE After OCV Holding
Figure 10 shows the change in the S–S curve of e-PTFE support after OCV holding, and the change in mechanical properties is shown in Table 2. After OCV holding, The Young’s modulus of the e-PTFE support decreased from 382 to 301 MPa, yield stress decreased from 11.3 MPa to 6.9 MPa, and ultimate stress decreases from 100.2 MPa to 82.2 MPa. The yield strain decreases from 2.5% to 1.8%, ultimate strain decreases from 54.9 to 41.0%. This trend is similar to that observed after the Fenton test, however, the reduction rate is greater. This result correlates with the result of FT-IR. It is the result that the more chemical degradation leads the more decrease of mechanical properties. When the elasticity and toughness of the support decrease, it becomes easily deformable and cannot withstand mechanical stresses. Therefore, the chemically degraded support can easily lead failure caused by mechanical stress during PEMFC operation.
Consequently, our results show that the e-PTFE support is deteriorated chemically during PEMFC operation, and this chemical degradation lead reduction of mechanical strength. In the reinforced membrane, the mechanical properties of the support are important to enhance the membrane of mechanical strength. However, the chemical durability of the membrane support during PEMFC operation is also crucial because the chemical and mechanical degradation occur simultaneously during PEMFC operation. Therefore, we suggest that to achieve the long-term durability targets of PEMFC, the chemical durability as well as the mechanical durability of the membrane support should be studied.
Conclusions
In this study, the Fenton test and OCV holding were conducted to confirm the chemical degradation of the e-PTFE support in reinforced membrane. After the Fenton test of e-PTFE support, significant changes were observed in both its chemical and physical structures. The CF2 bonds of e-PTFE were broken. The fibrils of the e-PTFE support adhered to each other, the nodes thickened, and the micropores were enlarged. These changes significantly impacted the mechanical properties of the e-PTFE support. The Young’s modulus, yield stress, and ultimate stress of e-PTFE decrease after the Fenton test. In addition, after OCV holding of reinforced MEA, the degradation of the e-PTFE support appeared similarly with the Fenton test. The chemical degradation of the membrane support results in a significant collapse of its physical structure and a substantial decrease in mechanical strength. Although the support is present in the middle of the membrane, the long-term degradation was sufficient to deteriorate the membrane support. Our results show that the e-PTFE support is chemically degraded during long-term PEMFC operation, resulting in a decrease in mechanical properties. Therefore, we suggest that further research on the durability of the support in the reinforced membrane during long-term operation is needed to achieve the PEMFC durability targets.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
J. Larminie, A. Dicks, Fuel Cell Systems Explained (John Wiley and Sons, Chichester, England, 2000)
S.J. Peighambardoust, S. Rowshanzamir, M. Amjadi, Int. J. Hydrog. Energy 35(17), 9349–9384 (2010)
R.K. Pachauri, Y.K. Chauhan, Renew. Sustain. Energy Rev. 43, 1301–1319 (2015)
J. Lee, K. Hyun, Y. Kwon, Korean J. Chem. Eng. 40, 1775–1782 (2023)
DOE U.S. DOE fuel cell technologies office multi-year research, development, and demonstration plan. 3.4 fuel cells (2016), https://www.energy.gov/sites/default/files/2017/05/f34/fcto_myrdd_fuel_cells.pdf. Accessed 24 Jan 2024
DOE U.S. Fuel cell technical team roadmap (2017), https://www.energy.gov/eere/vehicles/articles/us-drive-fuel-cell-technical-team-roadmap. Accessed 24 Jan 2024
DOE U.S. 2020 DOE hydrogen and fuel cells program review presentation, fuel cell system analysis (2020), https://www.hydrogen.energy.gov/pdfs/review20/fc163_james_2020_o.pdf. Accessed 24 Jan 2024
DOE U.S. 2021 DOE hydrogen and fuel cells program review presentation, fuel cell system analysis (2021), https://www.hydrogen.energy.gov/pdfs/review21/fc163_james_2021_o.pdf. Accessed 24 Jan 2024
S.H. Woo, S. Kim, S. Woo, S. Park, Y.S. Kang, N. Jung, S. Yim, Korean J. Chem. Eng. 40, 2455–2462 (2023)
X.X. Wang, M.T. Swihart, G. Wu, Nat. Catal. 2, 578–589 (2019)
S. Ohyagi, T. Sasaki, Electrochim. Acta 102, 336–341 (2013)
S. Hidai, M. Kobayashi, H. Niwa, Y. Harada, M. Oshima, Y. Nakamori, T. Aoki, J. Power. Sources 196(20), 8340–8345 (2011)
L. Dubau, F. Maillard, M. Chatenet, J. André, E. Rossinot, Electrochim. Acta 56(2), 776–783 (2010)
M. Zatoń, J. Rozière, D.J. Jones, Sustain. Energy Fuels 1, 409–438 (2017)
Y. Tang, A. Kusoglu, A.M. Karlsson, M.H. Santare, S. Cleghorn, W.B. Johnson, J. Power. Sources 175(2), 817–825 (2008)
A. Kusoglu, M.H. Santare, A.M. Karlsson, S. Cleghorn, W.B. Johnson, J. Electrochem. Soc. 157(5), 705–713 (2010)
A. Kusoglu, A.M. Karlsson, M.H. Santare, S. Cleghorn, W.B. Johnson, J. Power. Sources 170(2), 345–358 (2007)
DOE U.S. 2022 Annual merit review and peer evaluation report: fuel cell technologies (2022), https://www.hydrogen.energy.gov/pdfs/review22/2022-amr-05-fuel-cell-technologies.pdf. Accessed 24 Jan 2024
A.R. Hobson, S.J. MacKenzie, US Patent, 6,613,203 (2009)
S. Ebnesajjad, Expanded PTFE Applications Handbook: Technology, Manufacturing and Applications (Elsevier Science, New York, 2016)
Y.H. Lai, C.K. Mittelsteadt, C.S. Gittleman, D.A. Dillard, J. Fuel Cell Sci. Technol. (2009). https://doi.org/10.1115/1.2971045
D. Spernjak, P.P. Mukherjee, R. Mukundan, J. Davey, D.S. Hussey, D. Jcobson, R.L. Borup, ECS Trans. 33(1), 1451–1456 (2010)
S.M. MacKinnon, T.J. Fuller, F.D. Coms, M.R. Schoeneweiss, C.S. Gittleman, Y.H. Lai, R. Jiang, A.M. Brenner, Fuel Cells – Proton-Exchange Membrane Fuel Cells | Membranes: Design and Characterization, in Encyclopedia of Electrochemical Power Sources. (Academic Press, Boston, 2009), pp.741–754
C.S. Gittleman, F.D. Coms, Y.H. Lai, Polymer Electrolyte Fuel Cell Degradation Chapter 2 - Membrane Durability: Physical and Chemical Degradation (Academic Press, Boston, 2012)
M. Crum, W. Liu, Trans. ECS Trans. 3(1), 541 (2006)
S. Oh, D. Lim, Y. Han, S. Lee, D. Yoo, K. Park, Korean J. Chem. Eng. 41, 545–552 (2024)
N.S. Khattra, Z. Lu, A.M. Karlsson, M.H. Santare, F.C. Busby, T. Schmiedel, J. Power. Sources 228, 256–269 (2013)
L. Junsheng, X. Yang, H. Tang, M. Pan, J. Membr. Sci. 361(1–2), 38–42 (2010)
W.D. Callister, Fundamentals of Materials Science and Engineering (Wiley, London, 2000)
E. Endoh, S. Terazono, H. Widjaja, Y. Takimoto, Electrochem. Solid-State Lett. 7(7), A209-211 (2004)
F.A. de Bruijin, V.A.T. Dam, G.J.M. Janssen, Fuel Cells 8(1), 3–22 (2008)
B.C. Hwang, S.H. Oh, M.S. Lee, D.H. Lee, K.P. Park, Korean J. Chem. Eng. 35, 2290–2295 (2018)
Z. Wang, H. Tang, J. Li, M. Pan, J. Appl. Polym. Sci. 121(3), 1464–1468 (2011)
D. Yoo, B. Hwang, S. Oh, K. Park, Korean J. Chem. Eng. 40(8), 2004–2009 (2023)
N. Hasegawa, T. Asano, T. Hatanaka, M. Kawasumi, Y. Morimoto, ECS Trans. 16(2), 1713 (2008)
T. Kim, H. Lee, W. Sim, S. Kim, T. Lim, K. Park, Korean J. Chem. Eng. 26(5), 1265–1271 (2009)
Acknowledgements
This work was supported by the Technology Innovation Program (20017400, Development of technology for thinning and widening the reinforced electrolyte membrane and improving durability) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea)
Funding
This article is funded by Ministry of Trade, Industry and Energy, 20017400, Kwon Pil Park.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoo, D., Oh, S., Han, Y. et al. Change of Mechanical Properties of e-PTFE Support by Electrochemical Degradation in Polymer Electrolyte Membrane Fuel Cell. Korean J. Chem. Eng. 41, 2433–2440 (2024). https://doi.org/10.1007/s11814-024-00182-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00182-6