Abstract
Mass transfer of ozone in a jet loop reactor was investigated in this study. Different parameters such as ozone gas concentration, gas flow rate, circulation flow rate, and spray nozzle diameter were examined for their effect on mass transfer. In experiments with ozone gas concentrations ranging from 3.83 to 17.1 g/m3, the KLa values remained unchanged, with an average of 28.7 ± 2.2 h−1. As the ozone gas concentration increased, the saturation values of ozone in the liquid phase also increased. Experiments were carried out with gas flow rates ranging from 50 to 250 L/h in the jet loop reactor, and increasing gas flow rates increased the KLa value up to five times. When the effect of the liquid circulation flow rate was examined for a spray nozzle diameter of 15.5 mm, increasing the flow rate from 40 L/min to 80 L/min increased the KLa value by approximately 41%. However, the power consumed per reactor volume for this process increased 8 times and reached 2.65 kW/m3. KLa values in the JLR vary between 6.1 and 37.3 h−1 depending on operating parameters and it has been concluded that it performs well compared to its counterparts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution problems have increased parallel to the rapid population growth and industrialization in the world which has caused an increase in the quantity and types of pollutants causing environmental pollution. When these pollutants are not treated with appropriate purification technologies, it can have negative effects on the environment in the long term. Due to their resistance and some being toxic even at low concentrations, it is quite difficult to completely purify these pollutants with conventional systems such as biological treatment. In addition to that the increasingly stringent regulations, new treatment processes and strategies are needed for the purification of these pollutants [1,2,3,4]. Ozonation, which is one of the effective methods in water and wastewater treatment, is utilized as an oxidizing agent to eliminate organic pollutants in water [5, 6]. Since ozone and ozone-based advanced oxidation processes were first used in drinking water and wastewater treatment applications, they have shown a significant increase in number and these methods are widly used in drinking water, urban wastewater, and industrial wastewater treatment [7,8,9,10]. The low solubility of ozone cause to low gas–liquid mass transfer efficiency, limiting the effectiveness of ozone in removing pollutants. Low mass transfer efficiency increases costs at an economic level. Enhancing the gas–liquid transfer of ozone and increasing the saturation concentration of ozone in water is particularly important [11].
Improvement of mass transfer of ozone will enhance the efficiency and reduce the cost of the ozonation process [12]. The transfer efficiency of ozone from the gas phase to the liquid phase is controlled by physical parameters such as temperature, gas flow rate, partial pressure of ozone, and reactor geometry. In addition, chemical parameters such as pH, ionic strength, and composition of aqueous solution also affect ozone transfer. A partial mass transfer coefficient can be used, to explain the effect of physical parameters on ozone mass transfer from the gas phase to the liquid phase [13].
Ozone is generally produced by on-site electrical discharge in pure oxygen or dry air flow which is expensive to produce and highly unstable (degrades quickly, decomposes in water by reacting with many compounds, including itself). Therefore, in the ozonation process, the ozone gas must be used as it is produced [8, 9, 14]. Ozone is a highly oxidative agent that reacts with organic compounds and inorganic compounds, converting them into intermediate compounds or CO2 and H2O. Depending on the ozonation conditions, direct reactions between pollutants and molecular ozone or indirect reactions between pollutants and hydroxyl free radicals occur [15]. Ozonation can be a potential pretreatment process to convert refractory compounds into substances that can be more easily removed by traditional methods (such as biological treatment), due to its high oxidaton power [9, 14, 16]. Effective use of ozone in wastewater treatment is essential due to its low solubility in water and high production cost [17]. Mass transfer occurs from the gas phase to the liquid phase, during the contact between ozone gas and water, which the liquid film controls this transfer. The mass transfer rate of ozone in the liquid phase depends on the size of the bubbles formed and thus the size of the gas–liquid interfacial area [18, 19]. Therefore, the use of reactors that can enable a more effective transfer of ozone gas to water is important. Various contactors, such as fixed beds, bubble columns, rotating bar reactors, mechanical stirrers, and contact towers, have been investigated for this purpose [22,23,24,25,26,27]. However, these reactors have various disadvantages that negatively affect ozone performance and bring some complexities to their application and maintenance. In general, these disadvantages include operating in small volumes at lab-scale, requiring high energy input, needing extra circulation, facing challenges in scaling up from laboratory to pilot scale, having complex operating procedures, and requiring maintenance.
Jet Loop Reactors (JLRs) are known for their high mass transfer capabilities and have simple construction, low investment, and operating costs compared to conventional reactors. They also have advantages such as low energy consumption with more circulation, high heat and mass transfer coefficients, excellent gas dispersion, homogeneous concentration, and temperature profile. The absence of any moving parts inside the reactor reduces energy requirements and maintenance needs. In addition to that, pilot-scale facilities have many advantages such as easy transition from pilot to industrial scale [20, 25,26,27]. Therefore, JLRs appear to be reliable alternatives as they avoid such problems.
The literature review shows that there is no study regarding the mass transfer of ozone in JLRs characterized by ease of construction from an architectural perspective, low operational costs, low power consumption, and high mass transfer capability. In this context, an investigation was conducted on the determination of the volumetric mass transfer coefficient (KLa) of ozone in JLRs, focusing on the effects of ozone gas concentration, gas flow rate, and liquid circulation rate on the KLa.
Experimental
Experimental Setup and Procedures
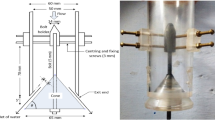
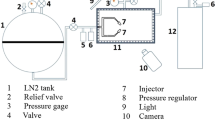
The JLR used in the experimental study is composed of two concentric cylindrical tubes made of stainless steel (SS304). The reactor (outer) and draft pipe (inner) have inner diameters of 10 cm and 4 cm, respectively, and a wall thickness of 2 mm for both cylindrical tubes. The wider compartment above the reactor is called the gas uptake tank and has an inner diameter of 20 cm. With a total height of 110 cm (including the degassing tank). The draft pipe, located 35 cm below the top of the gas intake tank and 10 cm above the base of the reactor (impact plate), is aligned with the reactor through support. The liquid, which is pressurized by a circulation pump, and gas from a separate line are sprayed into the draft pipe through a nozzle. The two-phase flow moving inside the draft pipe moves downward and hits the reactor’s impact plate before rising through the gap between the reactor’s wall and the draft pipe. Some of the liquid-containing gas bubbles at the level of the draft pipe are drawn back into the draft pipe due to the pressure differential, and the cycle continues in this way. The nozzle consists of two concentric cylindrical tubes, with an inner diameter of 3.7 mm and an outer diameter of 6.3 mm for the inner tube through which ozone gas passes, and an inner diameter of 15.5 mm for the outer cylinder through which the liquid flows. The other two nozzles inner diameters of 8.8 mm and 10 mm with 7.5 cm lengts. The liquid volume is in the reactor is 18 L. A schematic representation of the JLR used in the experimental studies is shown in Fig. 1.
There are various measurement systems in the experimental system to measure dissolved ozone, ozone gas, pH, temperature, liquid flow rate, and gas flow rate. The liquid flow rate in the system was measured with an analog inline flowmeter and adjusted to the desired values with manually controlled valves. The gas flow rate was adjusted to the desired value through a flow meter located in front of the ozone generator. Ozone gas was produced from dry air. The air coming from a compressor (Dalgakiran, DKC-150) was passed through an air dryer to remove moisture as much as possible. With a moisture and dust collector (not shown in the table) before the dryer. Then, the dry air passing through the pressure regulator was introduced into the ozone generator (Anseros, COM AD-08) with a maximum pressure of 1.2 bar. The ozone gas concentration was adjusted by changing the operating capacity of the ozone generator at a constant dry air-flow rate. The pH value of the solution inside the reactor was measured and controlled using a pH controller (Eutech, Alpha pH500). And the reactor temperature was kept constant at 20 °C with a heat exchanger placed in the gas collection chamber. A PLC-based control panel was used to record and control the produced ozone gas and the values of pH, temperature, and dissolved ozone concentration in the reactor. The panel communicates with a computer through an RS485 interface, transmitting all data collected from the system and receiving commands from the computer. Moreover, this system can perform all operations without a computer through its touch screen as well.
Analysis
The measurements of dissolved ozone were carried out using a potentiostatic dissolved ozone probe with the model number AuAu-600-OO-2–1-PG. Before starting the ozone absorption experiments, the accuracy of the ozone probe measurements was tested using the iodometric method and the concentration of ozone in the gas phase was measured using an ozone gas analyzer (Anseros, GM-6000-OEM) that utilizes non-dispersive ultraviolet (NDUV) absorption technology.
Modeling of the Mass Transfer
Different mathematical models have been developed and experimental studies have been conducted to determine the ozone mass transfer performance of many contactors in the literature [12, 23, 28,29,30,31]. Two film theories were used in this study to model the ozone mass transfer. Equation 1 was used to calculate the KLa of ozone gas. Since the spreading power of ozone gas in the gas phase is much higher than in the liquid phase, the resistance to mass transfer in the gas phase is negligible. Since ozone gas is a gas with low solubility in water, the gas phase resistance is neglected [32,33,34]. Thus, the KLa of ozone in the liquid phase can be calculated using Eq. 1;
where C represents the dissolved ozone concentration (mg/L), CS represents the saturation concentration of ozone (mg/L), KLa represents the volumetric mass transfer coefficient, and kd represents the self-decomposition constant of ozone. The kd value can be determined based on the decrease in dissolved ozone over time when ozone gas is introduced into the environment. The decomposition constant of ozone gas, which is known to decrease at a first-order rate, is calculated using Eq. 2.
The experiments conducted to determine the mass transfer coefficients in this study, pure water was used due to difficulties in determining the decay constant kd of ozone [23]. In addition, low pH conditions were used as the kd value can be neglected at low pH levels [31, 35]. Therefore, Eq. 3 can be used to calculate the KLa of ozone in JLR.
Results and Discussion
The Effect of Ozone Gas Concentration on Mass Transfer Coefficient
The reactor temperature was kept constant at 20 ℃ and the jet loop reactor was operated at a circulation flow rate of 60 L/min during the experiments. The nozzle diameter used was 15.5 mm and the dry air-flow rate sent to the ozone generator for ozone gas production was 250 L/h. The ozone gas concentrations sent to the JLR were obtained by operating the ozone generator at different capacities (Fig. 2). Figure 2a shows the time evolution of the average ozone gas concentrations obtained by operating the ozone generator at different capacities. It can be said that the ozone gas concentration produced by the ozone generator remained constant during the experiment depending on the capacity of the ozone generator. The average ozone gas concentration sent to the reactor was obtained by taking the average of these values measured over time (Fig. 2b). For example, the ozone gas value of 3.83 g/m3 is the average value obtained by operating the ozone generator at 10% capacity. The average ozone gas concentrations produced by increasing the capacity of the ozone generator to 20%, 40%, 60%, 80%, and 100% are 8.97, 13.6, 14.8, 17.1, and 16.2 g/m3, respectively. Increasing the capacity results in an increase in the voltage used by the generator. This results in an increase in the gas ozone concentration [36]. Although the capacity of the ozone generator is increased linearly, the concentration of ozone gas produced does not increase linearly. As seen in Fig. 2, the rate of increase slows down, especially after 40% operating capacity. This can be explained by the insufficient concentration of oxygen in the dry air used for ozone production or by the formation of nitrogen oxides due to nitrogen in the dry air. Therefore, the use of pure oxygen is recommended for higher ozone gas concentrations [36].
The changes in dissolved ozone values over time for different concentrations of ozone gas sent to JLR are shown in Fig. 3, where the KLa was calculated using a non-linear method with Eq. 3, based on the time variation of dissolved ozone concentration given in Fig. 3a. The variation of KLa values calculated with ozone gas given to JLR at constant flow rate and different concentrations is given in Fig. 3b. When Fig. 3b is examined, it is clearly seen that the ozone gas concentration does not have an effect on the KLa value. The expected result, which explains the dominant factor in mass transfer with the increase in the liquid–gas interface, is that the KLa s are 28.5, 30.0, 29.0, 32.4, 25.3, and 27.3 h−1, respectively. Therefore, when the gas flow rate is constant, the interface does not change, and the mass transfer does not increase [23]. The equilibrium concentration of ozone in the liquid phase increases with increasing gas ozone concentration. The equilibrium concentration of ozone in the liquid phase due to increasing ozone gas concentration is 3.1, 6.9, 11.2, 12.0, 11.0, and 10.7 mg/L, respectively. According to Henry’s Law, an increase in gas phase concentration causes an increase in the concentration of dissolved ozone in the liquid phase under stable conditions. This occurs due to the high concentration of gas phase ozone resulting in an increase in the equilibrium concentration of dissolved ozone in the liquid [12, 26, 28]. Kukuzaki et al. (2010) reported that increasing gas ozone concentrations from approximately 1 mol/m3 to 4 mol/m3 raised the equilibrium concentrations in the liquid phase from 0.1 mol/m3 to 0.5 mol/m3. Furthermore, it has been reported that increasing ozone gas concentrations did not change the mass transfer coefficient [23]. Al-Abduly et al. (2014) reported that investigating ozone mass transfer in an oscillatory bed reactor, increasing the ozone gas concentration from 11.3 mg/dm3 to 33.3 mg/dm3 resulted in an increase in the ozone equilibrium concentration from 3.34 mg/dm3 to 9.39 mg/dm3. However, they found that the increased ozone gas concentrations did not affect KLa and Henry’s constant [21].
The Effect of Gas Flow Rate on the Mass Transfer Coefficient
Ozone gas was sent to the reactor at flow rates of 50, 100, 150, and 250 L/h through a flow meter located in front of the ozone generator to investigate the effect of gas flow rate on ozone mass transfer in JLR. During the experiments, the reactor temperature was kept constant at 20 ℃, and the JLR circulation flow rate was maintained at 60 L/min. The ozone generator was operated at 20% capacity, and the diameter of the nozzle used for the experiments was 15.5 mm. The variation in the measured average gas ozone concentrations is shown in Fig. 4. The ozone concentrations produced at gas flow rates of 50 and 100 L/h are approximately the same, at 12.8 and 12.7 g/m3, respectively. The ozone gas concentrations produced with an increase in the input gas flow rate to 150 and 250 L/h have decreased to 11.3 and 7.90 g/m3, respectively. A decrease in the input gas flow rate means an increase in the residence time of the gas in the electrical field inside the ozone generator. This results in an increase in the produced ozone gas concentration [36, 37].
The changes in calculated KLa values and dissolved ozone concentrations at different gas flow rates are shown in Fig. 5a shows that higher gas flow rates decrease the time required to reach the equilibrium concentration of dissolved ozone in JLR. As the gas flow rate increases, the concentration of ozone in the gas phase decreases (Fig. 4), which also affects the equilibrium concentrations in the liquid phase. Figure 5b clearly shows that increasing the input gas flow rate increases the KLa values. When the gas flow rate is increased from 50 L/h to 250 L/h, the KLa values increase from 6.1 h−1 to 30.0 h−1. The increase in gas flow rate increases turbulence in both gas and liquid phases, which leads to an increase in interfacial area and consequently an increase in mass transfer [18, 30, 33, 38,39,40]. In the literature, studies investigating ozone mass transfer have reported an increase in mass transfer coefficients with an increase in ozone gas flow rates. Graça et al. (2020) investigated ozone mass transfer with an oscillatory flow reactor and reported that when the gas flow rate was increased from 60 Ncm3/min to 150 Ncm3/min, the KLa value increased by 4.07 min−1 [30]. Gao et al. (2022) conducted experiments with varying reactor configurations for ozone mass transfer using a rotating bar reactor. In each configuration, increasing gas flow rates (20–100 L/h) increased KLa values (0.001–0.0045 s−1) [22]. The saturation concentrations of ozone in the liquid phase are calculated to be 9.2, 9.3, 8.5, and 6.9 mg/L at gas flow rates of 50, 100, 150, and 250 L/h, respectively. Decreasing the input gas flow rate increases the concentration of ozone in the gas phase, which leads to an increase in the saturation ozone values [12, 23, 39].
The Effect of Circulation Flow Rate and Nozzle Diameter on Mass Transfer Coefficient
Liquid drawn from the upper gas collection chamber of the JLR is sprayed into a draft tube through a nozzle at a predetermined flow rate from the main circulation line. As the flow rate increases (due to an increase in liquid velocity through the nozzle), it is known that mass transfer also increases. This is caused by the kinetic energy of the liquid, which creates smaller bubbles of gas from the same source, leading to an increase in gas–liquid interfacial area. Thus, the transfer surface area increases, increasing mass transfer coefficients. In addition, the increased liquid velocities will induce the formation of a turbulent regime in the fluid flow, further enhancing mass transfer coefficients [30]. However, increasing the liquid circulation rate is not always desirable because it also means an increase in the power transferred per unit volume of the reactor [20, 25,26,27]. Therefore, experiments were conducted at JLR to determine the effect of liquid circulation flow rate on the mass transfer coefficient and equilibrium concentration of dissolved ozone (Fig. 6). The nozzle diameter used in the experiments was 15.5 mm. During the experiments, the reactor temperature was 20 ℃, the dry air-flow rate sent to the ozone generator for ozone gas production was 250 L/h, and the ozone generator operated at 20% of its capacity. In Fig. 6a, the change in dissolved ozone concentration over time is shown for experiments conducted at different liquid circulation rates in the JLR. As seen in Fig. 6a, increasing liquid circulation rates increased KLa values of 26.5, 30.0, and 37.3 h−1, respectively. Increasing liquid circulation rates create strong turbulence, leading to an increase in interfacial area and KLa [40]. The equilibrium dissolved ozone concentration values also increased with increasing circulation rates, reaching 6.6, 6.9, and 7.3 mg/L, respectively. As noted by Fadavi and Chisti, co-injection of gas and liquid enhances turbulence and reduces bubble size [41]. Therefore, higher liquid circulation rates indicate that more power is delivered to the gas by mixing and resulting in smaller bubble sizes. According to the Young–Laplace equation, the internal pressure of a smaller bubble will be higher. As a result, an increase in the internal pressure of a bubble improves the equilibrium concentration of ozone in the liquid phase according to Henry’s law [39, 42]. In studies examining ozone mass transfer in the literature, an increase in liquid circulation has been reported to increase mass transfer coefficients. Gao et al. (2022) investigated ozone mass transfer using a rotating bar reactor, was reported that increasing liquid circulation rates (20–50 L/h) also led to an increase in KLa values. They reported that an increase in liquid flow rate has influenced the degree of liquid turbulence in the reactor, thereby increasing surface renewal efficiency, enhancing the driving force, and reducing mass transfer resistance [18]. Graça et al. (2020) study to determine the mass transfer coefficient of ozone, and conducted experiments for liquid flow rates ranging from 60 to 180 mL/min. They reported an increase in liquid flow rate to 180 mL/min resulting in an increase in KLa values from 0.20 to 0.35 min−1 for the packed bed column and from 0.11 to 0.22 min−1 for the bubble column [30].
The amount of energy (E) transferred to the JLR system is measured as the kinetic energy of the liquid velocity at the nozzle’s type and calculated using Eq. 4 [43]:
where U is the velocity of the fluid passing through the nozzle, ρL is the density of the fluid, ΔP is the pressure drop in the nozzle (converted into kinetic energy), and QL is the liquid circulation flow rate.
According to Eq. 4, increasing the liquid circulation velocity leads to an increase in the power transferred to the reactor per unit volume. The highest achievable liquid velocity using a nozzle with a diameter of 15.5 mm is 8.46 m/s. To achieve higher liquid velocities, it is necessary to decrease the nozzle diameter. To this end, experiments were carried out using two nozzles with diameters of 10 mm and 8.8 mm. The maximum circulation flow rates that can be achieved with the two different nozzles, as well as the changes in nozzle liquid velocities, calculated power transferred per unit volume, and KLa values are given in Table 1. It can be seen from Table 1 that the power consumption per unit volume increases with an increase in nozzle velocities. However, when examining the KLa values, it can be observed that there is no increase in KLa values after a power consumption of 2.65 kW/m3; rather, they decrease. The decrease in KLa values is thought to be due to changes in the flow regime, the coalescence of ozone gas bubbles due to high kinetic energy, and a decrease in circulation around the draft tube due to high velocity. The decrease in the number of ozone gas bubbles entering the draft tube due to the increased liquid jet velocity also leads to a decrease in KLa values. Therefore, increasing the liquid circulation velocity does not always lead to a desirable situation, as it results in an increase in the power transferred per unit volume to the reactor.
Comparison with Other Reactors
The comparison of calculated KLa values with some reactors in the literature are given in Table 2. For this comparison attempted in Table 2, KLa values of all studies were corrected for a 100 L reactor. This is because the transferred liquid volume must be taken into account in the mass transfer coefficient. The term “a” in KLa represents the specific surface area, expressed as A/V, defined as the gas and liquid contact surface area per unit reactor volume. Thus, increasing the contact surface area (A) and decreasing the liquid volume (V) increases the KLa value. To take this into account during comparison, this situation was attempted to be considered. In addition, the power consumption per unit volume must also be included in this evaluation. In mass transfer studies, how much energy is spent per unit mass of gas transferred to water is more important. Therefore, the most accurate way to compare is to compare based on the amount of gas transferred to water per unit of energy spent. However, since energy consumption is not given in most of the literature, the comparison could not be made from an energy consumption perspective.
The mass transfer performance of the reactors is evaluated according to the amount of gas transfer per unit of power consumption. For example, the capacity of oxygenation devices is compared in terms of kgO2/kWh. The amount of dissolved gas in the reactor per unit of energy consumed, which includes volume, mass transfer coefficient, and gas concentrations, is a term commonly used for comparison. However, for reactors used for ozonation, the mass of ozone gas that can be transferred per energy consumption is not provided, so in this study, only the corrected KLa values for a 100 L volume are compared. When Table 2 is examined, the JLR corrected KLa value for 100 L used in this study is considered to perform well, with a range of 1–6.7 h−1. In addition to its high mass transfer performance, JLR is also seen as advantageous in ozonation processes due to its simple architectural structure, suitability for continuous operation, compatibility with both pilot and full-scale installations, lack of clogging problems, no need for additional mixers, and ease of operation.
Conclusions
The parameters such as the concentration of ozone gas, gas flow rate, nozzle diameter, and circulation flow rate were examined to determine the ozone mass transfer performance of the jet loop reactor. It was determined that different ozone gas concentrations did not affect KLa values, but dissolved ozone saturation values increased with increasing gas concentration. There was a linear relationship between the gas flow rate was sent to the JLR and KLa values. Two different situations were studied to determine the effect of liquid circulation flow rates in the experiments. In the first, different liquid circulation flow rates were studied with the same nozzle (15.5 mm), and KLa values increased from 26.5 h−1 to 37.3 h−1 with liquid circulation flow rates ranging from 40 L/min to 80 L/min. In this process where the KLa value increased by 41%, the power consumed per unit reactor volume (E/V) increased by approximately 8 times to reach a value of 2.65 kW/m3. In the second part, the effect of different liquid velocities obtained with different nozzles were examined. The KLa values decreased due to reasons such as decreasing in the number of bubbles entering the cycle and the coalescence of bubbles due to the increase in liquid and gas velocities around the draft tube at these liquid velocities. As a result of comparing the obtained KLa values with other ozonation systems in the literature, it was observed that the Jet loop reactor showed a good performance that could be considered as an effective tool for future ozonation studies.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- C:
-
Dissolved ozone concentration mg/L
- Cs :
-
Saturation ozone concentration mg/L
- KLa:
-
Volumetric mass transfer coefficient h−1
- kd :
-
The self-decomposition constant of ozone h−1
- E:
-
The amount of energy kW
- U:
-
Velocity of the fluid m/s
- ρL :
-
Density of the fluid kg/m3
- ΔP:
-
The pressure drop
- QL :
-
The liquid circulation circulation flow rate L/min
- A/V:
-
The specific surface area m−1
- A:
-
Surface area m2
- V:
-
Volume m3
References
M. Priyadarshini, I. Das, M.M. Ghangrekar, L. Blaney, J. Environ. Manage. 316, 115295 (2022)
S. Lim, J.L. Shi, U. von Gunten, D.L. McCurry, Water Res. 213, 118053 (2022)
P.H. Presumido, R. Montes, J.B. Quintana, R. Rodil, M. Feliciano, G.L. Puma, A.I. Gomes, V.J.P. Vilar, J. Environ. Chem. Eng. 10, 108671 (2022)
Y. Amini, J. Karimi-Sabet, M. Nasr Esfahany, Can. J. Chem. Eng. 95, 535 (2016)
L. Tang, J. Huang, C. Zhuang, S. Zhou, L. Sun, H. Lu, Chem. Eng. J. 476, 146518 (2023)
X. Wang, W. Ping, A.S. Al-Shati, Eng. Appl. Artif. Intell. 123, 106380 (2023)
S. Li, Y. Yang, H. Zheng, Y. Zheng, T. Jing, J. Ma, J. Nan, Y.K. Leong, J.S. Chang, Chemosphere 297, 134214 (2022)
E. Bein, I. Zucker, J.E. Drewes, U. Hübner, Chem. Eng. J. 413, 127393 (2021)
J. Wang, H. Chen, Sci. Total. Environ. 704, 135249 (2020)
Y. Amini, J. Karimi-Sabet, M. Nasr Esfahany, M. Haghshenasfard, A. Dastbaz, Sep. Sci. Technol. 54, 2706 (2019)
Y. Liu, B. Wang, D. Zhao, W. Jin, F. Xu, Y. Gao, W. Shi, H. Ren, J. Environ. Chem. Eng. 11, 110805 (2023)
B. Wang, W. Shi, H. Zhang, H. Ren, M. Xiong, J. Environ. Chem. Eng. 9, 106115 (2021)
G. Tiwari, P. Bose, Chem. Eng. J. 132, 215 (2007)
C. Wu, W. Chen, Z. Gu, Q. Li, Sci. Total. Environ. 762, 143131 (2021)
S.N. Malik, P.C. Ghosh, A.N. Vaidya, S.N. Mudliar, J. Water Process Eng. 35, 101193 (2020)
M. Sung, C.P. Huang, J. Hazard. Mater. 141, 140 (2007)
X. Yang, Z. Liu, D. Manhaeghe, Y. Yang, J. Hogie, K. Demeestere, S.W.H. Van Hulle, Chemosphere 283, 131217 (2021)
H.L. Gao, Z.N. Wen, B.C. Sun, H.K. Zou, G.W. Chu, Chem. Eng. Process. 176, 108946 (2022)
B. Wang, X. Xiong, Y. Shui, Z. Huang, K. Tian, Chem. Eng. J. 357, 678 (2019)
M.S. Barlak, N. Değermenci, I. Cengiz, H. Ucun Özel, E. Yildiz, J. Environ. Chem. Eng. 8, 104402 (2020)
A. Al-Abduly, P. Christensen, A. Harvey, K. Zahng, Chem. Eng. Process. 84, 82 (2014)
G. Boczkaj, A. Fernandes, Chem. Eng. J. 320, 608 (2017)
M. Kukuzaki, K. Fujimoto, S. Kai, K. Ohe, T. Oshima, Y. Baba, Sep. Purif. Technol. 72, 347 (2010)
A. Mahyar, H. Miessner, S. Mueller, D. Moeller, Chem. Eng. Res. Des. 121, 287 (2017)
M. Petruccioli, J. Cardoso Duarte, A. Eusebio, F. Federici, Process Biochem. 37, 821 (2002)
E. Yildiz, B. Keskinler, T. Pekdemir, G. Akay, A. Nuhoǧlu, Chem. Eng. Sci. 60, 1103 (2005)
N. Değermenci, İ Cengiz, E. Yildiz, A. Nuhoglu, J. Environ. Manage. 184, 441 (2016)
M. Sabelfeld, S.U. Geißen, J. Memb. Sci. 574, 222 (2019)
A.K. Biń, B. Duczmal, P. Machniewski, Chem. Eng. Sci. 56, 6233 (2001)
C.A.L. Graça, R.B. Lima, M.F.R. Pereira, A.M.T. Silva, A. Ferreira, Chem. Eng. J. 389, 124412 (2020)
A.K. Biń, Exp. Therm. Fluid Sci. 28, 395 (2004)
C. Tizaoui, Y. Zhang, Chem. Eng. J. 162, 557 (2010)
M.T. Gao, M. Hirata, H. Takanashi, T. Hano, Sep. Purif. Technol. 42, 145 (2005)
E. Cogo, J. Albet, G. Malmary, C. Coste, J. Molinier, Chem. Eng. J. 73, 23 (1999)
K.K. Panda, A.P. Mathews, J. Environ. Eng. 134, 860 (2008)
A.W. Kinandana, E. Yulianto, A.D. Prakoso, A. Faruq, A. Qusnudin, M. Hendra, E. Sasmita, M. Restiwijaya, S.H. Pratiwi, F. Arianto, M. Nur, J. Phys. Conf. Ser. 1217, 012010 (2019)
Z. Buntat, I.R. Smith, N.A.M. Razali, J. Phys. D Appl. Phys. 42, 235202 (2009)
C.A. Jakubowski, B.W. Atkinson, P. Dennis, G.M. Evans, Ozone Sci. Eng. 25, 1 (2003)
Y.C. Yang, S.S. Zeng, Y. Ouyang, L. Sang, S.Y. Yang, X.Q. Zhang, Y.Y. Huang, J. Ye, M.T. Xiao, N. Zhang, Sep. Purif. Technol. 257, 117909 (2021)
T. Liu, Y. Liu, D. Wang, Y. Li, L. Shao, Chem. Eng. Res. Des. 152, 38 (2019)
A. Fadavi, Y. Chisti, Chem. Eng. J. 131, 105 (2007)
T. Zheng, Q. Wang, T. Zhang, Z. Shi, Y. Tian, S. Shi, N. Smale, J. Wang, J. Hazard. Mater. 287, 412 (2015)
B. Farizoglu, B. Keskinler, Chem. Eng. J. 133, 293 (2007)
J.A. Rhim, J.H. Yoon, Korean J. Chem. Eng. 22, 201 (2005)
O. Chedeville, M. Debacq, M. Ferrante Almanza, C. Porte, Sep. Purif. Technol. 57, 201 (2007)
Acknowledgements
The authors are grateful for the financial support provided by the Scientific and Technological Research Council of Türkiye (TUBITAK- Project No: 107Y298).
Author information
Authors and Affiliations
Contributions
IC: conceptualization, methodology, writing—original draft, investigation. ND: conceptualization, methodology, investigation, writing—original draft. EY: supervision, conceptualization, writing—review and editing, project administration, funding acquisition, resources. MSB: writing—original draft, investigation.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cengiz, I., Değermenci, N., Yildiz, E. et al. Investigation of Mass Transfer of Ozone in Jet Loop Reactor. Korean J. Chem. Eng. 41, 1045–1053 (2024). https://doi.org/10.1007/s11814-024-00147-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00147-9