Abstract
Utilization of the biomass with simple preparation method is one of the keys to prepare cost-effective photocatalysts. Photo-catalysts such as oxidized activated carbon (OAC), Ni-, Co- doped OAC (MOAC) were prepared from bio-waste Calophyllum inophyllum (punnai) shells via physico-chemical activation. The characterization of photo-catalysts using SEM-EDAX, XRD and FT-IR analysis. The band-gap energy of photo-catalysts and their photo-catalytic activity were determined by DRS and PL analysis, and the surface morphology of the catalysts was confirmed using SEM-EDAX analysis. The insertion of metals on the surface of the OAC and the effective functional groups of the catalysts were confirmed by the FT-IR analysis. The photo-catalytic degradation of Rhodamine-B (Rh-B) was performed using prepared photo-catalysts under 16-W low-pressure mercury lamp-UV-light exposure. To attain maximum efficiency, experimental parameters were optimized, such as solution pH (8), exposure time (100 min), photo-catalyst dosage (150 mg), and initial dye conc. (30 mg/L). Ni- and Co-doped OAC have achieved higher degradation efficiency (91.99% for NOAC and 97.28% for COAC) than OAC (35.55%) because of metal dopants acted as semiconductors, which play a vital role in dye-degradation process. Kinetics and isotherm parameters were evaluated using the effects of exposure time and initial dye conc. experimental data, respectively. Based on the experimental result, a degradation mechanism has been predicted. Metal-doped OAC is an efficient material for degradation of dye-molecules from polluted-water due to its active adsorption sites. The formation of super oxide anion radicle to produce reactive hydroxyl radicles on metal surface, which enhanced the catalytic activity on the surface of the catalysts in the process of dye-degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural resources such as air, water, and soil are crucial amenities to all living organisms and could be indiscriminately polluted by human activities via the discharge of harmful toxins or pollutants into the natural environment. The toxic pollutants damaged the ecological functions in the ecosystem, which creates an ecological imbalance between the biotic and abiotic components. In the last few decades, the discharge of toxic pollutants, especially dyes and heavy metals, into water resources has become a major issue in the environment. Water quality parameters (colour, odour, pH, taste, temperature, and conductivity) are disturbed by the discharging of pollutants such as inorganic and organic pigments into the water bodies from the textile industries. In recent modern developments in the textile and paper industries, many colour variations are used for the process of dying to produce textile products that are in demand, which leads to excess water consumption. After the dying process, a huge amount of polluted water is produced by the textile industry. Therefore, the treatment of industrially polluted water is an essential requirement to decant the toxic pollutants from the polluted water and achieve sustainable health. Especially Rh-B is a carcinogen agent for human health because consumption of such water could be bio-magnified the dye molecules in the tissue of the organism and damage the reproductive systems in the organs of the human body as well as those of other animals [1,2,3]
To overcome these issues, one of the most effective methods is required to decant the Rh-B from the wastewater. Many researchers and industrialists have developed different technologies to decant pollutants from wastewater, such as bio-catalytic degradation, photo-catalytic degradation, aqua-sonolysis, metal–organic frameworks, microbial degradation, separation through membranes, and adsorption [4,5,6,7,8,9,10]. Furtherly, heterogeneous catalysis involves the use of catalysts in a different phase from the reactants, often solid catalysts in gaseous or liquid reactions. This approach offers easy catalyst recovery and reuse, making it cost-effective and environmentally friendly. Within these techniques, photo-catalytic degradation technology is an effective method to decant pollutants such as dyes and metal ions in an solution medium by using photo-catalysts such as non-metal (g-C3N4), metal oxides (MoS2, TiO2), metal-doped metal oxides loaded activated carbon (Ag-TiO2-AC), Metal/porous carbon composites nanomaterials (BiOX), aerogels (BiPO4-graphene) [11,12,13,14,15,16,17] etc. through UV lig ht, visible light, a nd sun light.
Furthermore, because of their affordability, biomass-based materials [18, 19] are frequently employed in the wastewater treatment in industries. They are capable to efficiently absorbing a wide range of contaminants, such as metals, dyes, and chemical compounds, from contaminated water. However, photocatalysis is better than adsorption in several ways. By using catalysts that can be activated by light source that is photocatalysis can converts pollutants into less toxic forms. So, photocatalysis is a promising choice for water purification because it has advantages over adsorption, including entirety pollutant degradation, the ability to target a wider range of contaminants, and catalyst regeneration but adsorbed adsorbents become solid waste in bulk in adsorption technology, which may pollute land. The implementation of thermal activation of biomass is a better route to achieve mesoporous and microporous structures in the facet of the activated carbons. Improving the properties of the material occurs through thermal activation at elevated temperatures (400–600 °C and 600–800 °C). Few researchers are focusing on developing metal-doped activated carbon [20, 21] photo-catalysts from biomass because of their recyclability and enhanced catalytic degradation activity. Through metal loading and doping on activated carbon, AC is a useful semiconductor material for eliminating organic pollutants. It also can lower band gap energy and enhance photocatalytic performance [22]. Oxidized-AC and metal-doped oxide-AC have significantly higher efficiency compared to bare activated carbon in degradation of pollutants in polluted water [23]
Taking this into consideration, the textile sector faces a significant problem with water pollution caused by dye molecules contaminated during processing and washing. This problem is being solved by applying photocatalysts via photo-degradation technology for effective wastewater treatment. Calophyllum inophyllum (Punnai), is a plant with high oil content in its seeds and in seed’s shells also, making it a potential for oils and as biofuels. The plant’s seed’s shells wasting during oil processing, which can be utilized as a carbon source for activated carbon production, providing an opportunity for manure utilization [24]. In this article, by utilizing Calophyllum inophyllum to prepare activated carbon (AC) and photo-catalysts were prepared through physico-chemical activation process. By acid activation of AC to obtain oxidized activated carbon (OAC). Ni and Co are considered excellent dopants among various transition metal ions, such as Ni+2, Zn+2, Nd+3, Fe+2, Co+2, and Zr+2, due to their crystalline nature and high surface area. Ni ions can enhance the activity of photocatalysts used for breaking down organic effluents, while Co ions are effective in improving the photocatalytic activity of metal oxide semiconductors [25, 26]. Therefore, metals (Ni and Co) doped to the OAC to attain metal doped oxidized activated carbon (NOAC and COAC), which were utilised as photo-catalysts. The photo-catalytic performance of photo-catalysts in degradation of Rh-B dye-molecules followed by adsorption was studied using UV-analysis of the final solution. SEM-EDAX, FTIR, XRD, PL (Photoluminescence), UV-DRS (diffusion-reflectance spectroscopy) were utilized to characterise the photo-catalysts. The catalytic performance of photo-catalysts on Rh-B was investigated through a photo-catalytic reactor, and degradation efficiency was calculated.
Materials and Methods
Required Materials
Rhodamine-B (Rh-B) (Fig. 1) and deionized water (DIW) were obtained from Vijaya-Scientific Company, India. NaOH, HCl, ethanol, H2SO4, Co (NO3)2.6H2O, and NiSO4 were brought from Thermo Fisher Scientific, India. Naturally obtained bio-waste; Calophyllum inophyllum (punnai) dried seed shells converted to activated carbons (AC), oxidized AC, and Co- and Ni-doped oxidized AC. A 1000 ppm solution of Rh-B (stock solution) is prepared and diluted to appropriate conc. before being used as a polluted solution.
Photo-Catalysts Preparation from Biomass
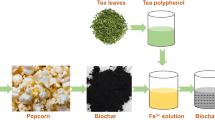
Calophyllum inophyllum (punnai) seed shells were collected and dried well over two days in sunlight after being washed with DIW. The shells were activated by a physico-chemical activation method. The dried shells were heated at 600 ℃ for 1 h in a muffle furnace to get bare activated carbons (AC). AC washed using DIW, treated with 20% H2SO4 for 12 h to achieve oxidized AC (OAC), and washed by DIW for decant excess acid present on the surface of AC using the Soxhlet apparatus. The OAC carbon that was obtained dried well at 110 ℃ to decant moisture content and was stored. OAC were again treated with 0.1 M nickel sulphate (NiSO4) and cobalt nitrate (Co (NO3)2.6H2O) solutions individually and stirred for 24 h to get NOAC and COAC, respectively. They were filtered, dried up well at 150 ℃ to decant moisture content, and stored. The preparation of photo-catalysts is shown in Fig. 2. Finally, the obtained AC, OAC, NOAC, and COAC were used as photo-catalysts for degradation of Rh-B using a inbuilt lamp photo-catalytic reactor box.
Characterization of Photo-catalysts
The prepared photo-catalysts were characterized by SEM-EDAX (Philips, model: XL-30) to analyse surface morphology, XRD (BRUKER ECO D8 ADVANCE) for analysis of the crystallinity and amorphous nature of photocatalysts, FTIR (IRAffinity-1S SHIMADZU) in the range 450–4000 cm−1 using the KBr pellet method for identification of functional moieties, UV-DRS (UV-2600 SHIMADZU) to examine the reflectance and band-gap of the photo-catalysts and PL spectroscopy for analysis of re-combination rate of electron-holes. The dye (Rh-B) solution conc. after and before of the photo-catalytic event were examined using a UV–visible Spectro-photometer (Perkin-Elmer 1640) at 555 nm.
Photo-catalytic Analysis
Photo-catalytic performance of the NOAC and COAC was studied by degradation of Rh-B using UV-irradiation in inbuilt lamp photo reactor box, which includes a 16-W low-pressure mercury lamp source (16 W LPML) and an emission maximum of 254 nm. Leakage of UV light is controlled by covering it with aluminium foil to enhance the intensity of the UV light source inside box [23]. Through a conventionally modified procedure, 20 millilitres of a 30 ppm Rh-B dye solution with 100 mg of photo-catalysts (NOAC, COAC) were placed individually in the dark room overnight with continuous agitation to achieve an adsorption–desorption equilibrium condition [27]. Finally, UV irradiation takes place for 60 min at room temperature, filtered using HM 2 filter paper and analysed by a UV Spectro-photometer in the range 380–780 nm to check the final conc. of the dye solution. The final and initial colour of the dye solution analysed, and degradation efficiency were calculated using Eq. 1 [28]. Under repeated experiments, the results deviated by nearly 3%. Furthermore, the photo-catalytic performance of each catalyst was studied by other factors such as pH adjustment of the initial dye solution (20 ml, 30 ppm) by 0.4 g in 100 millilitres of sodium hydroxide solution and 0.1 molarity of hydrochloric acid solution, exposure time by taking the initial dye solution through increments of time, dosage of photo-catalysts by taking the initial dye solution with different weights of photo-catalysts, and initial dye conc. variation with photo-catalysts studied by individual experiments. The UV-photo-catalytic experimental setup is shown in Fig. 3.
where Co is the initial conc. of dye (before the photo-catalytic process) and Cf is final conc. of dye solution (after photo-catalytic process) (mg/L).
After operating under UV for photo-catalytic degradation, filtered photo-catalysts were recycled for repeatability testing of the catalyst. The catalysts were washed by ethanol, and DIW, dried using hot oven at 120 °C and used to the next tests.
Reaction kinetics
Kinetics models is crucial for determining the routes of adsorption and photo-catalytic processes. The rate of the process of adsorption of dye by photo-catalysts is described using kinetic models with varying pH and contact [29,30,31,32]. The Langmuir–Hinshelwood (LH) kinetic equation was utilized to determine the photo-catalytic degradation rate of Rh-B to determine the reaction pathway of catalysis with dye-molecules. The LH model equation is represented in Eq. 2.
where Cf and Co are the final and initial conc. of the Rh-B dye solution, respectively; k is the rate constant of the degradation of Rh-B (min−1) and time t at which the reaction takes place in minutes.
Results and Discussion
Characteristic Analysis of Photo-catalysts
The uniform flake-like structured of activated carbon (AC) with an indefinite shape presented in Fig. 4a, which is very facilitate to easy oxidation during the acid activation process. After acid activation, the roughness surface of OAC was observed and shown in the Fig. 4b. Which clearly suggest that oxidation was occurred and formed the amorphous nature on the surface of OAC. Further on metal (Ni and Co) doping process, the crystallinity and porous of the catalyst increased due the effect of metal characteristics induced on the surface of OAC is called NOAC and COAC, respectively. Which is resulted to explain that availability of porous sites (red circled) on the surface of NOAC and COAC shown in Fig. 4c and 4d, respectively. Porous sites create a large surface area on the catalyst for the Rh-B degradation followed by adsorption through photo-catalytic test under UV light. The elemental composition percentage results of the photo-catalysts were reported and shown in Fig. 4. The results confirmed the incorporation of metal ions could be enhanced the activity of the photocatalyst for the dye-degradation.
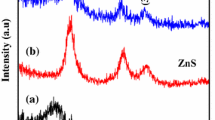
XRD plots displayed in Fig. 5. In that, the crystallinity of OAC decreases due to effect of H2SO4 activation of AC but the porous nature of OAC has been increased. In metal doping process, crystallinity increases due to crystal nature of metal precursors after metal (Ni and Co) doped on the surface of OAC. The stars mentioned in Fig. 5 about to NOAC and COAC refers to the crystallinity formed in the photocatalyst. The crystallinity surface nature of the photocatalyst may be responsible to effective in degradation of pollutants. Finally, after utilization of photocatalyst in degradation, some of the dye molecules locked in pores of photocatalyst led to increased crystallinity shown in Fig. 5 as NOAC 01 and COAC 02. Crystallinity nature of rhodamine-B was compared to reported literature [33]. The 2-theta values and nature of crystallinity of photocatalysts before and after degradation provided in Table 1.
The FTIR spectra of AC, OAC, NOAC, and COAC are displayed in Fig. 6, and their data are aligned in Table 2. From the FTIR spectrum of AC, –OH and N–H of stretching vibrations appeared at from 3600 to 3200 cm−1, and broad and sharp peaks of O–H groups, –C = C– (1528 cm−1), strong bond of –C = O (1705 cm−1), –C≡C– (2342 cm−1), –C-H (1338 cm−1) observed. After modification of AC, similar functional groups were observed along with new stretching vibrations. The FT-IR spectra of Ni and COAC show that Ni+‒O– stretching vibration occurs at 662 cm−1 (matched with reported literature) [34] and Co+‒O– bending vibration occurs at 3610 cm−1 [35] respectively (Fig. 6), which confirms presence of metal on the surface of OAC. In both NOAC and COAC, the peaks at 1549–1107 cm−1 refers to aromatic (aromatic carbon) ester bond could be connected to metal (Ni and Co). But in COAC, intensity high, means number of metal bonding to carbon aromatic bonds are high. Hydroxyl groups of photo-catalysts influenced the function and performance of the photo-catalysts by physico-chemical activation, which enhanced and facilitated the degradation process of Rh-B.
A UV–visible diffusion reflectance spectrum (UV–visible DRS) is a tool that provides a material's optical properties, electronic transitions, and band-gap energies. The diffused reflectance spectra of photo-catalysts are shown in Fig. 7. The strong absorption peak observed at 314 nm for AC, OAC, NOAC, and at 333 nm for COAC have been caused by the charge transfer from VB to CB throughout the UV region, suggests that the catalysts are photoactive materials. Due to the presence of OAC, the absorption peak of COAC occurs at 333 nm, which is shifted from 314 nm, as shown in Fig. 7a. The band-gap energy is calculated using the equation Eg = 1240/λ where, Eg is the band-gap energy calculated from wavelength to eV (hν), α is an absorption co-efficient, λ represents wavelength and bandgap calculated by using Tauc plot by extrapolating the straight portion of (αhν)2 Vs. (hν) [36]. The estimated band-gap energies are 3.41 eV, 3.26 eV, 3.11 eV, and 2.38 eV for AC, OAC, NOAC, and COAC respectively are shown in Fig. 7b. Comparing to OAC, COAC exhibited low band-gap energy and would act as semiconductor due to their rich in electron separation on the surface under photon absorption, which led to high catalytic activity. NOAC exhibited high band-gap energy due to low electron separation, which led to low catalytic activity compared to COAC. UV-DRS results shows that, the enhanced photo-absorption in the UV region benefits in degradation of dye.
Efficient generation and separation of charge carriers produced by light are crucial for photo-catalytic reactions. These processes can be studied using photoluminescence (PL) emission spectroscopy. Generally, the reduction in photoluminescence (PL) intensity attributed to the efficient generation and separation of charge carriers in the semiconductor materials. When charge carriers are efficiently separated and do not recombine as readily, the PL intensity decreases [37]. The excitation and emission wavelength are shown in Table 3. Figure 8 shows that the intensities of PL emission peaks at 422 nm and 486 nm for NOAC and COAC are significantly lower compared to AC and OAC (COAC < NOAC < OAC < AC). The modification of the band structure of the semiconductor material due to its composition or doping affected the energy levels and electronic transitions, leading to changes in the emission properties and a decrease in PL intensity. This decrease in PL intensity indicates a reduced rate of recombination, suggesting that the photo-generated electrons and holes are remaining on the surface [38] of the NOAC and COAC led to highly activity in dye degradation mechanism. The result indicates that the photo-catalyst COAC is more efficient in degrading dye molecules compared to NOAC and other catalysts.
Photo-catalytic Evaluation
First, an evaluation was conducted on the degradation of Rh-b dye solely under UV-irradiation without photocatalyst. The analysis of the UV spectrum results indicated that very little degradation occurred because no photoactive catalyst was utilized, as expected in the reaction mechanism section. Secondly, the degradation of Rh-b dye was tested in darkness with photocatalysts such as AC, OAC, NOAC, and COAC. The adsorption property and porous nature of the COAC and NOAC catalysts resulted in 63.3% and 62% removal, respectively and results are shown in Fig. 9. Under dark conditions, there was low degradation and low removal due to sufficient dye molecules covered the surface of the catalyst. Beyond this, the photo-catalytic degradation of Rh-b using prepared photo-catalysts (AC, OAC, NOAC, and COAC) was determined by a photo-catalytic reactor using 16 W LPML. After the process, the final conc. of the dye solution filtered and analysed using a UV Spectro-photometer. The photo-catalytic activity could be affected by parameters such as pH, exposure time, dosage of photo-catalyst, and initial dye conc.
Effect of pH
The investigation of the impact of pH, is crucial variable to predict the activities of the photo-catalyst in both low pH and high pH medium. 20 millilitre of 30 ppm Rh-B solution and 100 mg of photo-catalysts was used to examine the effects of a solution with a pH range of 2–12. The tests were carried out for 60 min at 28 °C in a photo-catalytic reactor using 16 W LPML and the final solution was filtered using HM 2 filter paper. The efficiency of degradation by the photo-catalyst was confirmed by the examination of the final conc. of the dye solution.
The impact of pH on photo-catalytic degradation is illustrated in Fig. 10a. Initially, very little dye-degradation was seen in the acidic setting (2 > pH). The solution pH increased along with a gradual increase in the dye-degradation percentage. The maximum dye-degradation was observed at pH 8, and thereafter there was an equilibrium or decline in the dye-degradation process. Beyond the pH 6, little bit more degradation occurred. According to the influence of pH, the presence of H+ (in an acidic medium) and OH− (in a base medium) ions changes the photo-catalytic activity. The conclusion is that H+ ions have a stronger influence on photo-catalytic activity than OH− ions. Based on the findings, when compared to metal- doped OAC (NOAC and COAC), AC and OAC showed a significantly lower percentage of Rh-B degradation, as illustrated in Fig. 10b.
Effect of Exposure Time
The effect of exposure time studies can influence the photo-catalytic activities, and the data of the experiment is used to evaluate the rate of dye-degradation. The Rh-B dye-degradation experiment was carried out using 20 ml of dye solutions (30 ppm) and 100 mg of catalysts, with solution pH (6) at 28 °C, and constant interval exposure times ranging from 10 to 100 min under 16 W LPML. The solution was taken at the prescribed intervals of 10 and 20 min, the catalysts were decanted by filtration using HM 2 filter paper, and the filtrate was used to examine the final conc. of the dye solution. Figure 11a displays the findings of the effect of exposure time dye-degradation experiment. Initially, low Rh-B degradation was obtained for up to 30 min. After 30 min, Rh-B degradation gradually increased with increasing exposure time. This experiment showed that equilibrium occurred after 60 min. After the equilibrium period of 60 min, constant Rh-B degradation was observed. The ideal contact duration in this investigation was determined to be 60 min, and the experimental data were used to examine the kinetic studies. The degradation efficiency observed at maximum condition is 91.99% and 97.28% for NOAC, and COAC, respectively, as shown in Fig. 11b. OAC exhibited lower degradation efficiency when compared to NOAC and COAC photo-catalysts. Thus, Ni and Co are doped on OAC, which effectively increase the photo-catalytic degradation efficiency. Results show that these photo-catalysts are efficient in degradation compared to previous reported photo-catalysts shown in Table 4.
Effect of Dosage of Photo-catalysts
The effect of photo-catalyst dosage studies can be used to predict standard dye-degradation. In this experiment, photo-catalyst dosage varied from 25 to 150 mg with 20 ml of a 30-ppm dye solution, a constant pH of 6. The experiment was carried out in a photo-catalytic reactor for 60 min of exposure time at 28 °C under 16 W LPML UV light irradiation. The dye employed as a photo-catalyst was decanted from the solution after the degradation process using HM 2 filter paper, and the filtrate was examined to find the final dye conc. Figure 12 depicts the impact of photo-catalyst dosage on dye deterioration.
According to Fig. 12a, an excess of adsorption as well as photo-catalytic sites resulted from increasing the photo-catalyst dose, which increases the photo-catalytic activity with increasing the photo-catalytic dosage with constant dye-molecules present in the solution. The maximum dye-degradation was achieved with 100 mg of photo-catalyst dosage. No appreciable changes were observed in the addition dosage after 100 mg because excess of adsorption and photo-catalytic sites are present on the surface of the photo-catalysts. Therefore, the results of this experiment confirm that the maximum degradation is achieved at 100 mg catalyst dosage with constant parameters (30 ppm, 20 ml of Rh-B dye). The degradation percentage at optimized condition shown in Fig. 12b.
Effect of Dye Concentration
The impact of dye conc. studies is a crucial tool to examine the nature of the photo-catalytic degradation response. The Rh-B degradation experiment was conducted using 20 millilitres of Rh-B solutions at various conc. ranging from 25 to 200 mg per litre, 100 mg of catalysts, and a solution pH of 8 for 60 min at 28 °C under 16 W LPML UV light irradiation. The final solution filtered using HM 2 filter paper and analysed using UV spectrophotometer to find the concentration of the solution. Figure 13a depicts the experimental findings of the effect of dye conc. The highest dye-degradation percentage was obtained at low dye conc. because there were more adsorption and photo-catalytic sites on the catalyst surface and fewer dye-molecules in the solution. Due to more adsorption and photo-catalytic sites, all the dye-molecules undergo complete degradation, as shown in Fig. 13b. By increasing the Rh-B conc., the constant adsorption and photo-catalytic sites are present in the solution, which could reduce the percentage of Rh-B degradation with increasing the initial dye concentration. The maximal Rh-B degradation could be attained with a modest dye conc., according to this experimental finding.
Degradation Kinetics
Degradation kinetics is a key factor in determine the types of degradation reactions between the dye-molecules and photo-catalysts. The effect of exposure time data was used to evaluate the kinetic parameters using the empirical equation of the kinetics. In this process, there may be three different processes possible, either adsorption or photo-catalytic degradation, or adsorption followed by photo-catalytic degradation. The adsorption sites of catalysts are playing a crucial role in achieving maximum photo-catalytic degradation because they could adsorb more and more dye molecule towards the adsorption sites and transfer them to the metal surface for a fast degradation process. From which Elovich, and intra-particle diffusion pseudo-second order, pseudo-first order kinetic models [39,40,41,42] were employed to analyse the reaction kinetics. Equation 3 explains that the amount of dye-adsorbed at a time ‘t’ is called a pseudo-first order kinetic equation and demonstrated by Lagergren.
Figure 14a displays the pseudo-first order kinetic plots of log (qe – qt) Vs t. The pseudo-first order kinetic parameters k1 (constant), R2 (correlation coefficient), and qe (equilibrium adsorption capacity) were derived from the slope and intercept of the plots using the empirical Eq. (4). The obtained values are listed in Table 4. The experimental data R2 values are found to be 0.88, and 0.89 and qe values are found to be 340 mg/g, 337 mg/g for NOAC and COAC, respectively. The observed qe value and the estimated pseudo-first-order kinetic qe value did not match, and the pseudo-second-order kinetic qe value had a higher R2 value.
Pseudo-second order kinetic equation is represented in the Eq. 5.
The pseudo-second order kinetic parameter: rate constant k2 (g mg−1 min−1), R2 (correlation coefficient), and equilibrium adsorption capacity (qe), which were calculated using the plot of t/qt Vs. t and shown in Fig. 14b. The derived values are listed in Table 4. The obtained R2 values for NOAC and COAC are 0.972, 0.976, and qe values are 303 mg/g, 333 mg/g respectively and nearly fit well to experimental data, which indicates that the degradation of dyes on the surface of photo-catalysts is dependent on dye-molecules as well as the photo-catalysts. The linear form of second order kinetic equation after integration is represented as Eq. 6
The Elovich-kinetic model, which is described in Eq. 7, was applied to the experimental data.
The Elovich kinetic plot was drawn using qt on the y-axis and ln (t) on the x-axis were displayed in Fig. 14c. The Elovich kinetic model parameters such as α, β, R2 which were calculated from the slope and intercept using empirical Eq. (7), and the obtained values are listed in Table 4. The initial adsorption rates (α) are 7.56, and 8.73 mgg−1 min for NOAC and COAC, respectively, which results that the adsorption process is a chemi-sorption by photo-catalysts. The surface coverage activation energies (β) are 1.44, 1.51 (gram per milligram) and the R2 values are 0.9297, 0.9471 for NOAC, COAC respectively. The whole results of the Elovich kinetic model were used to describe the heterogeneous nature of photo-catalysts. α and β values of the Elovich kinetic model explain the presence of functional groups and active sites of the photo-catalyst and influence of dye-molecules in photo-catalytic degradation process.
Intra-particle diffusion model is derived by Weber and Morris and represented in Eq. 8.
The intra-particle diffusion kinetic parameters such as rate constant (kp), R2 and C were calculated from the plot of t1/2 Vs. qt, the results shown in Fig. 14d, and the derived values are listed in Table 4. The degradation kinetics results show by photo-degradation of dye using NOAC and COAC catalysts is feasible [43] and the photo-catalytic degradation could be explored by three processes. In the first process, photo-catalysts adsorbed dye-molecules on their exterior surfaces. In the second step, dye-molecules diffuse on the photo-catalyst surface. In the final step, degradation occurred, and equilibrium was obtained. Finally, the findings of the adsorption kinetic experiment revealed that the pseudo-second-order kinetic model is used to describe the photo-degradation process of the photo-catalyst-dye system.
Where, qe, qt, time (t), k1(min−1), k2 (g mg−1 min−1), α (milligram per gram min), β (gram per milligram), kp (mg/g min) are the quantity of dye-adsorbed on the surface of the photo-catalyst at equilibrium, amount of dye-adsorbed at time, kinetic rate constant, second order kinetic rate constant, intra-particle diffusion rate constant t. The equation integration and rearrangement yield the linear form, initial adsorption rate constant, respectively.
Adsorption Isotherms
The study of adsorption isotherm modelling is crucial to predict the adsorption behaviour of the photo-catalysts towards Rh-B molecules [44]. Effect of dye conc. results were utilised for analysis of the surface functions of the photo-catalysts using the isotherm’s parameters. In this study, Langmuir (Eqs. 9, 10), Freundlich (Eq. 11) and Temkin (Eq. 12) adsorption isotherms were evaluated by using linear empirical equations.
where R, T (°C), ‘b’, qm (mg/g−1), KF (mg g−1) (L mg−1), KL (L mg−1), n, α, ‘β (β = RT/b)’ are the gas constant, temperature and Temkin-constant (TC), maximum adsorption capacity, Freundlich constant (FC), Langmuir constant (LC), related to adsorption capacity, heterogeneity factor, is Temkin-isotherm constant, exponent, respectively.
Utilising the Langmuir-isotherm linear equation, the mono-layer ad-sorption performance of Rh-B onto photo-catalysts is determined. The obtained qe and Ce values, which are the effects of initial dye conc., were utilised to linear plot Ce/qe against Ce and displayed in Fig. 15. The linear equation (y = mx + c) was used to calculate the Langmuir-isotherm parameters calculated value which are mentioned in Table 5. When RL (separation factor) is between 0 and 1, which means that adsorption is advantageous (1 > RL < 0). Adsorption is un-favourable if RL > 1. While RL = 0 adsorption is irreversible, the RL = 1 process is linear. In present work,\({R}_{L}\) value is between 0 to 1 that is 0.110 to 0.330 and 0.109 to 0.328 for NOAC and COAC photo-catalysts, respectively. Which indicates that the favoured adsorption. The maximal mono-layer ad-sorption capability by Rh-B onto NOAC and COAC photo-catalysts was found to be 322.58 and 333 mg g−1 respectively. According to the Langmuir adsorption isotherm data, monolayer adsorption was reportedly seen on the photo-catalyst surfaces.
The Freundlich isotherm linear equation is used to explain heterogeneous surface and multilayer adsorption. The plotted of log qe Vs. Ce using Ce and qe values of the effect of dye conc. experimental data and displayed in Fig. 16. The Freundlich parameters were calculated using the straight-line equation and are mentioned in Table 5. The value of ‘n’ range 1–10 indicates favourable multi-layer adsorption. In this adsorption system, heterogeneity factor (n) was found to be 4.36 and 4.79 for NOAC and COAC photo-catalysts, which shows that the multilayer adsorption occurs on the surface of photo-catalysts and is supplied over the metal surface sites to occur degradation process.
The Temkin isotherm model is used to anticipate how the adsorbent and adsorbate interact. The plotted of qe Vs Ce using Ce and qe values of the effect dye conc. experimental data and is shown in Fig. 17. Temkin isotherm parameters such as α, β and R2 were calculated, and the values are in Table 5. The results of the adsorption isotherm model indicated that the surfaces of the photo-catalysts had both monolayer and multi-layer adsorption. When compared to published literature, the maximal adsorption capacity of prepared photo-catalysts qe is greater and is shown in Table 6.
Mechanism of Photo-catalytic Dye-Degradation
According to the above experimental results and reported literature, the expected dye-degradation mechanism was predicted. There are two different degradations that may occur during the process, one is self-photo degradation of dye-molecules, and another is catalytic degradation of dye-molecules. To elucidate the possible mechanism of Rh-B dye-degradation, the role of self-photo dye-degradation, the role of OAC in the degradation process, and the effect of metal ion (Ni and Co) dopants on OAC were discussed in detail below.
Rh-B Self-Photo-Dye-Degradation
In the self-photo dye-degradation process, Rh-B molecules undergo an electronic transition from their ground state to an excited state under UV light absorbance. This transition involves the promotion of electrons from the lower energy orbital HOMO level to the higher energy orbital LUMO level. The excited state of Rh-B is typically referred to as Rh-B* present in either a singlet or triplet state [27] resulting in the self-oxidation and formation of OH• radicles leading to self-degradation in presence aqueous dye-molecules in nature. Furthermore, the photo induced electrons could transfer from Rh-B to the conduction band (CB) of the photo-catalysts (M+(Ni (or) Co)-OAC) given Eq. 14, which results in the Rh-B* being converted into the Rh-B*+ radicle cation. Finally, Rh-B*+ reacts with OH• radicle species and instantaneously degraded to by-products but not in effective degradation occur. The possible self-photo-dye-degradation reaction mechanism is predicted and given in Eqs. 13–15.
OAC Activity in Degradation Process
The acid activated AC (OAC) was used as photo-catalysts in the degradation process. In this process, the formation of superoxide anion radicles on the surface of oxidised activated carbon is a crucial species for the degradation of Rh-B dye-molecules. The processes occur in the three steps and are explained as follows: in the first step, Rh-B dye-molecules are adsorbed on the surface of the OAC to form dye-adsorbed-OAC because a strong interaction is formed between the functional moieties present on the surface and dye-molecules. In the second step, dye-adsorbed-OAC absorbs photons from UV irradiation, which leads to excitation and transfer of electrons on the surface of the dye-adsorbed-OAC. This process produced the electron rich catalyst, electron holes (h+) in CB, and positively charged Rh-B on the surface of dye-adsorbed-OAC, which are involved to reacting with molecular oxygen to form superoxide anion radicles and hydrogen peroxide, resulting in the formation of OH• radicles [45]. In the third step, the reaction takes place between the OH• radicles [46] and Rh-B dye-molecules on the surface of the adsorbed-OAC catalyst to degrade Rh-B dye-molecules into degradation products. However, a maximum 35.55% degradation was observed based on exposure time, which is the lowest degradation in the experiment and AC also lower (27.7%) than that of OAC. The mechanism of degradation steps is predicted in Eqs. 16–20 and as shown in Fig. 18.
Effect of Metal Doped on OAC in Degradation Process
Metals such as Ni and Co were successfully doped on OAC individually. In this, Ni2+ and Co2+ ions are reduced to Ni+ and Co+ due to the formation of metal OAC because of the lone pair of electrons of oxygen present in the functional moieties of OAC is shared with metal ions, which reduces to Ni+ and Co+-OAC, respectively confirmed by the FTIR, the presence of metal connected to ester type (carbon aromatic) bonds (1549–1107 cm−1) as mentioned illustration Fig. 19 was confirmed. Doping of metal impurities could increase the photo-catalytic activity by absorption of excess photons in rich media [11]. Which enhanced the degradation behaviour of photo-catalysts when compared to bare OAC. Whereas OAC act as mediator for transfer of photo induced electrons to the metal surface to enrich the photo-catalytic activity of the photo-catalysts. The obtained band-gap energies of the photo-catalysts NOAC and COAC are 3.41 eV and 2.29 eV, respectively. Here, COAC act as semiconductors. Under illumination of UV light, photons are absorbed by the photo-catalysts, which could be activated the self-photo dye sensitization as well as the activity of OAC. This activation played a key role to achieve maximum photo-catalytic degradation on the surface of metal (M+-OAC) effectively. Through the metal surface, activity of active sites and separation of electrons in VB to the CB occur by photon absorption. OAC facilitated to reduce the re-combination rate of e−-hole pairs by acting as a mediator lead to allowing more charge carriers to participate in the photo-degradation process. The main active species are O2−•, –OH•, and h+ which helped in the degradation [22] of Rh-B dye-molecules [11, 47]. The degradation showed pseudo-second order kinetics. The plausible mechanism of Rh-B dye-degradation in the presence of photo-catalysts (M+-OAC. M+- Ni, Co) given in Eqs. 21–25 and as shown in Fig. 19.
-
1.
Photoexcitation occurs on metal surface.
$${M}^{+}-Ox-ACs+h\nu \to {M}^{+}-Ox-ACs\left({h}_{VB}^{+}+ {e}_{CB}^{-}\right).$$(21) -
2.
Iono-sorption and formation of hydrogen peroxide takes place by O2 molecule.
$${ O}_{2}+ {e}^{-}+ {2H}^{+} \to {O}_{2}^{-\cdot }+ {H}_{2}{O}_{2}.$$(22) -
3.
Oxidation of Hydroxyl ion
$$O{H}^{-}+ {h}^{+}\to {OH}^{\cdot }.$$(23) -
4.
Formation of hydroxyl radicles
$${O}_{2}^{-\cdot }+ {H}_{2}{O}_{2} \to O{H}^{-}+ {OH}^{\cdot }+{O}_{2}.$$(24) -
5.
Degradation occurs by active species.
$$Rh-B\left(*\right)+ {h}^{+} \to Rh-{B}^{\cdot +}+{OH}^{\cdot } \to \text{degradation products }\quad \left[\mathrm{12,34}\right].$$(25)
Recyclability and Reusability of Photo-catalysts
The stability of the NOAC and COAC was studied by a reusability test. In each cycle, the recycled photo-catalysts were centrifuged to separate them from the dye solution and immersed in ethanol for 120 min, washed DIW, and dried in a vacuum hot oven at 80 for 12 h [28], which is used again as photo-catalyst in dye-degradation. A very less significant percentage of dye-degradation changes were observed, which suggests that the outstanding performance and stability of the photo-catalysts (NOAC, COAC) were achieved even after five cycles. The recyclability results show that the photo-catalysts are more efficient and effective for the Rh-B dye-degradation, and they could be a promising photo-catalyst for industrial wastewater treatment. Figure 20 shows the photo-degradation performance of Rh-B on photo-catalysts for five runs under UV irradiation.
Conclusion
Our findings are summarized as follows: the effective Ni- and Co-doped OAC photo-catalysts were prepared using punnai seed shells for cost effective by the physico-chemical activation method. In SEM analysis, the porous nature of catalyst COAC confirmed which effectively worked in degradation process. The nature of the catalysts surface and effective functional groups such as –OH, C = O, C–O were confirmed by the SEM-EDAX and FT-IR analytical techniques, which suggested that porous and functional moieties could enrich the adsorption sites on the surface of the photo-catalysts. The crystallinity nature of the photocatalyst through XRD data have discussed that crystalline peaks obtained after metal induced on the surface of OAC. The band-gap energies of NOAC and COAC were found to be 3.11 eV, 2.38 eV, respectively, which suggested that Ni- and Co-doped OAC photo-catalysts could act as a semiconductor and be used as effective photo-catalysts for dye-degradation process. The experimental results of the dye-degradation process reveal that adsorption sites are a key factor in adsorbing dye-molecules towards adsorption sites, and dye-molecules could be transported to metal surface, which promotes higher dye-degradation (maximum dye-degradation was found to 97.28%) in the process. The kinetic study suggested that the experimental data well fit pseudo-second order kinetics because catalysts and dye-molecules are influenced in the degradation process. The results of isotherm models confirmed the types of adsorption process and revealed that monolayer and multilayer adsorption were obtained on the surface of the catalysts. From the Longmuir isotherm study, the maximum mono-layer adsorption capacity was found to be 322.58 mg/g for NOAC and 333 mg/g for COAC. Overall, the current study concluded that the Ni and Co doped OAC are promising photo-catalysts for the effective dye-degradation process and could be a cost effective and eco-friendly material for the wastewater treatment process.
Data Availability
The authors assure that all necessary data to support the study's findings are included in the submitted manuscript. Additional data clarifications can be obtained from the corresponding author upon reasonable request.
References
R. Jain, M. Mathur, S. Sikarwar, A. Mittal, J. Environ. Manage. 85, 956–964 (2007)
P. Qi et al., Food Chem. 164, 98–103 (2014)
T. Santhi, A.L. Prasad, S. Manonmani, Arab. J. Chem. 7, 494–503 (2014)
R. Parales, Curr. Opin. Biotechnol.. Opin. Biotechnol. 15, 374–379 (2004)
J. Lifka, B. Ondruschka, J. Hofmann, Eng. Life Sci. 3, 253–262 (2003)
M.F. Abid, M.A. Zablouk, A.M. Abid-Alameer, Iran. J. Environ. Health Sci. Eng. 9, 17 (2012)
C.-C. Wang, J.-R. Li, X.-L. Lv, Y.-Q. Zhang, G. Guo, Energy Environ. Sci. 7, 2831–2867 (2014)
A. Oren, P. Gurevich, M. Azachi, Y. Henis, Biodegradation 3, 387–398 (1992)
A. Murugesan, M. Divakaran, P. Raveendran, A.B. Nitin Nikamanth, K.J. Thelly, J. Polym. Environ.Polym. Environ 27, 1007–1024 (2019)
P. Jiang et al., An easily sedimentable and effective TiO2 photocatalyst for removal of dyes in water. Sep. Purif. Technol.Purif. Technol. 122, 128–132 (2014)
Md. Ahmaruzzaman, S.R. Mishra, Mater. Res. Bull. 143, 111417 (2021)
M. Cantarella et al., J. Photochem. Photobiol. A Chem. 321, 1–11 (2016)
M. Hasanpour, M. Hatami, Photocatalytic performance of aerogels for organic dyes removal from wastewaters: review study. J. Mol. Liq. 309, 113094 (2020)
K. Sathishkumar et al., Chemosphere 302, 134844 (2022)
Y. Yang et al., Adv. Colloid Interface Sci. 254, 76–93 (2018)
W. Yang et al., Chem. Eng. J. 421, 129720 (2021)
Y. Cao, S. Mao, M. Li, Y. Chen, Y. Wang, ACS Catal.Catal. 7, 8090–8112 (2017)
Y. Dai et al., Chemosphere 211, 235–253 (2018)
Md.T. Uddin, Md.A. Islam, S. Mahmud, Md. Rukanuzzaman, J. Hazard. Mater. 164, 53–60 (2009)
A. Taha, M. Ben Aissa, E. Da’na, Molecules 25, 1586 (2020)
B. Zhu, H. Cheng, J. Ma, Y. Kong, S. Komarneni, Chemosphere 237, 124547 (2019)
D. Thirumoolan et al., J Photochem Photobiol A Chem 448, 115300 (2024)
A. Murugesan, M. Loganathan, P. Senthil Kumar, D.-V.N. Vo, Sustain. Chem. Pharm. 21, 100406 (2021)
Fayanto, S. et al. in Proceedings of the The 1st International Conference on Computer Science and Engineering Technology Universitas Muria Kudus (EAI, 2018). https://doi.org/10.4108/eai.24-10-2018.2280629
W.W. Anku, S.O.-B. Oppong, S.K. Shukla, E.S. Agorku, P.P. Govender, Progress Nat. Sci.: Mater. Int. 26, 354–361 (2016)
A. Irshad et al., Physica B Condens Matter 636, 413873 (2022)
M. Sundararajan, V. Sailaja, L. John Kennedy, J. Judith Vijaya, Ceram. Int. 43, 540–548 (2017)
S. Maqbool et al., Environ. Sci. Pollut. Res.Pollut. Res. 30, 7121–7137 (2023)
J.-Y. Fang, L. Ling, C. Shang, Kinetics, and mechanisms of pH-dependent degradation of halonitromethanes by UV photolysis. Water Res. 47, 1257–1266 (2013)
M.A. Hossain, M. Kumita, Y. Michigami, S. Mori, J. Chem. Eng. Jpn.Jpn. 38, 402–408 (2005)
M.V. Shankar et al., Indian J. Eng. Mater. Sci. 8, 104–109 (2001)
L. Wang, R. Niu, B. Chen, L. Wang, G. Zhang, Chem. Eng. J. 330, 1326–1333 (2017)
I.S. Yahia, Y.S. Rammah, K.F. Khaled, J. Mater. Environ. Sci. 4(3), 442–447 (2013)
A.M. Azharudeen, R. Karthiga, M. Rajarajan, A. Suganthi, Arab. J. Chem. 13, 4053–4064 (2020)
G. Allaedini, A. Muhammad, J. Nanostruct. Chem. 3, 77 (2013)
A. Ahmed, M. Usman, B. Yu, Y. Shen, H. Cong, J. Mol. Liq. 339, 116729 (2021)
M. Shakil et al., Optik (Stuttg) 272, 170353 (2023)
B. Chai, J. Yan, C. Wang, Z. Ren, Y. Zhu, Appl. Surf. Sci. 391, 376–383 (2017)
S. Mallakpour, V. Behranvand, J. Clean. Prod. 190, 525–537 (2018)
R.S. Aliabadi, N.O. Mahmoodi, J. Clean. Prod. 179, 235–245 (2018)
L.A. Shah et al., Superabsorbent polymer hydrogels with good thermal and mechanical properties for removal of selected heavy metal ions. J. Clean. Prod. 201, 78–87 (2018)
M. Loganathan, A.S. Raj, A. Murugesan, P.S. Kumar, Chemosphere 304, 135332 (2022)
A. Murugesan, M. Divakaran, P. Senthilkumar, Desalin. Water Treat.. Water Treat. 122, 176–191 (2018)
O. Merka, V. Yarovyi, D.W. Bahnemann, M. Wark, J. Phys. Chem. C 115, 8014–8023 (2011)
L.F. Velasco et al., Appl. Catal. A Gen. 452, 1–8 (2013)
S. Ragupathy et al., Ceram. Int. 49, 17776–17783 (2023)
T.S. Saleh, A.K. Badawi, R.S. Salama, M.M.M. Mostafa, Materials 16, 2170 (2023)
N. Chakinala, P.R. Gogate, A.G. Chakinala, Mater. Today Proc. 43, 3066–3070 (2021)
T. Wang et al., J. Hazard. Mater. 389, 121827 (2020)
M. Lal, P. Sharma, L. Singh, C. Ram, Results Eng. 17, 100890 (2023)
L.T.T. Nguyen et al., Environ. Technol. Innov.Innov. 25, 102130 (2022)
S. Sharma, S. Basu, Appl. Mater. Today 23, 101033 (2021)
M.S. Adly, Sh.M. El-Dafrawy, S.A. El-Hakam, J. Market. Res. 8, 5610–5622 (2019)
T.K. Ngan Tran et al., Open Chem. Chem. 20, 52–60 (2022)
A.A. Yadav, S.W. Kang, Y.M. Hunge, J. Mater. Sci. Mater. Electron. 32, 15577–15585 (2021)
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
There are no conflicts to declare.
Ethical Approval
Not applicable because in the manuscript report there is no use of any animal or human data or tissue.
Informed Consent
I, as the corresponding author, take full responsibility for the submission of this paper on behalf of all co-authors. I hereby confirm that all authors mentioned on the title page have made substantial contributions to the research, have thoroughly reviewed the manuscript, and affirm the accuracy and integrity of the data and its interpretation. Furthermore, all authors consent to the submission of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Munireddy, R., Murugesan, L., Arukkani, M. et al. Studies on Effective Photo-catalytic Degradation of Rhodamine-B Using Metal-Doped Oxidized-Activated Carbon: Kinetics, Isotherm Models and Degradation Mechanism. Korean J. Chem. Eng. 41, 2183–2200 (2024). https://doi.org/10.1007/s11814-024-00145-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00145-x