Abstract
Two distinct deep eutectic solvents (DESs) were prepared by combining allyltriphenylphosphonium bromide (ATPPB) as a hydrogen bond acceptor (HBA) and ethylene glycol as a hydrogen bond donor (HBD) at molar ratios of 6:1 and 8:1, respectively. The study investigated the solubility of these DESs in supercritical carbon dioxide, with ethanol serving as a cosolvent, under varying conditions of temperatures 308.2, 313.2, and 318.2 K and pressures up to 18.89 MPa. Solubilities were determined by measuring both bubble point and cloud point pressures in ternary mixtures comprising CO2, ethanol, and DES, utilizing a phase equilibrium apparatus equipped with a high-pressure variable-volume view cell. Higher molar ratios between ethylene glycol and ATPPB resulted in significantly higher pressures required for the solubility of DES. The occurrence of either bubble point or cloud point depended on the molar ratios between the cosolvent (ethanol) and the solvent (scCO2) for both DESs. Increasing system temperature at a constant DES mole fraction led to an elevation in both bubble point and cloud point pressures. Furthermore, an increases in the amount of cosolvent (ethanol) resulted in a substantial decrease in both bubble point and cloud point pressures. The experimental data exhibited robust correlation with three models: the modified Chrastil equation, the Kumar–Johnston equation, and the Adachi–Lu equation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supercritical carbon dioxide (scCO2), deep eutectic solvents (DESs), and ionic liquids have gained significant attention in recent years due to their unique properties and versatile applications in various fields. scCO2 refers to CO2 at a temperature and pressure above its critical point. In this state, CO2 exhibits both gas and liquid-like properties, making it an environmentally friendly solvent for a wide range of substances. Researchers have been exploring the solubility of different compounds in scCO2 to develop environmentally friendly process. Unfortunately, although it can solubilize low molecular weight, volatile compounds, polar and high-molecular weight materials, CO2 is a feeble solvent, as Beckman reported [1]. Great progress has been made in disclosing hydrocarbon-based CO2-philic containing carbonyl groups, which are known to interact through a Lewis acid–Lewis base interaction with CO2 molecules [2,3,4]. In addition, phosphorus compounds, particularly phosphonium-based salts, are known to enhance CO2 solubility [5, 6].
DESs are often composed of inexpensive and readily available components of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA). This makes them more cost-effective compared to many ionic liquids, which often involve more complex and costly synthesis processes [7]. DESs, in many cases, exhibit higher biodegradability compared to ionic liquids. The use of natural or bio-derived components in DES contributes to their more environmentally friendly profile. In particular, the selection of allylphosphonium salts for preparing DESs is driven by their versatile and tunable properties, polar nature, and compatibility with green chemistry principles [8,9,10]. Dialcohols, such as, ethylene glycol, are favored for preparing DES due to hydrogen bond donor capability, tunable properties, biodegradability, and low toxicity [11,12,13].
As far as extension of scCO2 usage is concerned higher solubility of versatile chemicals can also be easily accomplished in the presence of polar cosolvent with hydrogen bond capacity. Brennecke et al.[14]. investigated the phase behavior of systems combining scCO2 with ionic liquids. The findings revealed unexpectedly high solubility of CO2 in five ionic liquids, while the solubility of ionic liquids in scCO2 was notably low, approximately on the order of 10–7. Numerous studies [15,16,17,18] have explored the impact of cosolvents on the solubility of ionic liquids in scCO2. These investigations revealed that the solubility of ionic liquids in scCO2 was remarkably low. However, the addition of cosolvent proved to be a significant factor in substantially augmenting the solubility of ionic liquids in scCO2. Although the solubility of DES in scCO2 surpasses that of ionic liquid in scCO2 [18,19,20], there remains considerable potential for improvement in the presence of a cosolvent.
Emerging computational methodologies, such as artificial intelligences [21] and group theory [22] offer the capability to predict solubility data with exceptional accuracy. However, the effective training of these models necessitates extensive and precise experimental information, which is often scarce across various temperature and pressure ranges. Consequently, empirical and semi-empirical models have proven successful in correlating solubility data with high efficacy [23,24,25,26]. These models, particularly empirical ones, offer simplicity in application, where the solubility of a substance is influenced by density, pressure, and temperature of scCO2 in the presence of a cosolvent.
The mole ratios between HBD and HBA play a crucial role in determining the formation of DES and influencing the appropriate viscosity range [19]. In this study, two DESs were formulated employing ethylene glycol as the HBD and allytriphenylphosphonium bromide as the HBA, with mole ratios specified as 6 to 1 (E6A1) and 8 to 1 (E8A1), respectively. Typically, extractions are conducted at pressures of 7–14 MPa and temperatures of 308–343 K [27]. To assess the solubility of these DESs, evaluations were carried out in scCO2 as the solvent, along with ethanol as a cosolvent. The evaluations encompassed a temperature range of 308.2 to 318.2 K at intervals of 5 K to explore temperature effects. Furthermore, the study delved into the impact of system density and mole ratio between the solvent and cosolvent on DES solubility. Experimental results were then correlated using distinct empirical models: the modified Chrastil equation [24], the Kumar–Johnston equation [25], and the Adachi–Lu equation [26].
Experimental
Chemicals and preparation of the DESs
Ethylene glycol, ethanol, and allyltriphenylphosphonium bromide (ATPPB) were purchased from Merck Inc, as shown in Table 1. The supercritical fluid chromatography grade carbon dioxide was sourced from Sebotech Inc. (Sejong, Korea). All chemicals were utilized as received without further purification. Two DESs, denoted as E6A1 and E8A1, were formulated using ethylene glycol and ATPPB with mole ratios of 6:1 and 8:1, respectively. The molecular weight of the prepared DES was calculated based on the individual compounds, as per Eq. (1).
where Mi and xi refer to the molecular weight and mole fraction of compound i. Molecular weights (g⋅mol−1) of E6A1 and E8A1 DESs are 107.95 and 97.96, respectively, as shown in Table 2. The water content in the prepared DESs was determined using a Karl-Fisher titrator (Model V20). The analysis indicated that the water content for E6A1 and E8A1 is 0.07% and 0.09%, respectively.
Experimental Apparatus and Method
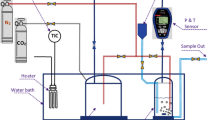
The experimental apparatus and methodology employed to ascertain the phase behavior have been previously elucidated [28,29,30]. In brief, the apparatus comprises a variable volume view cell, hand pump, syringe pump, and pressure indicator. The cell's temperature was regulated using a water bath. Initially, a fixed quantity of DES and ethanol were loaded into the view cell, replacing the air with CO2. As CO2 was compressed into the cell, the pressure varied. Solubility, in this study, was defined as the mole fraction of DES in the system. Given the negligible amount of DES, the density of CO2 and ethanol was computed using Aspen Plus with the Peng--Robinson equation of state, as shown in Table 3.
The pressure was adjusted until a visible phase change occurred. Under fixed composition and temperature, the bubble point pressure was established as the pressure at which the first bubbles appeared. Cloud points were also identified when the solution displayed cloudiness, indicating a phase transition from a single phase to liquid–liquid phases. To ensure consistency in the experimental results, each measurement was performed at least three times for each condition.
Experimental Data Fitting Model
In general, there are three primary approaches to calculate the solubility of solutes in scCO2: equation of states, solubility parameter models, and empirical models. The first two methods necessitate the determination of physical parameters for DESs, which can be challenging to precisely ascertain, resulting in a lower degree of precision. Conversely, empirical models, while not reliant on the physical parameters of DESs, provide relatively reliable correlation accuracy. In this study, three models—the modified Chrastil model [24], the Kumar–Johnston model [25], and the Adachi–Lu model [26]—were employed to correlate the DES solubility data. The models varied in the number of adjustable parameters, ranging from 3 to 5. Specifically, the Kumar–Johnston model stands out with the fewest adjustable parameters at 3. However, it overlooks the influence of cosolvent presence in the system, focusing solely on exploring the impact of solvent density and system temperature. In contrast, the modified Chrastil model takes into account the effects of solvent density, cosolvent content, and temperature on the solubility of DES with 4 parameters. Meanwhile, the Adachi–Lu model addresses the first two effects only but employs 5 parameters.
A semi-empirical model, originally proposed by Chrastil [23] to depict the relationship between solute solubility and the density of the supercritical fluid, was further refined by Gonzalez et al. [24]. This modification involves the assumption that solute molecule form complex molecule, as shown in Eq. (2)
, where y (g⋅lit−1) is the solubility of DESs, ρ (g⋅lit−1) is density of scCO2, and m (g⋅lit−1) is the concentration of the ethanol. The constant k signifies an average association number for a specific solute and supercritical solvent, while r designates an average association number for the cosolvent. a is a constant linked to the enthalpies of vaporization and solvation of the solute, and b denotes model parameter. The obtained results are presented in Table 4.
Kumar and Johnston [25] introduced an empirical model wherein the density of solvent in supercritical state is correlated with the mole fraction of the solute. The expression of the model is shown in Eq. (3)
y (g⋅lit−1) is the solubility of DESs in solvent, ρ (g⋅lit−1) is density of solvent of scCO2. c0, c1, and c2 are model parameters. The results are shown in Table 5.
In general, alternations in density did not exhibit a linear correspondence to changes in pressure. To account for this nonlinearity in density concerning pressure variations, the Adachi–Lu model [26] was also subjected to correlation. Taking into account the presence of a cosolvent, the Adachi–Lu model is expressed in Eq. (4).
, where y (g⋅lit−1) is the solubility of DESs, ρ (g⋅lit−1) is density of solvent of supercritical CO2, y* (g⋅lit−1): mass concentration of the cosolvent of ethanol. e0, e1, e2, e3, and e4 are model parameters. The results are shown in Table 6.
The effectiveness of the models was assessed using the average absolute relative deviation (AARD (%)), as indicated in Eq. (5).
, where yical represents the calculated value of the DES solubility, yiexp signifies the experimentally measured value, N denotes the number of data points, and Z represents the correlation parameters following the suggestion of Garlapati and Madras [31]. This metric represents the average deviation between experimentally determined DES solubility and that calculated by the correlation model. The parameters of the models were optimized through non-linear regression.
Results and Discussion
The bubble point and cloud point pressures for mixtures of CO2 + ethanol + DES with varying compositions were experimentally determined over a temperature range spanning from 308.2 K to 318.2 K with a 5 K interval. The experimental data for two distinct ternary systems, namely CO2 + ethanol + E6A1 and CO2 + ethanol + E8A1, are presented in Table 3. In the CO2 + ethanol + E6A1 ternary system, both bubble point pressures and cloud point pressures were observed. Specifically, when the ratios between cosolvent (ethanol) and solvent (CO2) exceeded 0.6, bubble points were observed, and when these ratios were less than 0.5, cloud points were observed. Similar phase change patterns were observed for the CO2 + ethanol + E8A1 system. However, when the ratios of cosolvent to solvent exceeded 0.5, bubble points were observed. Significantly, no phase transition pressures have been observed at these temperatures in the absences of ethanol, a cosolvent, up to 30 MPa, the experimental apparatus operating limit.
Effect of temperature on solubility
Both the bubble point and cloud point pressures increased with increasing the system temperature at a fixed DES mole fraction. This suggests that the solubility of DES in CO2 and ethanol mixture decreased at higher temperature corresponding to exothermic processes [32]. When the mole fraction of E6A1 increased isothermally, the bubble point pressure correspondingly increased slightly. For example, while the bubble point pressure of 8.01 MPa was observed for 0.040 mol fraction of E6A1, that of 7.89 MPa for 0.030 at 313.2 K. To dissolve the same amount of DESs in CO2 and ethanol mixture lower system temperature seems to be better condition. Typically, with a rise in temperature, the saturation vapor pressure of solute increases, promoting an increase in solubility [33]. However, the density of scCO2 decreases as temperatures rise, leading to a reduction in the solubility of DES. The impact of temperature is contingent on the combined effects of these two opposing factors.
As far as cloud point pressures are concerned, similar results were also observed. For instance, the cloud point pressure of 13.69 MPa at 308.2 K increased 16.21 MPa at 318.2 K at 0.040 mol fraction of E6A1. Not only the bubble point but also the cloud point pressures for E8A1 were also followed similar trends, as shown in Fig. 1.
Effect of Ratio of Cosolvent to Solvent on Solubility
As the mole ratio between ethanol and CO2 decreased, the cloud point pressure correspondingly increased significantly. For instance, at 308.2 K while the mole ratio was 0.502, the cloud point pressure was 7.65 MPa, and when the mole ratio was 0.412, it was 13.69 MPa at 308.2 K for E6A1. In case of E8A1, similar results were observed. Specifically, when the mole ratio was 0.696, the bubble point pressure was 8.26 MPa, and when the mole ratio was 0.500, it was 9.54 MPa at 318.2 K.
As the amount of cosolvent ethanol increased, the bubble point or cloud point pressure decreased significantly. This means that the solvation power of CO2 is considerably low that the addition of ethanol raises the solvation power significantly. CO2's solvent power is not strong enough to dissolve CO2-philic compounds, as Beckman reported [1]. So, it would be such a meaningful approach to add ethanol as a cosolvent to enhance the solubility of DESs.
Effect of Pressure on Solubility
In general, with an increase in pressure at constant temperature, the solubility of DESs demonstrated a corresponding increase. For instance, the solubility of E6A1 was 0.030 at 8.54 MPa, and it increased to 0.040 at 8.84 MPa at 318.2 K. The pressure effect can be attributed to the rising values of the variable leading to an increases in fluid density, consequently enhancing the solvent power of scCO2. The solvent densities at 8.54 MPa and 318.2 K, and 8.84 MPa and 318.2 K were 0.7002 and 0.7723 g⋅cm−3, respectively. However, the impact of density alone is not sufficient to elucidate the increase in DES solubility. A plausible explanation for this nonlinear dependence on solvent density on DES solubility could be that the density of scCO2 and the ethanol solvent converges toward the liquid phase with increasing pressure. However, as the compressibility of scCO2 diminishes when it approaches the liquid phase, the density of scCO2 and the ethanol solvent experiences a slight increase rising pressure in higher pressure ranges, as shown in Table 3.
Data Correlation
In this study, the experimental data were correlated with three models: the modified Chrastil equation, the Kumar–Johnston equation, and the Adachi–Lu equation, as briefly outlined in Sect. "Experimental data fitting model". Tables 4, 5, 6 present the correlation parameters and AARDs between the experimental and calculated solubility of two DESs. The result from the modified Chrastil model indicated AARD of 3.80% for CO2 + ethanol + E6A1 and 0.25% for CO2 + ethanol + E8A1. Notably, for CO2 + ethanol + E8A1, the modified Chrastil model demonstrated the lowest AARD values at 0.25%. In the case of CO2 + ethanol + E8A1, the Kumar–Johnston model exhibited the lowest AARD values at 2.02%. Despite having five parameters, the Adachi–Lu model demonstrated higher AARD values for both the CO2 + ethanol + E6A1 and CO2 + ethanol + E8A1 systems. As anticipated, the modified Chrastil model exhibited lower deviation in the case of CO2 + ethanol + E8A1 due to its consideration of solvent density, cosolvent content, and temperature effects on the solubility of DES. In the case of CO2 + ethanol + E6A1, the Adachi–Lu model yielded the lowest AARD values, indicating that solvent density and temperature effects may be predominant in E6A1 DES solubility. Nevertheless, all three models exhibited a high degree of fitting to the experimental data, with R2 values approaching unity and AARD values converging toward zero percent.
Conclusions
The high-pressure phase behavior of {CO2 + ethanol + E6A1} was investigated using a high-pressure variable-volume view cell, covering temperatures from 308.2 K to 318.2 K and pressures up to 18.89 MPa. At a constant temperature, the solubility of the DES in scCO2 + ethanol increased with pressure. Besides the bubble point pressure, cloud point pressures were observed when the mole ratios of ethanol to scCO2 were below 0.502. The phase behavior of {CO2 + ethanol + E8A1} was also examined to explore the impact of the mole ratio between ethylene glycol as a hydrogen bond donor and allytriphenylphosphonium bromide as a hydrogen bond acceptor. Similar to the {CO2 + ethanol + E6A1} ternary system, the {CO2 + ethanol + E8A1} ternary system exhibited both bubble point and cloud point pressures at high pressure. Generally, as the mole ratio between HBD and HBA increased from 6:1 to 8:1, both bubble point and cloud point pressures also increased. This outcome could be attributed to the heightened interaction between ethylene glycol (HBD) and ethanol (cosolvent) at the molecular level. The solubility of DESs in scCO2/ethanol mixture increased with rising pressure, possibly due to the enhanced solvent power of scCO2. The experimental data were effectively correlated with three models—the modified Chrastil equation, the Kumar–Johnston equation, and the Adachi–Lu equation—with average absolute relative deviations falling in the range of 0.25–4.98%.
Data availability
All data supporting the findings of this study are available within the paper.
References
E. Beckman, Chem. Comm., 1885 (2004).
P. Raveendran, S.L. Wallen, J. Am. Chem. Soc. 124, 12590 (2002)
Y. Park, R.B. Gupta, C.C. Curtis, C.B. Roberts, J. Phys. Chem. B 106, 9696 (2002)
P.W. Bell, A.J. Thote, Y. Park, R.B. Gupta, C.B. Roberts, Ind. Eng. Chem. Res. 42, 6280 (2003)
M.S. Manic, A.J. Queimada, E.A. Macedo, V. Najdanovic-Visak, J. Supercrit. Fluid 65, 1 (2012)
Y. Park, J. Supercrit. Fluids 36, 154 (2005)
J. Wang, Z. Song, L. Chen, T. Xu, L. Deng, Z. Qi, Green. Chem. Eng. 2, 431–440 (2021)
P.A. Mercadal, M.L. Picchio, A. Gonzalez, Food Hydrocolloids 147, 109414 (2024)
R. Al-Frasi, M. Hayyan, Renew. Sust. Energy. Rev. 184, 113505 (2023)
A.K. Lavrinenko, I.Y. Chernyshov, E.A. Pidko, ACS Sustainable Chem. Eng. 11, 15492 (2023)
Y. Park, Korean J. Chem. Eng. 38, 604 (2021)
M. Jung, Y. Park, J. Chem. Eng. Data 67, 416 (2022)
Y. Park, J. Lee, J. Solution Chem. 52, 413 (2023)
S.N.V.K. Aki, A.M. Scurto, J.F. Brennecke, Ind. Eng. Chem. Res. 45, 5574 (2006)
W. Wu, W. Li, B. Han, T. Jiang, D. Shen, Z. Zhang, D. Sun, B. Wang, J. Chem. Eng. Data 49, 1597 (2004)
G. Li, D. Zhou, Q.Q. Xu, G.Y. Qiao, J.Z. Yin, J. Chem. Eng. Data 63, 1596 (2018)
Z. Zhang, W. Wu, Z. Liu, B. Han, H. Gao, T. Jiang, Phys. Chem. Chem. Phys. 6, 2352 (2004)
M.B. Haider, P. Maheshwari, R. Kumar, J. Env. Chem. Eng. 9, 106727 (2021)
S. Sarmad, Y. Xie, J.P. Mikkola, X. Ji, New J. Chem. 41, 290 (2017)
X. Liu, B. Gao, Y. Jiang, D. Deng, J. Chem. Eng. Data 62, 1448 (2017)
M.S. Alhajeri, Z. Wu, D. Rincon, F. Albalawi, P.D. Christofides, Chem. Eng. Res. Des. 167, 268 (2021)
S. Malik, V.K. Sharma, Korean J. Chem. Eng. 40, 2581 (2023)
J. Chrastil, J. Phys. Chem. 86, 3016 (1982)
J.C. González, M.R. Vieytes, A.M. Botana, J.M. Vieites, K.M. Botana, J. Chromatogr. A 910, 119 (2001).
S.K. Kumar, K.P. Johnston, J. Supercrit. Fluids 1, 15 (1988)
Y. Adachi, B.C.-Y. Lu, Fluid Phase Equilib. 1983(14), 147 (1983)
Y. Ozbakir, C. Erkey, J. Supercrit. Fluids 98, 153 (2015)
S.Y. Hwang, Y. Park, K. Park, J. Chem. Thermodyn. 43, 339 (2011)
S.Y. Hwang, Y. Park, K. Park, J. Chem. Eng. Data 57, 2160 (2012)
M. Kim, J. Lee, S. Lee, Y. Park, J. Chem. Thermodyn. 121, 91 (2018)
C. Garlapati, G. Madras, Thermochim. Acta 500, 123 (2010)
F.P. Pelaquim, A.M.B. Neto, I.A.L. Dalmolin, M.C. da Costa, Ind. Eng. Chem. Res. 60, 8607 (2021)
X. Zha, S. Han, W. Wang, Z. Jiao, J. Chem. Thermodyn. 131, 104 (2019)
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2020R1F1A10488128). This work was supported by 2023 Hongik University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, Y. The Solubility of Deep Eutectic Solvents Derived from Allytriphenylphosphonium Bromide in Supercritical Carbon Dioxide in the Presence of Ethanol as a Cosolvent. Korean J. Chem. Eng. 41, 2091–2097 (2024). https://doi.org/10.1007/s11814-024-00123-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00123-3