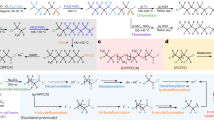
Abstract
Hexafluoropropylene oxide (HFPO) is a versatile fluorochemical widely used in the synthesis of various fluorinated compounds and fluorinated polymers. In this paper, we report on the successful synthesis of HFPO via the epoxidation of hexafluoropropylene (HFP) with NaOCl in a two-phase solvent system. Among the organic phase solvents tested, hydrofluoroethers such as C4F9OCH3, C4F9OC2H5, and C7F15OC2H5 showed high HFPO yields, indicating their potential to replace conventional CFCl2CF2Cl (CFC-113), which is ozone depleting and global warming chemical. When the reaction was carried out for 20 min at room temperature, the C4F9OCH3—water two-phase system produced HFPO with over 40% yield and over 70% selectivity. To optimize the reaction conditions, various reaction parameters were investigated, including the effects of NaOH and phase transition catalysts. Analysis of the by-products using 19F and 13C NMR and X-ray diffraction (XRD) showed that HFP/HFPO decomposes during oxidation to F¯, CO2, oxalate, trifluoroacetate, etc. Density functional theory (DFT) calculations elucidated the reaction pathway of this epoxidation: with a lower E-barrier of 12.8 kcal/mol, the nucleophilic attack of OCl¯ on the β-carbon of HFP is preferable to the α-carbon pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
E.I du Pont de Nemours & Co. first reported on hexafluoropropylene oxide (HFPO, 2,2,3-trifluoro-3-trifluoromethyloxirane) in 1959, and since then it has become one of the most important perfluoro compounds, as a raw material for various fluorinated chemicals and fluorinated polymers including Teflon, Nafion, and Krytox [1, 2]. Recently, it has attracted industrial attention again as a starting material for hexafluoroacetone, which is one of the precursors for fluoropolyimide, a film material suitable for foldable electronic devices [3].
HFPO can be synthesized from hexafluoropropylene (HFP) by electrophilic, radical or nucleophilic reaction with difference oxidants [1, 4,5,6]. In the electrophilic reaction, HFP reacts with KMnO4-anhydrous HF or Cr2O3–FSO3H at 70 °C to produce HFPO with yields of 30% and 55%, respectively [7, 8].
The second, radical reaction, involves HFP reacting with molecular oxygen in the presence of a radical generator such as benzoyl peroxide. According to the patents, very high yields of more than 90% were obtained using this process [1]. Recently, direct epoxidations of HFP using molecular oxygen have been developed [9,10,11]. The first reported system proceeded at high pressure and high temperature (around 200 °C) in the presence of fluorocarbons or chlorofluorocarbons (CFCs) as solvent [12]. This system achieved a 76% yield of HFPO but produced toxic COF2 as a side-product. Lokhat et al. introduced a continuous system for the synthesis of HFPO from HFP in the gas phase using O2 without any catalyst [9, 13, 14]. The reaction at around 200 °C reached 40% yield of HFPO.
Although these electrophilic reactions and radical reactions have recently been studied for the synthesis of HFPO, the first industrialized process for HFPO was a nucleophilic reaction with H2O2/NaOH or NaOCl [15]. The reaction between HFP and hydrogen peroxide H2O2 was carried out in a water–methanol mixture solvent system, where alkali metal bases such as KOH or NaOH were used to generate active oxidant species HOO¯. A higher yield of more than 50% HFPO could be achieved in this H2O2-KOH system by slowly adding these alkaline solutions to the reaction mixture, thereby reducing the decomposition of H2O2.
Meanwhile, a less expensive oxidant, hypochlorite (OClˉ), can also oxidize HFP to HFPO (Scheme 1). The reaction is carried out at pH 9–11 and 15–20 °C in the presence of water and a water-miscible organic solvent such as acetonitrile or diglyme, which increases the solubility of HFP in the reaction mixture. In order to increase the contact of hydrophobic HFP and hydrophilic NaOCl, Kazuyoshi et al. proposed that the HFP in organic solvent and NaOCl in the water be fed into a micro mixer such as a 1.0 mm diameter tube, where the reaction can happen. The advantages of this method are a short reaction time, no need for a phase transfer catalyst, and high product selectivity, of over 99%. However, this method does not seem easy to scale-up due to a clogging problem, caused by by-product salt, NaCl and side-product NaF in the micro mixer tube (Scheme 1 and Supplementary information S1).
Accordingly, one of the methods used for the synthesis of HFPO was to oxidize HFP using NaOCl in a two-phase solvent system in the presence of a phase transfer catalyst (PTC) such as quaternary ammonium salts (Scheme 2). Chlorofluorocarbons (CFCs) such as CFCl2CClF2 (CFC-113) and water have been used as solvents for HFP and NaOCl, respectively. As a PTC, trioctylmethylammonium chloride (TOMAC) has been used. With this method, HFPO can be synthesized with a yield of 69% and a selectivity of over 99% [16]. However, considering the ozone depletion and global warming potential of CFCs, there is a great need to replace traditional CFC-based solvents with an environmentally benign solvent.
Here, hydrofluoroethers (HFEs) such as C4F9OCH3, C4F9OC2H5, and C7F15OC2H5 are proposed as solvents for the epoxidation of HFP with NaOCl. These HFEs are known engineered fluids that can be used as a substitute for CFC, HCFC, HFC, and PFC as a solvent, dispersion medium, heat transfer agent, and aerosol, because they have no ozone depleting potential and very little global warming potential (Table 1). Furthermore, the acute toxicities of HFEs are also lower than that of CFC-113 [17,18,19].
In the HFP oxidation reaction, HFEs were found to be a viable alternative to traditional CFC solvents such as CF2ClCFCl2 (CFC-113). The effects of various reaction conditions such as reaction time, the amount of base, and phase transfer catalyst were investigated. In addition, for the first time, the side products formed during HFP oxidation were analyzed in qualitative and quantitative ways. Density functional theory (DFT) calculations were also conducted to understand the reaction mechanism of the HFP epoxidation.
Experimental
Materials
NaOCl (aq. 12 wt%) was purchased from Yakuri (Japan). 1,1,2-trichloro-2,2,1-trifluoroethane (CF2ClCFCl2, CFC-113) and hydrofluoroethers(HFEs) such as C4F9OCH3 (HFE-7100), C4F9OC2H5 (HFE- 7200), and C7F15OC2H5 (HFE-7500) provided by 3 M™. HFP and HFPO were supplied by Zhejiang Huanxin Fluoro Material Co.,Ltd. (China). Phase-transfer catalysts (PTC) and other chemicals were purchased from Sigma Aldrich.
Epoxidation of HFP
Epoxidation of HFP was carried out in pressure reaction vessels (Andrews glass) equipped with a thermocouple and pressure gauge. NaOCl solution, HFEs, NaOH, and PTC were added to the reactor and purged with N2. Afterward, the reactor was filled with HFP and stirred at room temperature for 20 min. Because the reaction is exothermic, a water bath was used to keep the reaction temperature constant. (Fig. S1).
The conversion of HFP (CHFP), the yield of HFPO (YHFPO), and the selectivity to HFPO (SHFPO) were determined, as in following equations.
where [HFP]i is the initial mole of HFP, [HFP]f and [HFPO] are the moles of HFP and HFPO after reaction, respectively. The yield of side product (Yside(%)) indicates the amount of HFP converted to side products such as CF3CO2¯, oxalate (C2O42¯), CO2, and polymeric materials. The details for quantitation and qualification for the product are shown in supplementary information S3–S6.
Instruments
The gas product was analyzed using GC–MS (Agilent 5973–7890 MSD) equipped with a PoraPlot Q column (Agilent, 25 m × 0.32 mm x 10 μm). The liquid product was analyzed by 19F NMR and 13C NMR (Bruker 400 MHz) to characterize the side products and determine the F content, and the solid remaining after the removal of all volatile components was analyzed by XRD (Bruker, D8 advance).
Computational Studies
The DFT calculations in this study were performed using the Gaussian16 suite with the Becke’s three-parameter hybrid exchange functional and the Lee–Yang–Parr’s correlation functional (B3LYP) with a polarization and diffusion combined basis set of 6–31 + g(d,p) for all atoms [20,21,22,23]. The convergence criterion of the total energy difference was set as 10–6 a.u for the stationary geometry optimizations. Harmonic vibrational frequency calculations were performed to confirm one imaginary frequency of the saddle point for the expected transition state (TS). Solvation correction was performed using the universal continuum solvation model (SMD) with water solvent parameters for all gas-phase optimized structures, and the relative free energy calculation was corrected by zero point vibrational energy (ZPVE), with thermal corrections at a temperature of 298.15 K, and a pressure of 1 atm [24].
Results and Discussion
Solvent Screening
The oxidation of hexafluoropropylene (HFP) with NaOCl (aq.) was conducted using 5 different kind of solvent, CF2ClCFCl2 (CFC-113), C4F9OCH3 (HFE-7100), C4F9OC2H5 (HFE-7200), C7F15OC2H5 (HFE-7500), CH2Cl2, and C6F14. The physical properties and environmental impact of these solvents are shown in Fig. 1. The reaction proceeded at room temperature for 20 min in the presence of a phase transfer catalyst, tetrabutylammonium bromide (TBAB). Figure 1 shows that hydrofluoroethers (HFEs) such as HFE-7100, HFE-7200, and HFE-7500 gave a HFPO yield comparable to CFC-113; the yield of HFPO obtained from CFC-113 was 47.7%, while the yields from HFE-7100, HFE-7200, and HFE-7500 solvent systems were 37.0, 41.2, and 35.2%, respectively.
In terms of selectivity to HFPO, the HFEs showed higher values than CFC-113. The selectivity to HFPO in the HFEs solvents were 67–74%, while it was 62% in the CFC-113 solvent. CH2Cl2, a versatile solvent used in lab and industry for two-phase reaction, showed a HFPO yield and selectivity similar to that of the HFEs. However, after the reaction, it was found that a large amount of CCl4 was present in the organic phase, which presumably was synthesized from the chlorination of CH2Cl2 by NaOCl or Cl2. CCl4 is also an ozone depleting and global warming chemical. Perfluorohexane (C6F14), which was expected to have high solubility for HFP and HFPO, resulted in a negligible amount of HFP due to the low solubility for HFPO and TBAB in the solvent.
One of the primary roles of a solvent in a chemical reaction is to drive the reaction by dissolving the reactants uniformly through solvation. In this respect, solubility of HFP in the organic solvent can be an important factor for this reaction. Although the exact solubility of HFP in each solvent at reaction temperature was not determined, the relative solubility could be estimated by observing the pressure drop of HFP in the reactor. Fig. S2 shows that in all solvents except C6F14, the pressure of reactor decreases rapidly in 5 min when the reaction is barely underway, and the pressure is maintained for 20 min when the reaction almost finish. In the case of C6F14, the pressure did not decrease as much as other solvent tested, which might be the main reason for the poor HFP conversion/HFPO yield in this reaction. For the other solvents tested, the conversion of HFP and the pressure drop did not correlate exactly, suggesting that other factors, such as the solubility of OCl¯/PTC in the organic phase and its ability to stabilize reaction intermediates, also play a role in the reaction.
Overall, the substitution of CFC-113 with HFEs seems quite promising in terms of HFPO yield and selectivity, and HFE-7200 showed the highest HFPO yield among the tested HFEs.
When the gas phase of the product was analyzed using GC and GC-Mass, the only components detected were HFP and HFPO (Supplementary information Fig. S3). However, the selectivity to HFPO was 50–80% in all the solvents tested, which indicates that some HFP and HFPO turn to non-gaseous materials during the reaction. One possible side product is a polymeric material formed from the polymerization of HFPO. The other side product could be over-oxidation species like CO2.
For a better understanding of the side products formation, the solution phases were analyzed after the reaction. The evaporation of all volatile components such as HFP, HFPO, and organic solvent from the organic phase left small amounts of viscous material, indicating polymeric compounds that were formed from HFPO during the reaction. Meanwhile, the analysis of the water phase showed the decomposition of HFP/HFPO had proceeded to a substantial degree to trifluoroacetate (CF3CO2¯), oxalate (C2O42¯), CO2, and fluoride (F¯).
When all of the volatile components were removed from the water phase after the reaction, a white powder remained. XRD analyses revealed the main components of the white powder were NaCl and NaF (Fig. 2). NaCl is the by-product of HFP oxidation, as shown in Scheme 1, while NaF is one of the side products, formed by the decomposition of HFPO during the oxidation.
When HFP is oxidized, over-oxidation produces trifluoroacetate (CF3CO2¯), oxalate (C2O42¯), and CO2 with the liberation of F¯ [1, 25]. The analyses of the aqueous phase using 13C and 19F NMR clearly showed the formation of these side products. 19F NMR shown in Fig. 3 reveals the peaks of CF3CO2¯ (at − 75.4 ppm) and F¯ (at − 121.6 ppm). The 13C NMR spectrum also shows the peaks of CF3CO2¯ (172 0.5 ppm for CO2¯, and 116 ppm for CF3), oxalate ion (172.8 ppm), and carbonate ion (168.6 ppm).
Based on the intensities of each component in the 19F NMR, the concentration of CF3CO2¯, F¯ and unknown fluorine components were quantitated according to the organic solvent used (Fig. S4 in SI). Among the tested solvents, CFC-113 showed the highest F¯ concentration, indicating over-oxidation happened quite heavily. At the same time, the F¯ concentrations of HFEs were less than that of CFC-113. When the entire F concentration is divided by 6, the amount of over-oxidized HFPO can be calculated. Figure 1 and Fig. S4 also show that the degradation of HFP/HFPO was more severe in CFC-113 than in the HFEs, which may be another advantage of HFEs, besides environmental effects.
Effect of NaOH
NaOCl is a basic material; the pH of aq. NaOCl (12 wt%) is 11.6. However, in the absence of NaOH, Cl2 is easily formed by the decomposition of NaOCl due to the decrease in pH during the reaction as Eq. (1) (the pH after the reaction without NaOH was 4.9) [26]. In the absence of NaOH, a yellow gas was observed in the reactor (Fig. S6 and Fig. S7) with the formation of CF3CFClCF2Cl, a Cl2 added product to CF3CF=CF2 (HFP). Accordingly, in the epoxidation of HFP to HFPO, it was found that the HFPO yield and side product formation were highly depended on the NaOH/NaOCl molar ratio.
Figure 4 shows that when the reaction was conducted in the absence of NaOH, it resulted in negligible HFPO formation. However, the addition of NaOH increased the HFPO yield and increased side product formation. When the molar ratio of NaOH/NaOCl was changed from 0.3 to 1.0 with a constant amount of NaOCl (30 mmol), the yield of HFPO increased linearly from 9.4 to 15.1% with increased side product yields; the HFP portion that decomposed to CF3CO2¯, (CO2)22¯, and CO32¯ increased from 5.5 to 10.2%. Interestingly, when the amount of NaOH was increased to NaOH/NaOCl = 1.3, a rapid increase in HFPO yield to 41.2% was observed, which is almost triple compared to the HFPO yield at NaOH/NaOCl = 1. However, a further increase in NaOH/NaOCl to 1.6 resulted in a decrease in yield to 32.8% due to the increased decomposition of HFPO. Overall, the optimum molar ratio of NaOH/NaOCl for the highest HFPO yield was 1.3 when the amounts of HFP and NaOCl were 20 mmol and 30 mmol, respectively.
Mechanism Study
The epoxidation of HFP to obtain HFPO is initiated by the nucleophilic attack of the hypochlorite ion on the sp2-carbon of HFP. The nucleophilic addition of O–Cl can occur at two different possible sites: (1) carbon-α and (2) carbon-β as shown in Fig. 5. Each of the sp2-carbons have different electrophilicity, caused by the presence of the electron withdrawing trifluoromethyl group on the carbon-β site. Previous relevant studies on the OCl-mediated epoxidation of fluoro-olefin predicted a reaction pathway according to the product configurations and their concentrations [27,28,29]. Although the two reaction paths resulted in the same product as in this HFP oxidation, the detailed reaction pathway was investigated via Density Functional Theory (DFT) study.
Two different propagating intermediates and transition states are incorporated in the overall kinetics of the reaction, as presented in Fig. 5. Depending on the reaction site, the intermediate states are observed in the form of intα and intβ, and the corresponding transition states, TSα and TSβ, are formed by the release of the chloride ion. The presence of a hydroxide ion adjacent to the chlorine helps the chloride ion release by abstracting a partially positively charged Cl from the electron rich oxygen atom. Thus, the addition of a base can accelerate the reaction rate, which is in line with the experimental results of NaOH addition.
Despite having similarly endergonic intermediates, the overall energy profile of each reaction pathway indicates that the route for HFP epoxidation prefers to follow the β-carbon pathway with a total energy barrier of 9.4 kcal/mol, whereas the α-carbon pathway requires 22.2 kcal/mol, as presented in Fig. 6.
The energy differences between the pathways can be explained using a geometrical analysis, as in S6 of SI. The impact of the –CF3 substituent on the interaction between HFP and a hypochlorite ion allows the C–O bond in the transition state to have a different bond distance and Wiberg bond index (WBI) depending on the reaction site carbon [30]. Because of the low electron density of C2, the C2–O bond in TSβ is relatively strong compared with the C1–O in TSα considering the short bond distance of C2–O in TSβ (1.394 Å). This leads to the O–Cl bond in TSβ being stretched further than the one in TSα. Thus, the simultaneous bond dissociation of O–Cl is rather undemanding in the β pathway, followed by the easy bond formation of C1–O.
Effect of PTC
Various kinds of quaternary ammonium salt-based phase transfer catalysts (PTCs) were tested and the results are shown in Fig. 7. Tetramethylammonium chloride (TMAC) showed no HFPO in the gas phase, presumably due to the low solubility of TMAC in the organic phase, HFE-7200. With the increasing length of the alkyl group in the ammonium salt, from ethyl to butyl, the yield of HFPO increased rapidly to 41.2% along with an increase of side products formation. Trioctylmethylammonium chloride (TOMAC) had HFP conversion similar to TBAC, but a lower HFPO yield due to the increased by-product formation. On the other hand, the difference between chloride and bromide as an counter anion in PTC was insignificant, as was also observed by Wang et al. with the oxidation of benzyl alcohol by NaOCl [31].
Like NaOH, PTC is an indispensable component in this oxidation reaction. Without PTC, the epoxidation reaction only marginally occurs (Fig. 8). Increasing the amount of PTC from 0.5 mol% to 3 mol% with respect to the amount of HFP led to an increase in HFP conversion, from 20.3% to 65.6%. However, the effect was more pronounced in the side product formation; it increased from 5.5% to 35.5%. As a result, the yield of HFPO reached a maximum value of 41.2% at 1.0% addition of TBAB, but decreased to 30.2% at 3 mol% of TBAB condition.
Effect of Reaction Time
As can be seen in Fig. 9, the yield of HFPO reached its peak at 20 min. Prolonging the reaction time did not increase the conversion but slightly decreased product selectivity, from 72.7% to 68.3%. After reaching the highest HFPO yield at 20 min, longer reaction times did not significantly increase over-oxidation products. In fact, HFPO itself was quite stable to NaOCl and NaOH. When HFPO was reacted with NaOH and NaOCl, only 1.9% of HFPO was oxidized. HFP was also very stable to NaOH, at least for 20 min. Although the pathway for the formation of the byproduct was presumed to begin with HFPO oxidation (Supplementary Information S1), these results indicate over-oxidation did not proceed via HFPO but via some transition species during HFP oxidation.
Conclusion
In conclusion, this study explored the synthesis of hexafluoropropylene oxide (HFPO) through the epoxidation of hexafluoropropylene (HFP) using NaOCl in a two-phase solvent system. The investigation of various organic phase solvents highlighted the efficacy of hydrofluoroethers such as C4F9OCH3, C4F9OC2H5, and C7F15OC2H5 in providing high yields of HFPO, suggesting their potential as environmentally friendly alternatives to conventional and environmentally harmful compounds such as CFC-113. Through a systematic investigation of reaction parameters, including the effects of NaOH and phase transition catalysts, we determined that the use of a C4F9OC2H5-water two-phase system, which achieved 41.2% yield and 72.7% selectivity for HFPO synthesis within a short reaction time at room temperature, stands out as a promising approach. Density Functional Theory (DFT) calculations to elucidate the reaction pathway add a theoretical dimension to the study, further enhancing our understanding of the underlying mechanisms.
Data availability
The data that support the findings of this study are available on request from the corresponding author (H. Lee).
References
H. Millauer, W. Schwertfeger, G. Siegemund, Angew. Chem. Int. Ed. Engl. 24, 161 (1985)
J.T. Hill, J. Macromol. Sci. Part A - Chem. 8, 499 (1974)
G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick, P. Kirsch, Fluorine compounds organic, in Ullmann’s encyclopedia of industrial chemistry. (Wiley, Hoboken, 2016)
D. Saunders, J. Heicklen, J. Am. Chem. Soc. 87, 2088 (1965)
G.G. Furin, Chem. Sustainable Dev. 14, 97 (2006)
O.V. Kuricheva, V.A. Dunyakhin, V.V. Timofeev, Y.N. Zhitnev, Russ. Chem. Bull. 48, 45 (1999)
M. Łgiewczyk, Z. Czech, Pol. J. Chem. Technol. 12, 1 (2010)
E. Meissner, A. Wróblewska, Pol. J. Chem. Technol. 9, 20 (2007)
D. Lokhat, D. Ramjugernat, M. Starzak, Chem. Eng. Commun. 201, 1173 (2014)
Z. Huang, Y. Zhang, C. Zhao, J. Qin, H. Li, M. Xue, Y. Liu, Appl. Catal. A 303, 18 (2006)
M. Dos Santos Afonso, R.M. Romano, C.O. Della Védova, J. Czarnowski, Phys. Chem. Chem. Phys. 2, 1393 (2000)
A. Wróblewska, E. Milchert, E. Meissner, Org. Process Res. Dev. 14, 272 (2010)
D. Lokhat, A. Singh, M. Starzak, D. Ramjugernath, Chem. Eng. Res. Des. 119, 93 (2017)
D. Lokhat, M. Starzak, D. Ramjugernath, Prog. React. Kinet. Mech. 41, 418 (2016)
H. S. Eleuterio, R. W. Meschke, US Patent, 3,358,003 (1967)
K. Ichihara, H. Nakaya, M. Hirai, Y. Senba, EP patent, 2,409,970 A1 (2010)
3MTM NovecTM 7100 Engineered Fluid | 3M United States, https://www.3m.com/3M/en_US/p/d/b40044867/
3MTM NovecTM 7200 Engineered Fluid | 3M United States, https://www.3m.com/3M/en_US/p/d/b40045142/
Novec, 3MTM NovecTM 73DE Engineered Fluid | 3M United States, https://www.3m.com/3M/en_US/p/d/b40045191/
P.J. Stephens, F.J. Devlin, C.F. Chabalowski, M.J. Frisch, J. Phys. Chem. 98, 11623–11627 (1994)
P.C. Hariharan, J.A. Pople, Theor. Chim. Acta 28, 213–222 (1978)
G.A. Petersson, A. Bennett, T.G. Tensfeldt, M.A. Al Laham, W.A. Shirley, J. Mantzaris, J. Chem. Phys. 89, 2193–2218 (1988)
G.A. Petersson, M.A. Al Laham, J. Chem. Phys. 94, 6081–6090 (1991)
A.V. Marenich, C.J. Cramer, D.G. Truhlar, J. Phys. Chem. B 113, 6378–6396 (2009)
The Conversion of Hexafluoropropylene (HFP) to Hexafluoropropylene Oxi—Fluoryx Labs, https://fluoryx.com/blogs/news/the-conversion-of-hexafluoropropylene-hfp-to-hexafluoropropylene-oxide-hfpo
L. Wang, M. Bassiri, R. Najafi, K. Najafi, J. Yang, B. Khosrovi, W. Hwong, E. Barati, B. Belisle, C. Cleri, M.C. Robson, J. Burns Wounds 6, e5 (2007)
Z.G. Zhang, H. Yin, X. Fang, J. Zhejiang Univ. Sci. A. 7, 325–329 (2006)
J. Ji, Z. Lu, Y. Lei, C.H. Turner, Catalysts 8, 421 (2018)
T. Hansen, P. Vermeeren, A. Haim, M.J.H. van Dorp, J.D.C. Codée, F.M. Bickelhaupt, T.A. Hamlin, Eur. J. Org. Chem. 25, 3822–28 (2020)
K.B. Wiberg, Tetrahedron 24, 1083–1096 (1968)
M.L. Wang, T.H. Huang, Chem. Eng. Commun. 194, 618 (2007)
Acknowledgements
This work was supported by the Industrial Strategic Technology Development Program (Project No. 20016071) through Korea Evaluation Institute of Industrial Technology (KEIT) funded by the Ministry of Trade, Industry and Energy (MOTIE), Republic of Korea, and was supported by KIST internal program (Atmospheric Environment Research Program, Project No. 2E32380).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, N.T., Cheong, S., Ramadhan, M.D. et al. Synthesis of Hexafluoropropylene Oxide from Hexafluoropropylene and Hypochlorite Using Hydrofluoroether Solvent. Korean J. Chem. Eng. 41, 1833–1840 (2024). https://doi.org/10.1007/s11814-024-00120-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00120-6